In Vitro Bioactivity of Australian Finger Lime Cultivars as an Initial Evaluation of Their Nutraceutical Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Extraction and Preparation of Crude Crystals
2.3. Determination of Antioxidant Capacity, Total Flavonoid Content, and TMAC
2.4. Determination of α-Glucosidase Inhibition
2.5. Determination of α-Amylase Inhibition
2.6. Determination of Acetylcholinesterase Inhibition
2.7. Determination of Tyrosinase Inhibition
2.8. Determination of Anti-Inflammatory Activity (COX-2 Inhibition)
2.9. In Silico Docking of Selected Polyphenols
2.10. Data Analysis and Statistics
3. Results
3.1. Antioxidant Capacity, TFC, and TMAC
3.2. Anti-Diabetic Activity
3.3. Anti-Alzheimer Activity
3.4. Anti-Tyrosinase Activity
3.5. Anti-Inflammatory Activity
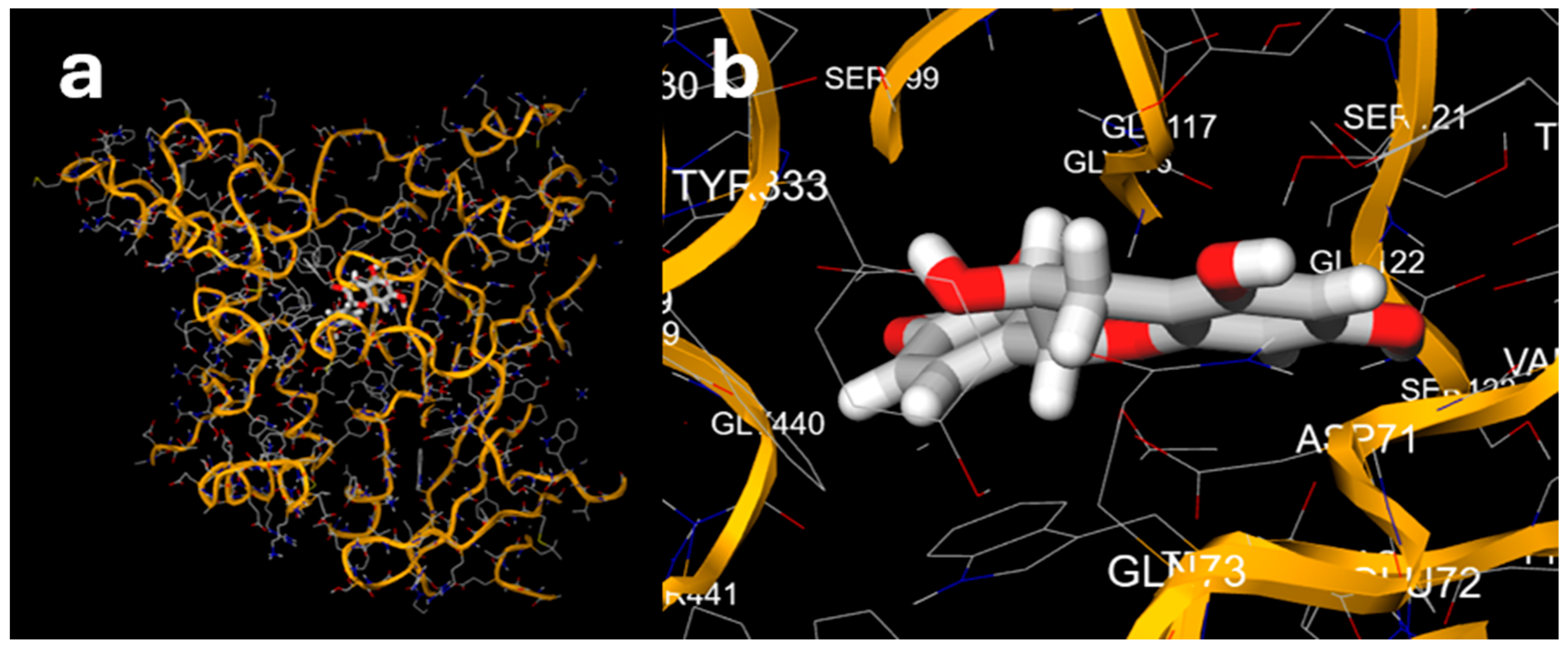
3.6. Docking of Selected Polyphenols Against Acetylcholinesterase
3.7. General Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agrifutures Australia. Finger Lime. Available online: https://www.agrifutures.com.au/farm-diversity/finger-lime/ (accessed on 8 March 2024).
- Mabberley, D.J. Australian Citreae with notes on other Aurantioideae (Rutaceae). Telopea 1998, 7, 333–344. [Google Scholar] [CrossRef]
- Delort, E.; Yuan, Y.-M. Finger lime/The Australian Caviar—Citrus australasica. In Exotic Fruits; Rodrigues, S., de Oliveira Silva, E., de Brito, E.S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 203–210. [Google Scholar]
- Johnson, J.; Mani, J.; Batley, R.; Hoyos, B.; Novello, N.; Thani, P.; Arachchige, C.; Neupane, P.; Naiker, M. Functional Foods or Over-Hyped? Observations on the Antioxidant and Phenolic Content of Australian Foodstuffs. Biol. Life Sci. Forum 2023, 26, 17. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C. Chemistry of the Antioxidant Effect of Polyphenols. Ann. N. Y. Acad. Sci. 2002, 957, 57–69. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Dadwal, V.; Joshi, R.; Gupta, M. A comparative metabolomic investigation in fruit sections of Citrus medica L. and Citrus maxima L. detecting potential bioactive metabolites using UHPLC-QTOF-IMS. Food Res. Int. 2022, 157, 111486. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Peleg, H.; Naim, M.; Rouseff, R.L.; Zehavi, U. Distribution of bound and free phenolic acids in oranges (Citrus sinensis) and Grapefruits (Citrus paradisi). J. Sci. Food Agric. 1991, 57, 417–426. [Google Scholar] [CrossRef]
- Johnson, J.B.; Batley, R.; Manson, D.; White, S.; Naiker, M. Volatile compounds, phenolic acid profiles and phytochemical content of five Australian finger lime (Citrus australasica) cultivars. LWT 2022, 154, 112640. [Google Scholar] [CrossRef]
- Delort, E.; Jaquier, A. Novel terpenyl esters from Australian finger lime (Citrus australasica) peel extract. Flavour Fragr. J. 2009, 24, 123–132. [Google Scholar] [CrossRef]
- Delort, E.; Jaquier, A.; Decorzant, E.; Chapuis, C.; Casilli, A.; Frérot, E. Comparative analysis of three Australian finger lime (Citrus australasica) cultivars: Identification of unique citrus chemotypes and new volatile molecules. Phytochemistry 2015, 109, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, R.; Câmara, J.S.; Malorni, L.; Amato, G.; Cannavacciuolo, C.; Masullo, M.; Piacente, S. Comparative Volatilomic Profile of Three Finger Lime (Citrus australasica) Cultivars Based on Chemometrics Analysis of HS-SPME/GC-MS Data. Molecules 2022, 27, 7846. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Dutt, M.; Vashisth, T. Comparative phytochemical analysis of the fruits of four Florida-grown finger lime (Citrus australasica) selections. LWT 2021, 135, 110003. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, S.; Zang, W.; Wang, N.; Cao, J.; Li, X.; Sun, C. Identification of phenolic compounds from a unique citrus species, finger lime (Citrus australasica) and their inhibition of LPS-induced NO-releasing in BV-2 cell line. Food Chem. Toxicol. 2019, 129, 54–63. [Google Scholar] [CrossRef]
- Cioni, E.; Migone, C.; Ascrizzi, R.; Muscatello, B.; De Leo, M.; Piras, A.M.; Zambito, Y.; Flamini, G.; Pistelli, L. Comparing Metabolomic and Essential Oil Fingerprints of Citrus australasica F. Muell (Finger Lime) Varieties and Their In Vitro Antioxidant Activity. Antioxidants 2022, 11, 2047. [Google Scholar] [CrossRef]
- Cáceres-Vélez, P.R.; Ali, A.; Fournier-Level, A.; Dunshea, F.R.; Jusuf, P.R. Phytochemical and Safety Evaluations of Finger Lime, Mountain Pepper, and Tamarind in Zebrafish Embryos. Antioxidants 2022, 11, 1280. [Google Scholar] [CrossRef]
- Aznar, R.; Rodríguez-Pérez, C.; Rai, D.K. Comprehensive Characterization and Quantification of Antioxidant Compounds in Finger Lime (Citrus australasica L.) by HPLC-QTof-MS and UPLC-MS/MS. Appl. Sci. 2022, 12, 1712. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Liu, F.; Cui, Y.; Li, X. Dietary citrus and/or its extracts intake contributed to weight control: Evidence from a systematic review and meta-analysis of 13 randomized clinical trials. Phytother. Res. 2020, 34, 2006–2022. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Effects of Citrus Fruit Juices and Their Bioactive Components on Inflammation and Immunity: A Narrative Review. Front. Immunol. 2021, 12, 712608. [Google Scholar] [CrossRef]
- Richmond, R.; Bowyer, M.; Vuong, Q. Australian native fruits: Potential uses as functional food ingredients. J. Funct. Foods 2019, 62, 103547. [Google Scholar] [CrossRef]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.B.; Skylas, D.J.; Mani, J.S.; Xiang, J.; Walsh, K.B.; Naiker, M. Phenolic Profiles of Ten Australian Faba Bean Varieties. Molecules 2021, 26, 4642. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Collins, T.; Power, A.; Chandra, S.; Portman, D.; Blanchard, C.; Naiker, M. Antioxidative properties and macrochemical composition of five commercial mungbean varieties in Australia. Legume Sci. 2020, 2, e27. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Johnson, J.; Collins, T.; Walsh, K.; Naiker, M. Solvent extractions and spectrophotometric protocols for measuring the total anthocyanin, phenols and antioxidant content in plums. Chem. Pap. 2020, 74, 4481–4492. [Google Scholar] [CrossRef]
- Ušjak, L.J.; Milutinović, V.M.; Đorđić Crnogorac, M.J.; Stanojković, T.P.; Niketić, M.S.; Kukić-Marković, J.M.; Petrović, S.D. Barks of Three Wild Pyrus Taxa: Phenolic Constituents, Antioxidant Activity, and in Vitro and in Silico Investigations of α-Amylase and α-Glucosidase Inhibition. Chem. Biodivers. 2021, 18, e2100446. [Google Scholar] [CrossRef]
- Zheng, B.; Yuan, Y.; Xiang, J.; Jin, W.; Johnson, J.B.; Li, Z.; Wang, C.; Luo, D. Green extraction of phenolic compounds from foxtail millet bran by ultrasonic-assisted deep eutectic solvent extraction: Optimization, comparison and bioactivities. LWT 2021, 154, 112740. [Google Scholar] [CrossRef]
- Johnson, J.B.; Neupane, P.; Bhattarai, S.P.; Trotter, T.; Naiker, M. Phenolic profiles and potential anti-Alzheimer activity of Australian adzuki bean. In Proceedings of the 72nd Australasian Grain Science Conference, Canberra, Australia, 24–26 August 2022; p. 48. [Google Scholar]
- Momtaz, S.; Mapunya, B.M.; Houghton, P.J.; Edgerly, C.; Hussein, A.; Naidoo, S.; Lall, N. Tyrosinase inhibition by extracts and constituents of Sideroxylon inerme L. stem bark, used in South Africa for skin lightening. J. Ethnopharmacol. 2008, 119, 507–512. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, version 4.2.3; R Foundation for Statistical Computing: Vienna, Austria, 2023.
- Kajaria, D.; Ranjana; Tripathi, J.; Tripathi, Y.B.; Tiwari, S. In-vitro α amylase and glycosidase inhibitory effect of ethanolic extract of antiasthmatic drug—Shirishadi. J. Adv. Pharm. Technol. Res. 2013, 4, 206–209. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products as α-Amylase and α-Glucosidase Inhibitors and their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Saravanan, R. Antidiabetic effect of d-limonene, a monoterpene in streptozotocin-induced diabetic rats. Biomed. Prev. Nutr. 2012, 2, 269–275. [Google Scholar] [CrossRef]
- Basak, S.S.; Candan, F. Effect of Laurus nobilis L. essential oil and its main components on α-glucosidase and reactive oxygen species scavenging activity. Iran. J. Pharm. Res. 2013, 12, 367. [Google Scholar]
- Benayad, O.; Bouhrim, M.; Tiji, S.; Kharchoufa, L.; Addi, M.; Drouet, S.; Hano, C.; Lorenzo, J.M.; Bendaha, H.; Bnouham, M.; et al. Phytochemical Profile, α-Glucosidase, and α-Amylase Inhibition Potential and Toxicity Evaluation of Extracts from Citrus aurantium (L) Peel, a Valuable By-Product from Northeastern Morocco. Biomolecules 2021, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Loh, S. In vitro antioxidant capacities and antidiabetic properties of phenolic extracts from selected citrus peels. Int. Food Res. J. 2016, 23, 211. [Google Scholar]
- Konrath, E.L.; Passos, C.d.S.; Klein-Júnior, L.C.; Henriques, A.T. Alkaloids as a source of potential anticholinesterase inhibitors for the treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2013, 65, 1701–1725. [Google Scholar] [CrossRef]
- Mehta, M.; Adem, A.; Sabbagh, M. New Acetylcholinesterase Inhibitors for Alzheimer’s Disease. Int. J. Alzheimer’s Dis. 2012, 2012, 728983. [Google Scholar] [CrossRef]
- Saito, T.; Nishida, Y.; Tabata, M.; Isobayashi, A.; Tomizawa, H.; Miyahara, Y.; Sugizaki, Y. Molecular Interactions between an Enzyme and Its Inhibitor for Selective Detection of Limonene. Anal. Chem. 2022, 94, 7692–7702. [Google Scholar] [CrossRef]
- Liu, C.; Hou, W.; Li, S.; Tsao, R. Extraction and isolation of acetylcholinesterase inhibitors from Citrus limon peel using an in vitro method. J. Sep. Sci. 2020, 43, 1531–1543. [Google Scholar] [CrossRef]
- Haider, S.; Liaquat, L.; Ahmad, S.; Batool, Z.; Siddiqui, R.A.; Tabassum, S.; Shahzad, S.; Rafiq, S.; Naz, N. Naringenin protects AlCl3/D-galactose induced neurotoxicity in rat model of AD via attenuation of acetylcholinesterase levels and inhibition of oxidative stress. PLoS ONE 2020, 15, e0227631. [Google Scholar] [CrossRef]
- Matsuura, R.; Ukeda, H.; Sawamura, M. Tyrosinase Inhibitory Activity of Citrus Essential Oils. J. Agric. Food Chem. 2006, 54, 2309–2313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, Y.; Tao, L.; Tao, X.; Su, X.; Wei, D. Tyrosinase inhibitory effects and inhibition mechanisms of nobiletin and hesperidin from citrus peel crude extracts. J. Enzym. Inhib. Med. Chem. 2007, 22, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Abirami, A.; Nagarani, G.; Siddhuraju, P. In vitro antioxidant, anti-diabetic, cholinesterase and tyrosinase inhibitory potential of fresh juice from Citrus hystrix and C. maxima fruits. Food Sci. Hum. Wellness 2014, 3, 16–25. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, G.-Y.; Choi, Y.H. Naringenin attenuates the release of pro-inflammatory mediators from lipopolysaccharide-stimulated BV2 microglia by inactivating nuclear factor-κB and inhibiting mitogen-activated protein kinases. Int. J. Mol. Med. 2012, 30, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P.; Roulfe, P.; Pavan, A. Health Benefits of Australian Native Foods: An Evaluation of Health-Enhancing Compounds; Rural Industries Research and Development Corporation: Barton, ACT, Australia, 2009. [Google Scholar]
- Xiao, J.; Ni, X.; Kai, G.; Chen, X. A Review on Structure–Activity Relationship of Dietary Polyphenols Inhibiting α-Amylase. Crit. Rev. Food Sci. Nutr. 2013, 53, 497–506. [Google Scholar] [CrossRef]
- Hua, F.; Zhou, P.; Wu, H.-Y.; Chu, G.-X.; Xie, Z.-W.; Bao, G.-H. Inhibition of α-glucosidase and α-amylase by flavonoid glycosides from Lu’an GuaPian tea: Molecular docking and interaction mechanism. Food Funct. 2018, 9, 4173–4183. [Google Scholar] [CrossRef]
- Sahnoun, M.; Trabelsi, S.; Bejar, S. Citrus flavonoids collectively dominate the α-amylase and α-glucosidase inhibitions. Biologia 2017, 72, 764–773. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, R.; Chen, J.; Cao, J.; Xiao, J.; Li, X.; Sun, C. Tangeretin maintains antioxidant activity by reducing CUL3 mediated NRF2 ubiquitination. Food Chem. 2021, 365, 130470. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Wei, C.-C.; Huang, J.; Pan, M.-H.; Shahidi, F.; Ho, C.-T. Anti-inflammatory effects of polymethoxyflavones from citrus peels: A review. J. Food Bioact. 2018, 3, 76–86. [Google Scholar] [CrossRef]
- Cornara, L.; Xiao, J.; Smeriglio, A.; Trombetta, D.; Burlando, B. Emerging Exotic Fruits: New Functional Foods in the European Market. eFood 2020, 1, 126–139. [Google Scholar] [CrossRef]
- Li, W.-Q.; Kuriyama, S.; Li, Q.; Nagai, M.; Hozawa, A.; Nishino, Y.; Tsuji, I. Citrus consumption and cancer incidence: The Ohsaki cohort study. Int. J. Cancer 2010, 127, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.G.d.; Yoshinaga, M.Y.; Glezer, I.; Chaves-Filho, A.d.B.; Santana, A.A.d.; Kovacs, C.; Magnoni, C.D.; Lajolo, F.M.; Miyamoto, S.; Aymoto Hassimotto, N.M. Orange juice intake by obese and insulin-resistant subjects lowers specific plasma triglycerides: A randomized clinical trial. Clin. Nutr. ESPEN 2022, 51, 336–344. [Google Scholar] [CrossRef] [PubMed]

| Variety | TPC (mg GAE/100 g DW) | FRAP (mg TE/100 g DW) | CUPRAC (mg TE/100 g DW) | TMAC (mg cyd-3-glu/100 g DW) | TFC (mg QE/100 g DW) |
|---|---|---|---|---|---|
| Pulp | |||||
| Durhams Emerald | 328 ± 6 f | 114 ± 15 g | 727 ± 0 g | 1 ± 2 ef | 100 ± 1 h |
| Chartreuse | 341 ± 2 f | 204 ± 4 f | 886 ± 5 g | 1 ± 1 ef | 112 ± 1 h |
| P1f2-10 hybrid | 344 ± 0 f | 234 ± 3 ef | 1235 ± 20 f | 4 ± 0 bcdef | 145 ± 1 h |
| Rhyne Red | 779 ± 22 e | 436 ± 5 bc | 1987 ± 55 e | 6 ± 1 bcde | 392 ± 5 fg |
| Red Champagne | 385 ± 3 f | 225 ± 5 f | 839 ± 18 g | 2 ± 0 def | 108 ± 0 h |
| Tahitian lime | 1043 ± 1 b | 422 ± 10 c | 7849 ± 160 c | 9 ± 1 b | 312 ± 10 g |
| Peel | |||||
| Durhams Emerald | 851 ± 32 d | 349 ± 9 d | 2063 ± 32 e | 7 ± 0 bcd | 526 ± 10 |
| Chartreuse | 1048 ± 8 b | 449 ± 8 bc | 8207 ± 27 b | 0 ± 0 f | 1786 ± 20 b |
| P1f2-10 hybrid | 755 ± 9 e | 259 ± 1 e | 1898 ± 12 e | 4 ± 1 bcdef | 511 ± 6 ef |
| Rhyne Red | 850 ± 24 d | 465 ± 14 ab | 2397 ± 74 d | 8 ± 4 bc | 1560 ± 63 c |
| Red Champagne | 966 ± 15 c | 495 ± 4 a | 2415 ± 5 d | 25 ± 0 a | 640 ± 16 d |
| Tahitian lime | 1704 ± 3 a | 491 ± 8 a | 11104 ± 140 a | 3 ± 0 cdef | 1935 ± 82 a |
| Variety | Crystal Concentration (mg/L) | α-Glucosidase Inhibition (%) | α-Amylase Inhibition (%) |
|---|---|---|---|
| Pulp | |||
| Durhams Emerald | 1450 | 9.5 | 20.2 |
| Chartreuse | 1490 | 0 | 19.3 |
| P1f2-10 hybrid | 1490 | 0 | 15.2 |
| Rhyne Red | 1520 | 0 | 12.0 |
| Red Champagne | 1520 | 0 | 8.9 |
| Tahitian lime | 1580 | 0 | 9.5 |
| Peel | |||
| Durhams Emerald | 1510 | 0 | 23.7 |
| Chartreuse | 1510 | 0 | 17.3 |
| P1f2-10 hybrid | 1450 | 0 | 26.9 |
| Rhyne Red | 1480 | 0 | 18.7 |
| Red Champagne | 1460 | 0 | 13.5 |
| Tahitian lime | 1490 | 0 | 14.2 |
| Variety | Equivalent Sample Concentration (mg/mL) | Acetylcholinesterase Inhibition (%) |
|---|---|---|
| Pulp | ||
| Durhams Emerald | 81.3 | 21.5 |
| Chartreuse | 75.7 | 45.3 |
| P1f2-10 hybrid | 69.2 | 7.5 |
| Rhyne Red | 76.8 | 24.1 |
| Red Champagne | 91.6 | 23.5 |
| Tahitian lime | 93.1 | 39.4 |
| Peel | ||
| Durhams Emerald | 71.9 | 54.6 |
| Chartreuse | 77.3 | 20.2 |
| P1f2-10 hybrid | 71.5 | 36.2 |
| Rhyne Red | 77.4 | 43.8 |
| Red Champagne | 71.5 | 11.4 |
| Tahitian lime | 82.8 | 71.9 |
| Variety | Crystal Concentration (mg/L) | Tyrosinase Inhibition (%) |
|---|---|---|
| Pulp | ||
| Durhams Emerald | 1450 | 0 |
| Chartreuse | 1490 | 0.4 |
| P1f2-10 hybrid | 1490 | 0 |
| Rhyne Red | 1520 | 0.9 |
| Red Champagne | 1520 | 0 |
| Tahitian lime | 1580 | 0 |
| Peel | ||
| Durhams Emerald | 1510 | 4.0 |
| Chartreuse | 1510 | 25.4 |
| P1f2-10 hybrid | 1450 | 4.5 |
| Rhyne Red | 1480 | 9.1 |
| Red Champagne | 1460 | 5.1 |
| Tahitian lime | 1490 | 11.2 |
| Variety | Crystal Concentration (mg/L) | COX-2 Inhibition (%) |
|---|---|---|
| Durhams Emerald pulp | 1450 | Not detected |
| Durhams Emerald peel | 1510 | Not detected |
| Compound | Docking Scores | |||
|---|---|---|---|---|
| Pose #1 | Pose #2 | Pose #3 | Pose #4 | |
| Gallic acid | −6.4 | −6.4 | −6.4 | −6.4 |
| Catechin | −10.0 | −9.0 | −8.9 | −8.7 |
| Gentisic acid | −6.2 | −6.2 | −6.1 | −6.0 |
| Catechol | −5.4 | −5.4 | −5.1 | −5.0 |
| Cyanidin-3-glucoside | −9.1 | −7.7 | −7.6 | −7.6 |
| Rutin | −8.8 | −8.8 | −8.6 | −8.5 |
| Quercetin-3-glucoside | −9.3 | −9.2 | −8.7 | −8.3 |
| Apigenin | −9.0 | −8.7 | −8.5 | −8.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, J.B.; Thani, P.R.; Chen, S.-Y.; Mani, J.S.; Netzel, M.E.; Naiker, M. In Vitro Bioactivity of Australian Finger Lime Cultivars as an Initial Evaluation of Their Nutraceutical Potential. Nutraceuticals 2024, 4, 596-610. https://doi.org/10.3390/nutraceuticals4040032
Johnson JB, Thani PR, Chen S-Y, Mani JS, Netzel ME, Naiker M. In Vitro Bioactivity of Australian Finger Lime Cultivars as an Initial Evaluation of Their Nutraceutical Potential. Nutraceuticals. 2024; 4(4):596-610. https://doi.org/10.3390/nutraceuticals4040032
Chicago/Turabian StyleJohnson, Joel B., Parbat Raj Thani, Si-Yuan Chen, Janice S. Mani, Michael E. Netzel, and Mani Naiker. 2024. "In Vitro Bioactivity of Australian Finger Lime Cultivars as an Initial Evaluation of Their Nutraceutical Potential" Nutraceuticals 4, no. 4: 596-610. https://doi.org/10.3390/nutraceuticals4040032
APA StyleJohnson, J. B., Thani, P. R., Chen, S.-Y., Mani, J. S., Netzel, M. E., & Naiker, M. (2024). In Vitro Bioactivity of Australian Finger Lime Cultivars as an Initial Evaluation of Their Nutraceutical Potential. Nutraceuticals, 4(4), 596-610. https://doi.org/10.3390/nutraceuticals4040032











