Abstract
Euglena gracilis (EG) microalgae has immune-modulating properties, partly due to its unique intracellular β-glucan-granules (paramylon). We evaluated the effects of EG consumption on immune status in vivo, ex vivo, and in vitro. A placebo-controlled cross-over study evaluated acute immune surveillance, followed by a 1-week open-label phase. Immune training was documented using ex vivo immune challenges and cytokine profiles. In vitro testing of monocytes compared the effects of EG to pure β-glucan. Compared to placebo, EG consumption triggered increased T cell numbers in the blood circulation (1 h: p < 0.01) and decreased monocyte numbers (2 h: p < 0.05). Natural killer cells showed increased CD25 expression (1 and 2 h: p < 0.01) and reduced CD69 expression (2 h: p < 0.01). T cells showed reduced CD25 and CD69 expression (p < 0.01). There were no significant changes to serum cytokines. After EG consumption, ex vivo cultures of peripheral blood mononuclear cells showed significant changes to spontaneous and inflammation-induced cytokine levels after 2 h (increased G-CSF: p < 0.01, reduced IL-1β and TNF-α (p < 0.05)) and one week (reduced TNF-α (p < 0.01) and increased IL-10 (p < 0.05)). In vitro, EG-trained monocytes responded differently to a second stimulus than β-glucan-trained monocytes (increased IL-1b: p < 0.1, TNF-α: p < 0.01). EG-mediated training of innate immunity, combined with long-term modulation of inflammation, suggests a nutraceutical strategy for preventive immune support.
1. Introduction
Euglena gracilis (EG) is a spindle-shaped unicellular microalga that lives in most freshwater habitats. EG can grow under both phototropic (autotropic) and heterotrophic conditions; when grown under phototropic conditions, it performs photosynthesis. EG is rich in storage granules called paramylon starch, a β-glucan known to engage Dectin-1 receptors on immune cells and contribute to immune-activating and immune-training events involving epigenetic and metabolic reprogramming [1].
β-glucans are natural fibers found in the cell walls of cereal grains, bacteria, and fungi, with significantly different physical and chemical properties depending on the source. β-glucans have the ability to bind to several different Pattern Recognition Receptors (PRRs), including Toll-Like Receptors (TLRs) [2] and Dectin receptors 1 and 2 [3,4]. Depending on the cell type and its epigenetic state, a cell may express different combinations of PRRs. The binding of β-glucans to different combinations of PRRs triggers selective downstream effects [5].
While paramylon has received much attention as an immune modulator, we have previously documented complementary effects on immune activation by the non-paramylon aqueous fraction of whole EG algae [6]. Both the aqueous fraction and paramylon triggered the activation of natural killer (NK) cells and NKT cells, as measured by increased expression of CD69. When cells were treated with whole EG algae, the increased CD69 expression was much more robust than for either fraction alone, suggesting synergistic effects between the paramylon and aqueous fractions. The regulation of additional cellular responses, such as reactive oxygen species production and resistance to oxidative stress, was strongly supported by both the whole EG algae and paramylon. The aqueous fraction contains antioxidants capable of entering into and protecting living cells from oxidative stress.
The classical understanding of long-term immune memory towards specific antigens involves the adaptive immune response, T and B lymphocyte activation, and hypermutation of genes for immunoglobulins, leading to long-term specific immunological memory toward highly-specific antigens, such as vaccine antigens. In addition, another type of immune memory involving the innate part of the immune system, responsible for immediate defense against invading pathogens, is becoming increasingly appreciated. This innate immune memory is also called trained immunity. β-glucans induce trained immunity, where immune cells that have been primed by binding of β-glucans to Dectin-1, and likely other cell surface receptors, respond more powerfully to a subsequent encounter with a pathogen [7,8]. To demonstrate ‘training’, it is necessary to document the magnitude of the second response to an immune trigger and compare it to the same stimulus on cells that were not previously stimulated (Figure 1) [9].
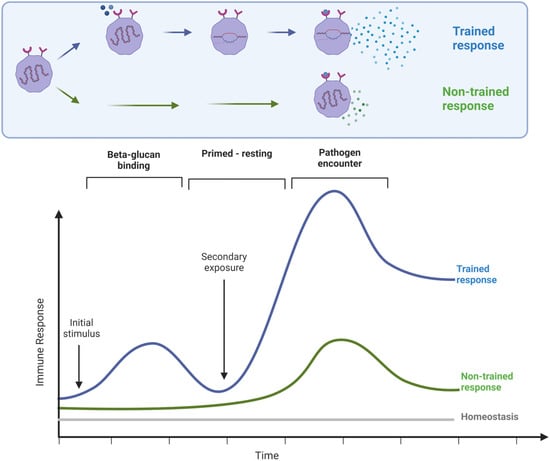
Figure 1.
Diagram illustrating the principle of trained immunity, where a primary stimulus triggers an initial response, accompanied by epigenetic re-programming. After a period of rest, the response to a second stimulus would be expected to be of the same magnitude as the initial response. However, when immune training has happened, the response to the second stimulus is more robust.
It has been demonstrated that EG and paramylon trigger cellular signaling in the intestinal tract and Peyer’s patches using intravital imaging; this is contrasted with the effects on intestinal sensory neurons, where only EG stimulated the cells while paramylon had no effect [10]. A placebo-controlled parallel-arm 90-day study showed that healthy adults consuming EG experienced fewer sick days and symptoms compared to those consuming placebo [11]. A placebo-controlled 8-week trial in severely stressed adults showed an increase in natural killer cell activity in participants consuming EG compared to participants consuming placebo [12]. A 12-week study showed that consuming EG had positive effects on reducing tension and easing irritability associated with performing stressful mental tasks [13]. A 4-week trial showed a significantly lower level of task-induced mental fatigue and a higher level of work efficiency when compared to the placebo group [14].
The clinical trial reported here evaluated the effects of consuming Euglena gracilis whole algae (EG) in 12 healthy subjects. Our overall approach was to document the effects of EG consumption using three distinct experimental models, based on the advantages that each methodological approach has to offer (Figure 2). First, the in vivo model examined the effects of a single dose of EG on immune surveillance and immune cell trafficking within the body. From each blood sample, the numbers and activation status of innate immune cells were documented, following our previously published protocol [15,16,17]. Secondly, as part of the clinical trial, an ex vivo model was applied to document the rapid re-programming of immune cells following EG consumption, where immune cells were cultured ex vivo, and the spontaneous versus inflammation-challenged cytokine production was evaluated. Lastly, the effects on isolated monocytes, as a model of the initiating events in the gut mucosa and Peyer’s patches, were studied in vitro and compared to the effects of pure EG-derived β-glucan. The results from this study are the first documentation of acute effects of EG consumption, evaluating the effectiveness of EG-induced trained immunity in humans using a combination of in vivo and ex vivo approaches.
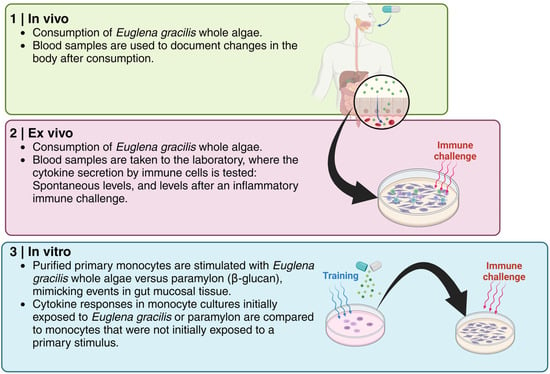
Figure 2.
The methodological approach for the work presented here involved three separate components: (1) Documentation of events happening in the body (in vivo) of a participant after consuming a single dose of Euglena gracilis whole algae (EG); (2) The immune cell re-programming that happened in the body of a participant was documented by taking a blood sample to the laboratory and exposing the immune cells to an inflammatory immune challenge; (3) Documentation of immune cell training on the blood sample exposed to EG in the lab and subsequent exposure of the immune cells to an inflammatory challenge.
2. Materials and Methods
2.1. Reagents
Blood collection tubes containing heparin (green-top, 6 mL vacutainer tubes, cat#367878), serum separator tubes (tiger-top, 8 mL vacutainer tubes, cat# 367988), and 21-gauge butterfly needles (cat# 367281) were purchased from Becton–Dickinson (Franklin Lakes, NJ, USA). Attune Focus fluid (cat# A24904), Performance tracking beads (cat# 4449754), wash and shut-down solutions (cat# A24974 and A24975), de-bubble buffer (cat# A10496), and High Yield Lysing buffer™ (cat# HYL250) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). These monoclonal antibodies were obtained from Thermo Fisher Scientific (Waltham, MA, USA): Anti-CD3-Superbright™ 645 (clone OKT3, cat# 64-0037-42), anti-CD56-phycoerythrin (clone CMSSB, cat# 12-0567-42), and anti-CD69-fluorescein isothiocyanate (clone FN50, cat# 11-0699-42). The monoclonal anti-CD25-Brilliant Violet421 antibody (clone 2A3, cat# 564033) was obtained from BD Biosciences (Franklin Lakes, NJ, USA). Bio-Plex Pro™ human cytokine arrays were purchased from Bio-Rad Laboratories Inc. (Hercules, CA, USA).
Roswell Park Memorial Institute 1640 medium (cat# 11835030), penicillin–streptomycin 100× (cat# 15140-122), Fetal Bovine Serum (cat# A38401-01) phosphate-buffered saline (cat# 14190-235), lipopolysaccharide (LPS) from E. coli 026:B6 (cat# 50-112-9325), and lipopolysaccharide (LPS-EK) from E. coli K12 (cat# tlrl-eklps) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Interleukin-2 (IL-2) (cat# 17908-10KU) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
2.2. Study Design
A placebo-controlled cross-over study design was used for this clinical study (clinical trial registration NCT05431751), which was conducted in accordance with the Declaration of Helsinki, and approved by the Argus Independent Review Board, Tucson, AZ, USA. The sample size was determined based on previous studies on β-glucan-rich nutritional supplements and we aimed for a minimum of 10 participants to complete the study [17]. People from the database at the study site, representing people who have previously indicated an interest in participating in clinical studies on natural products, were approached, and, if interested, were invited for screening. In the screening interview, we collected information on age, body mass index (BMI), medical/surgical history, diet and lifestyle, current health status, medication, and use of nutritional supplements. The following inclusion criteria were applied: healthy adult people of either gender, age 18–75 years (inclusive), body mass index (BMI) between 18.0 and 34.9 kg/m2 (inclusive), veins easily accessible for multiple blood draws, and willing to comply with the study requirements—maintaining a consistent diet and lifestyle routine throughout the study, a consistent habit of bland breakfasts on days of clinic visits, abstaining from exercising and nutritional supplements on the morning of a study visit, abstaining from the use of coffee, tea, and soft drinks for at least one hour prior to a clinic visit, and abstaining from music, candy, gum, computer/cell phone use, during clinic visits.
The following exclusion criteria were used: previous major gastrointestinal surgery (absorption of test product may be altered) (minor surgery not a problem, including previous removal of appendix and gall bladder); taking anti-inflammatory medications on a daily basis; currently in intensive athletic training (such as marathon runners); cancer during past 12 months; chemotherapy during past 12 months; currently treated with immune suppressant medication; diagnosed with autoimmune disorders, e.g., systemic lupus erythematosus, hemolytic anemia; donation of blood during the study or within the 4 weeks prior to the start of the study; received a cortisone shot within past 12 weeks; immunization during last month; currently taking antipsychotic, hypnotic, or anti-depressant prescription medication; ongoing acute infections (including teeth, sinus, ear, etc.); participation in another clinical trial study during this trial involving an investigational product or lifestyle change; an unusual sleep routine (examples: working graveyard shift, irregular routine with frequent late nights, studying, partying); unwilling to maintain a constant intake of supplements over the duration of the study; anxiety about having blood drawn; pregnant, nursing, or trying to become pregnant; known food allergies related to ingredients in active test product or placebo. If subjects met the inclusion criteria, they were informed that they qualified and were scheduled for clinic visits where they were enrolled into the study upon providing written informed consent. Fifteen people signed written informed consent, but one male and one female became unavailable due to a change in work schedule, and one female dropped out due to cold/flu onset. Twelve people finished the study participation (Table 1), and their data were analyzed (Figure 3).

Table 1.
Demographics of study population.
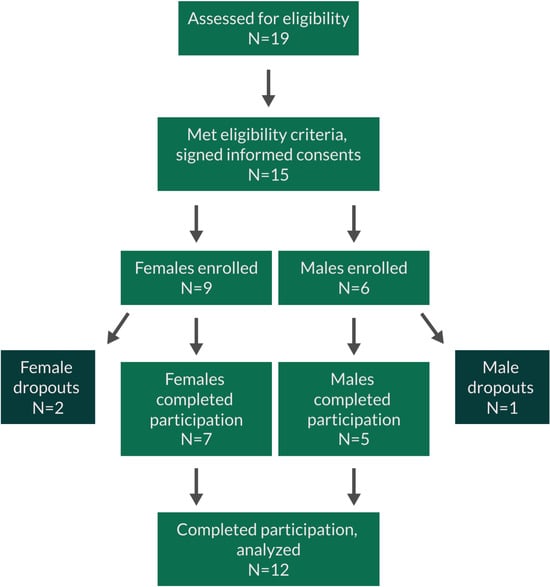
Figure 3.
CONSORT flow chart for the study.
Study participants were scheduled for three clinic visits 1 week apart (Figure 4). Due to the immune-training effects of β-glucans, the study was not randomized, and all participants received a placebo on the first clinic visit and EG on the second clinic visit. Through this non-randomized cross-over study design, we avoided biases that could result from EG-induced immune cell training affecting a subsequent visit where a placebo would be consumed. The participants received a single dose of placebo and the active product 1 week apart. After the second visit, participants consumed one daily dose of EG for 7 days and returned to the clinic for a blood draw to evaluate longer-term effects on the immune priming with daily consumption of EG. Below is a diagram illustrating the involvement of each participant.
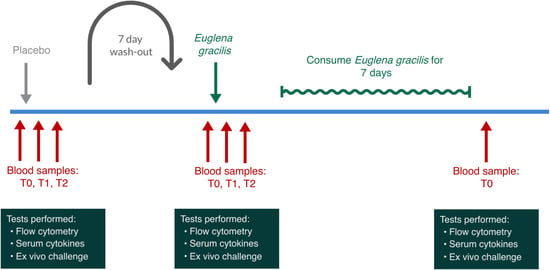
Figure 4.
Diagram showing the involvement of each participant. Study participants were tested on three different clinic days. Due to the known immune-training effects of β-glucans, the study was not randomized, and all participants received a placebo on the first clinic visit and Euglena gracilis whole algae (EG) on the second clinic visit. After the second visit, participants consumed one daily dose of EG for 7 days and then returned to the clinic for a blood draw to evaluate longer-term effects on immune priming associated with daily consumption of EG.
For each participant, the visits were scheduled at the same time of the day during the morning hours of 7–11 am to minimize the effect of circadian fluctuations [18,19,20,21]. The first clinic visit involved consuming a placebo and served as a control for the circadian variations in cytokine levels and immune surveillance for each participant. Since there is a well-documented interference from exercise [22] and stress [23,24,25] with the release versus homing of lymphocytes, the study environment was managed so that any physical and mental stress was minimized during each visit. Upon arrival at a clinic visit, participants completed a questionnaire to help monitor exceptional circumstances that may influence the stress level of that person on that day. Predetermined criteria for rescheduling a visit included sleep deprivation and acute anxiety. After completing the questionnaire, volunteers were instructed to remain calm and inactive for 3 h, comfortably seated in a chair. After the first hour, the baseline blood sample was drawn. Immediately after the baseline sample was drawn, an encapsulated test product was provided with water and consumed in the presence of the clinic staff. Blood samples were drawn at 1 h and 2 h after consumption of the test product or placebo. For each blood draw, 14 mL of blood was drawn: eight milliliters of blood was drawn into a serum separator tube and allowed to coagulate for 30–60 min after which the tube was centrifuged for 15 min at 1500× g at room temperature and the serum was harvested into a new tube, centrifuged for 10 min at 4 °C, aliquoted, and frozen at −80 °C for subsequent testing of cytokine profile. Six milliliters of blood were drawn into heparin vacutainer tubes for immunostaining, which was initiated within the hour of each blood draw. From each blood sample, the numbers and activation status of innate immune cells were documented [15,23,24], and immune cells were cultured overnight to document the re-programming of the participants’ immune cells after consuming a single dose of EG compared to placebo.
2.3. Production of the Microalgae Material
The whole unprocessed microalgae-based powder, BioGlena™, tested in this study was provided by Algatechnologies, Kibbutz Ketura, Israel. The single-celled alga Euglena gracilis is grown by fermentation in closed bioreactors using a technology designed for obtaining a high content of β-glucan (Figure 5). The powder contains a minimum of 50% linear β-glucan, complete protein, and essential vitamins and minerals (Table 2).
Figure 5.
Euglena gracilis. The single-celled algae are shown at 400× magnification. The white particles inside the cells are paramylon granules composed of β-1,3 glucan.

Table 2.
Composition of Euglena gracilis whole algae.
2.4. Consumable Products for the Clinical Trial
Capsules of BioGlena™ powder of Euglena gracilis and matching placebo capsules containing plain rice flour were supplied by Algatechnologies Ltd., Kibbutz Ketura Hevel Eilot, Israel. On each clinic day, immediately after the baseline blood draw, study participants were given a single dose of either a placebo or the active test product in the presence of the clinic staff. The capsules with the active test product contained 375 mg EG. Participants consumed the capsules with water and a few bland soda crackers to stimulate digestive function.
2.5. Immune Cell Evaluation by Flow Cytometry
From each blood draw, triplicate samples of heparinized whole blood were stained with a panel of monoclonal antibodies focused on identifying NK cells, NKT cells, T cells, and monocytes, and documenting the expression levels of the CD25 and CD69 activation markers on those cell types.
To perform the staining, triplicate samples of 50 µL heparinized whole blood were stained with fluorochrome-conjugated monoclonal antibodies towards CD3, CD25, CD56, and CD69 and were incubated for at least 15 min at room temperature in the dark followed by the addition of 1 mL of High-Yield Lysing buffer, mixing, and incubation for at least 10 min at room temperature in the dark. Samples were transferred to 2 mL deep-well 96-well plates and acquired by flow cytometry within 1 h of staining.
All samples were acquired using an acoustic-focusing Attune™ Nxt flow cytometer (Thermo Fisher Scientific), using a microplate-based Autosampler. Data analysis utilized gating on forward/side scatter to identify lymphocyte and monocyte populations, followed by gating based on CD3 and CD56 markers, to allow enumeration of CD3+ CD56− T cells, CD3+ CD56+ NKT cells, CD3− CD56+ NK cells, and CD3− CD56− non-NK non-T cells. Each cell subset was then analyzed for expression levels of the activation markers CD25 and CD69.
2.6. Ex Vivo Immune Challenges
From each blood draw performed in the clinical trial, peripheral blood mononuclear cells (PBMCs) were purified and plated at 106/mL in 200 µL volumes in two different sets in U-bottom 96 well plates (Thermo Scientific, cat# 163320) in culture medium (Roswell Park Memorial Institute (RPMI) medium + 10% fetal calf serum + Penicillin-Streptomycin). No immune challenge was performed on the first set of cultures, and these cultures served as a way to monitor the spontaneous release of cytokines ex vivo after product consumption in vivo. To the second set of cultures, an immune challenge was performed: 0.01 mL of the inflammatory bacterial toxin LPS (cat# 50-112-9325) was added (5 µg/mL in cell culture). Culture supernatants were harvested at 24 h and banked frozen for cytokine testing.
2.7. Serum Levels of Cytokines, Chemokines, and Growth Factors
Serum samples from all blood draws were used for evaluation of changes to blood levels of 27 cytokines and chemokines, quantified using a Bio-Plex Pro Human Cytokine Grp I Panel protein array (cat# M500KCAF0Y) (Bio-Rad Laboratories Inc.) and utilizing xMAP technology (Luminex, Austin, TX, USA). The following markers were tested: Interleukin-1 beta (IL-1β), interleukin-1 receptor antagonist (IL-1ra), interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-5 (IL-5), interleukin-6 (IL-6), interleukin-7 (IL-7), interleukin-8 (IL-8), interleukin-9 (IL-9), interleukin-10 (IL-10), interleukin-12 (protein 70) (IL-12 (p70)), interleukin-13 (IL-13), interleukin-15 (IL-15), interleukin-17 (IL-17), eosinophil chemotactic protein (Eotaxin), basic fibroblast growth factor (Basic FGF), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-gamma (IFN-γ), interferon gamma-induced protein 10 (IP-10), monocyte chemotactic protein 1 (MCP-1 (MCAF)), macrophage inflammatory protein 1 alpha (MIP-1α), macrophage Inflammatory protein 1 beta (MIP-1β), platelet-derived growth factor subunit beta (PDGF-BB), Regulated on Activation, Normal T cell Expressed and Secreted (RANTES), tumor necrosis factor-alpha (TNF-α), and vascular endothelial growth factor (VEGF).
2.8. Cytokines in Culture Supernatants from Ex Vivo and In Vitro PBMC Cultures
The culture supernatants from the 24 h ex vivo PBMC cultures from the clinical samples and the culture supernatants from the in vitro immune-training monocyte cultures were tested for the levels of seven cytokines and chemokines using Bio-Plex protein arrays (Bio-Rad Laboratories Inc.) and utilizing xMAP technology (Luminex, Austin, TX, USA). The following markers were tested: 24 h culture supernatants: IFN-γ, IL-1β, IL-6, IL-10, MIP-1β, TNF-α, and G-CSF; in vitro immune-training in monocyte cultures: IFN-γ, IL-1β, IL-1ra, IL-6, IL-10, TNF-α, and G-CSF.
2.9. Innate Immune Training In Vitro
A sample of bulk BioGlena™ powder of Euglena gracilis was supplied by Algatechnologies Ltd., Kibbutz Ketura Hevel Eilot, Israel. A stock solution was prepared fresh in phosphate-buffered saline. The stock solution was unfiltered and was introduced into cell cultures as a suspension of all algal components. Pure β-1,3-Glucan from Euglena gracilis was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA) (cat# 89862).
The immune-training effects of the whole algae EG were compared to those of pure EG-derived β-glucan in a classical in vitro model using purified monocytes (Figure 6) [9]. Peripheral blood mononuclear cells (PBMCs) were plated in sterile flat-bottom 96-well microplates and incubated for 2 h at 37 °C and 5% CO2 to allow monocyte adhesion. Lymphocytes were removed and the adherent monocytes were washed in warm PBS after which 0.18 mL fresh culture medium (Roswell Park Memorial Institute (RPMI) medium + 10% fetal calf serum + Penicillin-Streptomycin) was added to the wells. The initial stimulus for monocyte priming consisted of serial dilutions of EG, where each dose was tested in triplicate. One set of untreated cultures served as a negative control, where PBS was added instead of EG or LPS. Another set of untreated control wells was prepared so that the second stimulus could be added on Day 6.
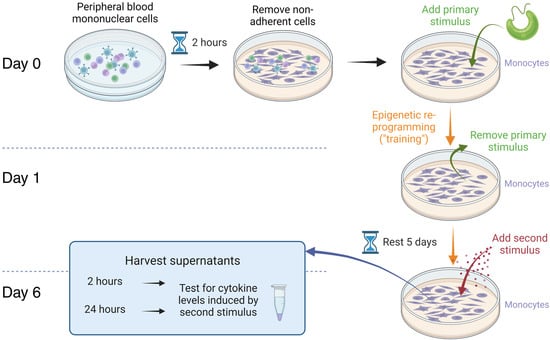
Figure 6.
Immune training of pure monocytes in vitro. Peripheral blood mononuclear cells were cultured for 2 h to allow monocytes to adhere to the plastic surface of the culture plates. Non-adherent cells were removed, and a primary stimulus was added. The primary stimuli compared were Euglena gracilis whole algae versus EG-derived pure β-glucan. After 24 h, the primary stimuli were removed, and the monocyte cultures were allowed to rest for 5 days. A second stimulus, in the form of the inflammatory bacterial toxin LPS, was added, and culture supernatants were harvested at 2 h and 24 h for cytokine testing.
After 24 h, the culture medium was exchanged with fresh medium without EG, thereby initiating the resting phase. After an additional 48 h, 0.08 mL culture medium was removed from each well, and 0.08 mL fresh culture medium was added. On day 6 after the culture was started, 0.1 mL medium was removed from each well and 0.09 mL fresh medium was added. Then, the second stimulus, 0.01 mL of the inflammatory bacterial toxin LPS-EK (cat# tlrl-eklps), was added (10 pg/mL in cell culture). Culture supernatants were harvested at 2 h and 24 h after the addition of the second stimulus and banked frozen for cytokine testing.
2.10. Statistical Analysis
The average and standard deviation for each data set were calculated using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). Post-consumption changes from baseline to later assessments were evaluated by between-treatment analysis for each time point. This allowed evaluation of changes on the day a person consumed the active product, in the context of the person’s circadian changes on the day he/she consumed a placebo. The evaluation used within-subject analysis and the two-tailed paired t-test where statistical significance was set at p < 0.05, and a high level of significance at p < 0.01.
For the analysis of in vitro data, the average and standard deviation for each data set were calculated using Microsoft Excel. Statistical analysis of in vitro data was performed using the 2-tailed, independent t-test for all data sets, except for the in vitro testing for IL-1β, where the one-tailed test was used, as values were close to baseline. Statistical significance was set at p < 0.05, and a high level of significance at p < 0.01.
3. Results
3.1. Rapid Increase in Immune Surveillance after Consumption
Consumption of EG triggered rapid changes in immune surveillance, as evidenced by rapid changes in blood circulation levels of certain types of immune cells (Figure 7). A single dose of 375 mg EG significantly reduced monocyte levels compared to a placebo (p < 0.05) 2 h after consumption (Figure 7A), indicating an increase in the trafficking of monocytes exiting the blood circulation. There was no significant difference between the numbers of CD3-CD56+ NK and NKT cells following EG consumption when compared to placebo (Figure 7B,C). However, despite not being statistically significant, a mild and transient increase in NKT cells was detected after EG consumption at 1 h which returned to similar levels to the placebo at 2 h (Figure 7C). Furthermore, at 1 h after EG consumption, an increase in CD3+ T cells was detected, reaching a high level of significance at 1 h (p < 0.01), and remaining significant at 2 h compared to placebo (p < 0.05, Figure 7D).
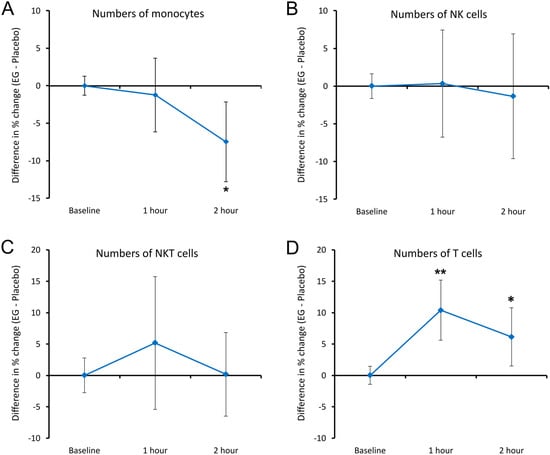
Figure 7.
Differences in changes to immune cell trafficking are reflected by the numbers of immune cells in the blood circulation within 2 h after consuming EG versus placebo. The results are shown as the group averages ± standard error of the mean of the individual percent changes from baseline after consuming EG where changes after consuming placebo are subtracted. (A) Monocyte numbers: a significant reduction was seen at 2 h after consuming EG. (B) NK cell numbers: there were no changes to the number of NK cells in the blood circulation associated with EG consumption. (C) NKT cell numbers: a mild and transient relative increase was seen after consuming EG compared to placebo; the change did not reach statistical significance. (D) Numbers of T cells: a rapid increase in the blood circulation reached a high level of significance at 1 h, remaining significant at 2 h. Levels of statistical significance are shown on the graphs where changes from baseline to a later time point are indicated by asterisks, where p < 0.05: *, and p < 0.01: **.
3.2. Rapid Changes to Expression of CD25 and CD69 on Immune Cells after Consumption
The expression of the activation marker CD25 was evaluated on monocytes, NK cells, NKT cells, and T cells (Figure 8). The expression of CD25 on monocytes showed no significant changes when compared to placebo (Figure 8A). However, a highly significant increase in CD25 expression was detected on NK cells at both 1 h and 2 h following EG consumption compared to placebo (p < 0.01, Figure 8B). In contrast, CD25 expression on NKT and T cells showed significant decreases following EG consumption at 1 h (p < 0.05), and a further decrease that reached high levels of statistical significance at 2 h compared to placebo (p < 0.01, Figure 8C,D).
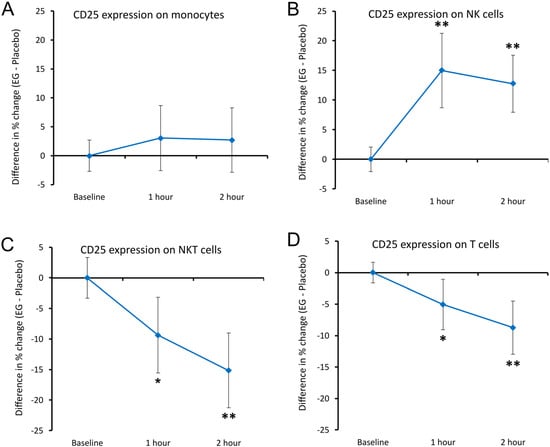
Figure 8.
Differences in changes to the expression of the CD25 activation marker on immune cells within 2 h after consumption of EG versus placebo. The results are shown as the group averages ± standard error of the mean of the individual percent changes from baseline after consuming EG where changes after consuming placebo are subtracted. (A) CD25 expression on monocytes: there were no differences after consuming EG compared to placebo. (B) CD25 levels on NK cells: an increase reached a high level of statistical significance at 1 h, remaining highly significant at 2 h. (C) CD25 expression on NKT cells: a decrease reached statistical significance at 1 h, and a high level of statistical significance at 2 h. (D) CD25 expression on T cells: a decrease reached statistical significance at 1 h, and a high level of statistical significance at 2 h. Levels of statistical significance are shown on the graphs where changes from baseline to a later time point are indicated by asterisks, where p < 0.05: *, and p < 0.01: **.
In response to a single dose of 375 mg EG, CD69 expression was reduced on different cell types (Figure 9). CD69 expression showed a decrease on monocytes and T cells, reaching a statistical trend at 1 h (p < 0.10, Figure 9A,D), and a high level of statistical significance at 2 h compared to placebo (p < 0.01). Similarly, CD69 expression on NK cells was reduced and reached a high level of significance at 2 h after EG consumption relative to the placebo (p < 0.01, Figure 9B). In addition, when compared to placebo, a transient decrease in CD69 expression was seen on NKT cells following EG consumption, reaching a high level of statistical significance at 1 h (p < 0.01) and returning towards levels seen after consuming placebo at 2 h, reaching a statistical trend (p < 0.10) (Figure 9C).
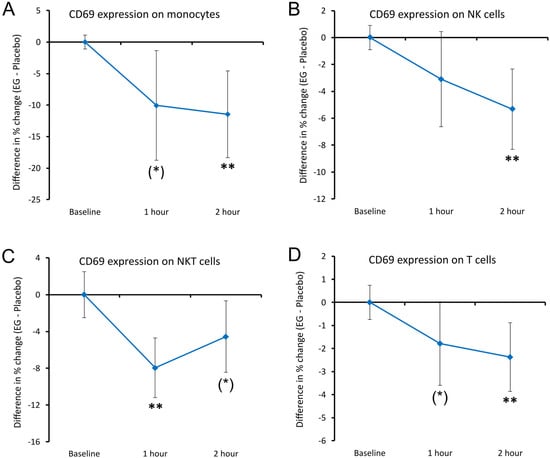
Figure 9.
Differences in changes in the expression of the CD69 activation marker on immune cells within 2 h after consumption of EG versus placebo. The results are shown as the group averages ± standard error of the mean of the individual percent changes from baseline after consuming EG where changes after consuming placebo are subtracted. (A) CD69 expression on monocytes: a relative decrease was seen, reaching a statistical trend at 1 h and a high level of statistical significance at 2 h. (B) CD69 expression on NK cells: a relative decrease was seen, reaching a high level of statistical significance at 2 h. (C) CD69 expression on NKT cells: a transient decrease reached a high level of statistical significance at 2 h and returned towards levels seen after consuming the placebo, reaching a statistical trend at 2 h. (D) CD69 levels on T cells: a relative decrease was seen, reaching a statistical trend at 1 h and a high level of statistical significance at 2 h. Levels of statistical significance are shown on the graphs where changes from baseline to a later time point are indicated by asterisks, where p < 0.10: (*) and p < 0.01: **.
3.3. Serum Cytokine Levels after Consumption
Serum from each blood sample was tested for cytokine levels. Due to normal circadian changes to immune status and cytokine levels, blood samples were taken at the same time in the morning on each clinic day, so that each person’s changes to cytokine levels after consuming EG were compared to the same person’s changes after consuming placebo. There were no statistically significant differences in serum cytokine levels at 1 h or 2 h after consuming EG when compared to the placebo.
3.4. Altered Immune Cell Responses Ex Vivo after Priming In Vivo
From each blood sample, peripheral blood mononuclear cells (PBMCs) were isolated and cultured for 24 h. Two culture conditions were compared: normal culture conditions, and inflamed conditions where the bacterial toxin LPS was used to induce inflammation.
The cytokine levels in the ex vivo cultures were analyzed for acute changes, where differences after consuming EG were compared to differences after consuming the placebo. Under normal culture conditions, the induction of cytokine production after consuming EG was compared to the induction after consuming the placebo (Figure 10). The differences in cytokine levels under normal culture conditions included a rapid and transient increase in the production of interferon-gamma (IFN-γ), reaching statistical significance at 1 h after consumption, and losing significance in cultures of PBMCs from blood samples taken 2 h after EG consumption (Figure 10A). There was also an increase in the production of granulocyte colony-stimulating factor (G-CSF), reaching a statistical trend at 1 h (p < 0.10), and a high level of statistical significance at 2 h after consumption (p < 0.01, Figure 10G).
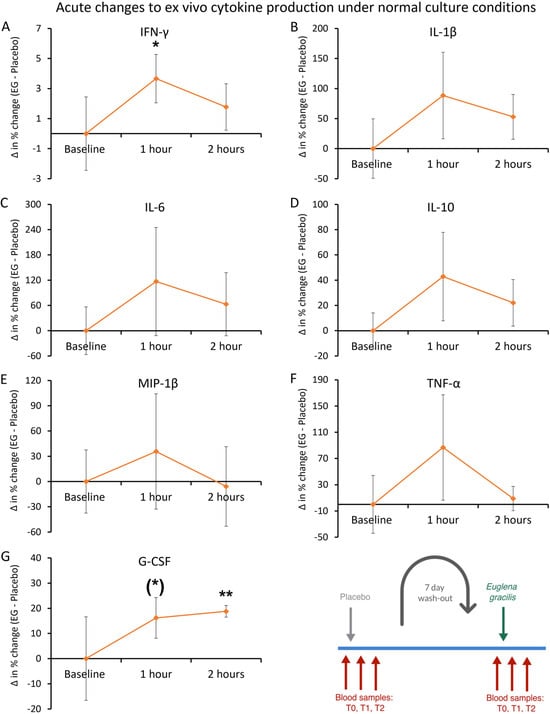
Figure 10.
Ex vivo cytokine production under normal culture conditions. Peripheral blood mononuclear cells were purified from each blood sample and cultured for 24 h in the absence of an immune challenge to document the cytokine production after consuming a single dose of Euglena gracilis (EG) compared to placebo. The results are shown as the group averages ± standard error of the mean of the individual percent changes from baseline after consuming EG where changes after consuming placebo are subtracted. (A) A transient increase in IFN-γ was seen at 1 h and reached statistical significance (p < 0.05). (B–F) Relative increases were also seen for IL-1β, IL-6, IL-10, MIP-1β, and TNF-α, but the increases were not significant when compared to placebo. (G) An increase was seen in G-CSF, reaching a high level of statistical significance at 2 h (p < 0.01). Levels of statistical significance are shown on the graphs where changes from baseline to a later time point are indicated by asterisks, where p < 0.10: (*), p < 0.05: *, and p < 0.01: **. The diagram in the bottom right corner is an excerpt of Figure 4 and serves as a reference to the acute crossover phase of the study.
In contrast, the cytokine levels in inflamed ex vivo cultures within 1 h and 2 h after consuming EG showed anti-inflammatory effects, where the production of six immune-activating inflammatory cytokines was reduced in the ex vivo cultures (Figure 11). The reduced levels of interleukin-1beta (IL-1β) were transient, reaching statistical significance at 1 h (p < 0.05) and approaching similar levels as placebo, reaching a statistical trend at 2 h (p < 0.10, Figure 11B). The reduced levels of tumor necrosis factor-alpha (TNF-α) were similar at 1 h and 2 h and reached statistical significance at 2 h (p < 0.05, Figure 11F). In contrast, the anti-inflammatory cytokine interleukin-10 (IL-10) showed an increase that did not reach statistical significance (p < 0.16, Figure 11D).
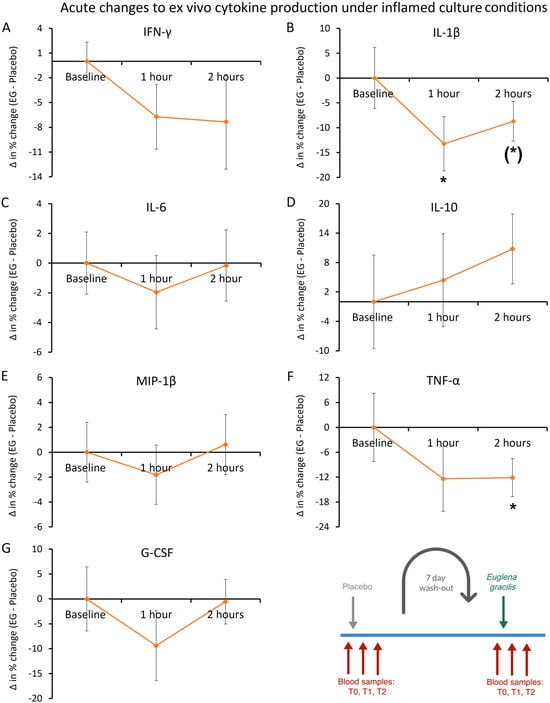
Figure 11.
Ex vivo cytokine production under inflamed culture conditions. Peripheral blood mononuclear cells were purified from each blood sample and cultured for 24 h in the presence of an immune challenge in the form of the bacterial toxin lipopolysaccharide (LPS) to document modulation of inflammation-induced cytokine production after consuming a single dose of Euglena gracilis (EG), compared to placebo. The results are shown as the group averages ± standard error of the mean of the individual percent changes from baseline after consuming EG where changes after consuming placebo are subtracted. Five immune-activating inflammatory cytokines were reduced in the ex vivo cultures after consuming a single dose of EG compared to placebo: IFN-γ, IL-1β, IL-6, MIP-1β, and TNF-α. The reduction in LPS-induced IL-1β was statistically significant at 1 h, and the reduction in TNF-a was significant at 2 h. In contrast, there was a transient increase in the production of the anti-inflammatory cytokine IL-10. Levels of statistical significance are shown on the graphs where changes from baseline to a later time point are indicated by asterisks, where p < 0.10: (*) and p < 0.05: *. The diagram in the bottom right corner is an excerpt of Figure 4 and serves as a reference to the acute crossover phase of the study.
The cytokine levels in ex vivo cultures were also analyzed for long-term changes, comparing the cytokine levels from blood samples taken immediately prior to consuming the first dose of EG to the final blood draw after 1 week’s daily consumption of EG. The cytokine levels under normal culture conditions showed reduced levels for IL-1β (−34%), interleukin-6 (IL-6; −71%, p < 0.25), macrophage inflammatory protein (MIP-1β; −44%), and TNF-α (−51%, p < 0.17), but the reductions did not reach statistical significance (Figure 12).
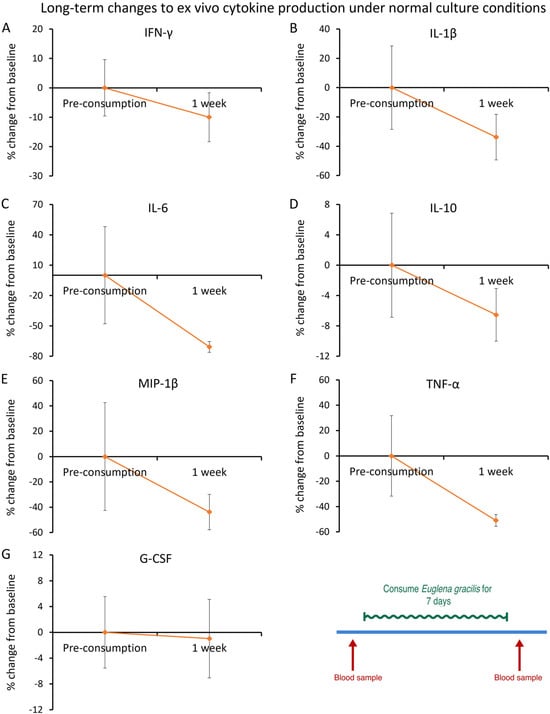
Figure 12.
After 1 week of consumption of Euglena gracilis (EG): ex vivo cytokine production under normal culture conditions. Peripheral blood mononuclear cells were purified from each blood sample and cultured for 24 h in the absence of an immune challenge, to document modulation of spontaneous cytokine production after consuming daily doses of Euglena gracilis (EG). The results are shown as the group averages ± standard error of the mean of the individual percent changes from baseline after consuming EG where changes after consuming placebo are subtracted. Five immune-activating inflammatory cytokines were reduced in cultures taken after consuming EG for one week, compared to levels before consuming EG: Interferon-gamma (IFN-γ), Interleukin-1beta (IL-1β), Interleukin-6 (IL-6), Macrophage inflammatory protein (MIP-1β), and tumor necrosis factor-alpha (TNF-α). There was also a mild but insignificant reduction in the anti-inflammatory cytokine IL-10. None of the changes achieved statistical significance. The diagram in the bottom right corner is an excerpt of Figure 4 and serves as a reference to the open-label 1-week phase of the study.
Under inflamed culture conditions, an anti-inflammatory effect was observed (Figure 13). The cytokine levels after 1 week of consumption of EG showed a statistically significant reduction in TNF-α levels (−39%, p < 0.006, Figure 13F). In the same cultures, there was a statistically significant increase in levels of the anti-inflammatory cytokine IL-10 (40%, p < 0.03, Figure 13D).
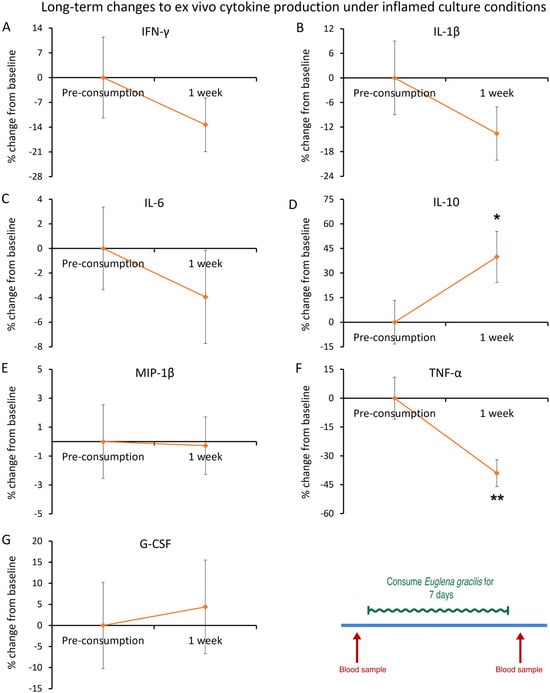
Figure 13.
After 1 week of consumption of Euglena gracilis (EG): ex vivo cytokine production under inflamed culture conditions. Peripheral blood mononuclear cells were purified from each blood sample and cultured for 24 h in the presence of an immune challenge, in the form of the bacterial toxin lipopolysaccharide (LPS), to document modulation of inflammation-induced cytokine production after consuming daily doses of Euglena gracilis (EG). The results are shown as the group averages ± standard error of the mean of the individual percent changes from baseline after consuming EG where changes after consuming placebo are subtracted. The reduction in LPS-induced production of TNF-α was highly significant after 1 week of consuming EG. In contrast, there was a statistically significant increase in production of the anti-inflammatory cytokine Interleukin-10 (IL-10). Levels of statistical significance are shown on the graphs where changes from baseline to a later time point are indicated by asterisks, where p < 0.05: * and p < 0.01: **. The diagram in the bottom right corner is an excerpt of Figure 4 and serves as a reference to the open-label 1-week phase of the study.
3.5. Trained Innate Immunity In Vitro
Cell cultures of primary human monocytes were used to document the effects of EG on innate immune training in vitro. Monocytes were treated with EG or pure EG-derived β-glucan as the primary stimulus for 24 h, then EG was removed, and the monocytes were allowed a 5-day rest period. Untreated cultures were cultured in parallel for the same duration. On Day 6, a second stimulus was added in the form of the inflammatory bacterial toxin lipopolysaccharide (LPS), and this was also added to the control cultures that had not received a primary stimulus. Culture supernatants were collected at 2 h and 24 h for cytokine testing (Figure 6).
Two hours after the second stimulus, EG-trained and β-glucan-trained monocytes produced higher levels of IL-6, TNF-α, IL-1ra, and IL-10 (Table 3). The increased levels of IL-6 and TNF-α were highly significant (p < 0.01) compared to the untrained control cultures for both EG and β-glucan, but only IL-6 showed comparable levels between EG-primed and β-glucan-primed cultures. For all other cytokines tested, the EG-primed monocytes showed higher levels than the β-glucan-primed monocytes. The increased level of TNF-α in EG-primed monocyte cultures was highly significant when compared to β-glucan-primed monocytes.

Table 3.
Cytokine levels in Euglena gracilis (EG)-trained monocyte cultures 2 h after second stimulus 1.
Twenty-four hours after the second stimulus, the EG-trained monocytes had produced higher levels of IFN-γ, IL-1β, and TNF-α than the β-glucan-trained monocytes (Table 4), where the difference between EG-primed and β-glucan-trained monocytes reached a high level of significance for TNF-α and a statistical trend for IL-1β. In contrast, the levels of IL-6, IL-1ra, and IL-10 were lower in the EG-trained cultures than in the β-glucan-trained cultures, reaching statistical trends for all three cytokines.

Table 4.
Cytokine levels in Euglena gracilis (EG)-trained monocyte cultures 24 h after second stimulus 1.
4. Discussion
This study evaluated the effects of consuming Euglena gracilis whole algae (EG) on immune cell activation, cellular trafficking, and cytokine levels, which are functions of the cumulative interplay between gut immune tissue, nerve stimulation, and immune cell communication. Secondly, as part of the clinical trial, ex vivo cell cultures were used to evaluate the effects of EG consumption on immune reprogramming and its impact on acute and long-term ex vivo cytokine production, spontaneously and in response to an inflammatory challenge. Finally, to understand the direct effects on purified macrophages, in vitro testing was conducted to evaluate the effects of EG- versus β-glucan-induced immune training on cytokine levels in inflammatory conditions as an in vitro model of the initiating effects at the gut level after consuming EG.
Consuming nutraceutical products represents a unique opportunity to provide rapid support for the natural processes of immune surveillance [17,26,27]. The clinical data presented here showed rapid and highly significant changes to activation markers on NK cells, where the CD25 activation marker was significantly elevated at 1 h after consuming a single dose of EG compared to placebo, suggesting increased NK cell proliferative activity [28], and possibly cytotoxic activity [29]. Correlations have been reported between CD25 up-regulation and increased sensitivity to IL-2, where the heightened responsiveness of NK cells to re-stimulation by cytokines and target cells is indicative of a memory-like reactivity [30]. Synchronous to our observations of increased CD25 expression, we also saw significantly reduced expression levels of CD69. This was not associated with changes to the total numbers of natural killer (NK) cells in the blood circulation, suggesting that, if changes to NK cell trafficking were induced, the number of NK cells migrating out of the blood was equal to the number entering back into the circulation. In addition, there was a rapid and highly significant increase in T cell mobilization after consuming a single dose of Euglena gracilis (EG) whole algae, where T cells in the blood circulation expressed significantly lower levels of CD25 and lower levels of CD69.
In previous studies on nutraceutical modulation of immune surveillance, the changes to cell numbers in the blood circulation were typically associated with changes to cytokine levels. The consumption of a blend of botanicals and medical mushrooms showed rapid increases in IP-10 and MCP-1, in association with monocyte surveillance and increased CD25 expression on NKT and T cells [17]. Consumption of an immunogenic yeast fermentate was associated with increased CD25 levels as well as T and NK cell migration, associated with significant changes in IFN-γ [27]. Both these previous studies involved β-glucan-rich ingredients. Therefore, it is unique for the EG study presented here that there were no statistically significant changes to serum cytokines at 1–2 h after consumption. There are several possible reasons for this, where either cytokine changes happened rapidly and lost significance at 1 h, or there may have been individual differences in timing between different participants so the group averages for these changes did not reach significance.
As part of the clinical study reported here, an ex vivo experimental approach was used to evaluate the effect of EG consumption on innate immune cells’ production of cytokines. Changes to spontaneous cytokine production ex vivo after consuming EG included a rapid increase in the levels of IFN-γ and G-CSF, demonstrating a swift epigenetic reprogramming of EG-primed immune cells. Contrary to the direct effects, ex vivo LPS-challenged PBMCs triggered a rapid decrease in the levels of IL-1β and TNF-α, with a concomitant increase in the level of the anti-inflammatory cytokine Interleukin-10 (IL-10) in response to EG priming in vivo. It is noteworthy that rapid and transient reductions in the levels of immune-activating cytokines such as IFN-γ, MIP-1β, and G-CSF were observed ex vivo following LPS-induced inflammation, despite not reaching statistical significance. This suggests the anti-inflammatory effects of EG consumption, resulting in the PBMCs being less responsive to inflammatory stimuli, such as LPS. After one week of EG consumption, the ex vivo PBMC cultures exhibited anti-inflammatory effects after an inflammatory challenge, as seen by a significant increase in the anti-inflammatory cytokine IL-10 and a highly significant decrease in the pro-inflammatory cytokine TNF-α. Our findings indicate the potential of EG to balance the beneficial and harmful effects of inflammation, and this modulation of the immune system may be key to the resolution of uncontrolled or excessive inflammation [31,32]. Collectively, this study suggests that priming immune cells with nonspecific stimuli, such as EG, may have protective effects during the inflammatory response to downstream challenges with pathogens unrelated to the original stimulus.
The immune-training effects of the whole algae EG were compared to pure EG-derived β-glucan, in a classical in vitro model using purified monocytes [9]. When monocytes received EG as the primary stimulus, the cytokine levels after 2 h of the second stimulus showed a more robust increase in the production of IFN-γ, IL-1β, TNF-α, IL-1ra, and IL-10 than when monocytes received pure EG-derived β-glucan as the primary stimulus. The higher level of TNF-α was highly significant when compared to β-glucan-primed monocytes. Only the cytokine IL-6 was induced to similar levels by both EG and β-glucan. This superior burst in cytokine production after the second stimulus of EG-trained monocytes seen at 2 h was less evident at 24 h after the second stimulus. In the 24-h supernatants, only TNF-α remained higher in the EG-trained monocytes than in the β-glucan-trained monocyte cultures. We suggest that multiple synergistic components in the whole-EG algae facilitated the rapid increase while also supporting a faster return to homeostasis than in cultures primed with pure β-glucan. Further testing is needed to elucidate the anti-inflammatory effects of the non-β-glucan fraction of EG.
A number of potential clinical implications may be associated with enhancing the body’s resistance to infections. It is possible that through the rapid epigenetic reprogramming of EG-primed immune cells, trained immunity may be extended beyond innate immune cells and likely impact the generation of more effective adaptive immune responses and antibody production [33]. Further research is required to understand how the rapid reprogramming of EG-primed immune cells impacts adaptive immunity as well as the potential for the development of effective therapeutic interventions using natural products [34]. Thus, this presents a unique opportunity for the development of EG-based nutraceutical interventions which may offer non-specific protection and potentially serve as a preventive therapy as well as enhancing immune resistance in immunocompromised populations.
5. Conclusions
In conclusion, priming the immune system by consuming EG triggered rapid and sustained changes in immune cell alertness, migration, and anti-inflammatory activity. Furthermore, EG consumption directly affected the immune cells by inducing a mild and transient increase in the spontaneous production of cytokines while, at the same time, showing resilience towards inflammation-induced cytokine production.
Author Contributions
Conceptualization, G.S.J., S.B. and O.G. methodology, G.S.J. and S.B.; formal analysis, I.I.; data curation, G.S.J. and K.S.; writing—original draft preparation, I.I., D.C. and G.S.J.; writing—review and editing, I.I., D.C., K.S., S.B., O.G. and G.S.J.; visualization, D.C., I.I., K.S. and G.S.J.; supervision, G.S.J.; project administration, G.S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was sponsored by AlgaTechnologies, a producer of commercially available microalgae products and concentrates.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Argus Institutional Review Board (protocol code 153-012).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author upon reasonable request.
Conflicts of Interest
G.S.J., I.I., D.C. and K.S. are employed in NIS Labs; these authors declare no conflicts of interest. S.B. and O.G. are employed by Algatechnologies, the sponsor of the study.
References
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. Toll-like receptors. Curr. Protoc. Immunol. 2015, 109, 14.12.1–14.12.10. [Google Scholar] [CrossRef] [PubMed]
- Brown, G. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Plato, A.; Willment, J.A.; Brown, G.D. C-type lectin-like receptors of the dectin-1 cluster: Ligands and signaling pathways. Int. Rev. Immunol. 2013, 32, 134–156. [Google Scholar] [CrossRef]
- Tukhvatulin, A.I.; Dzharullaeva, A.S.; Erokhova, A.S.; Scheblyakov, D.V.; Naroditsky, B.S.; Gintsburg, A.L.; Logunov, D.Y. NOD1/2 and the C-Type Lectin Receptors Dectin-1 and Mincle Synergistically Enhance Proinflammatory Reactions Both In Vitro and In Vivo. J. Inflamm. Res. 2020, 13, 357–368. [Google Scholar] [CrossRef]
- Phillips, F.C.; Jensen, G.S.; Showman, L.; Tonda, R.; Horst, G.; Levine, R. Particulate and solubilized β-glucan and non-β-glucan fractions of Euglena gracilis induce pro-and anti-inflammatory innate immune cell responses and exhibit antioxidant properties. J. Inflamm. Res. 2019, 12, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Rusek, P.; Wala, M.; Druszczyńska, M.; Fol, M. Infectious Agents as Stimuli of Trained Innate Immunity. Int. J. Mol. Sci. 2018, 19, 456. [Google Scholar] [CrossRef]
- Crisan, T.O.; Netea, M.G.; Joosten, L.A.B. Innate immune memory: Implications for host responses to damage-associated molecular patterns. Eur. J. Immunol. 2016, 46, 817–828. [Google Scholar] [CrossRef]
- Bekkering, S.; Blok, B.A.; Joosten, L.A.; Riksen, N.P.; van Crevel, R.; Netea, M.G. In Vitro Experimental Model of Trained Innate Immunity in Human Primary Monocytes. Clin. Vaccine Immunol. 2016, 23, 926–933. [Google Scholar] [CrossRef]
- Yasuda, K.; Nakashima, A.; Murata, A.; Suzuki, K.; Adachi, T. Euglena Gracilis and β-Glucan Paramylon Induce Ca2+ Signaling in Intestinal Tract Epithelial, Immune, and Neural Cells. Nutrients 2020, 12, 2293. [Google Scholar] [CrossRef]
- Evans, M.; Falcone, P.H.; Crowley, D.C.; Sulley, A.M.; Campbell, M.; Zakaria, N.; Lasrado, J.A.; Fritz, E.P.; Herrlinger, K.A. Effect of a Euglena gracilis Fermentate on Immune Function in Healthy, Active Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 2926. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, K.J.; Jo, S.M.; Jeon, J.Y.; Kim, B.R.; Hwang, J.E.; Kim, J.Y. Euglena gracilis (Euglena) powder supplementation enhanced immune function through natural killer cell activity in apparently healthy participants: A randomized, double-blind, placebo-controlled trial. Nutr. Res. 2023, 119, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Yasuda, K.; Murata, A.; Suzuki, K.; Miura, N. Effects of Euglena gracilis Intake on Mood and Autonomic Activity under Mental Workload, and Subjective Sleep Quality: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2020, 12, 3243. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Naito, J.; Nishioka, M.; Nishida, N.; Takahashi, M.; Kashiwagi, S.; Sugino, T.; Watanabe, Y. Effect of Food Containing Paramylon Derived from Euglena gracilis EOD-1 on Fatigue in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Trial. Nutrients 2020, 12, 3098. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Patel, D.; Benson, K.F. A novel extract from bovine colostrum whey supports innate immune functions. II. Rapid changes in cellular immune function in humans. Prev. Med. 2012, 54, S124–S129. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, C.; Benson, K.F.; Jensen, G.S. Rapid and selective mobilization of specific stem cell types after consumption of a polyphenol-rich extract from sea buckthorn berries (Hippophae) in healthy human subjects. Clin. Interv. Aging 2019, 14, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; McGarry, S.; Cruickshank, D.; Jensen, G.S. Rapid increase in immune surveillance and expression of NKT and γδT cell activation markers after consuming a nutraceutical supplement containing Aloe vera gel, extracts of Poria cocos and rosemary. A randomized placebo-controlled cross-over trial. PLoS ONE 2023, 18, e0291254. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, S.; Lange, T.; Nohroudi, K.; Born, J. Number and function of circulating human antigen presenting cells regulated by sleep. Sleep 2007, 30, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Lefta, M.; Wolff, G.; Esser, K.A. Circadian rhythms, the molecular clock, and skeletal muscle. Curr. Top Dev. Biol. 2011, 96, 231–271. [Google Scholar] [CrossRef]
- Narasimamurthy, R.; Hatori, M.; Nayak, S.K.; Liu, F.; Panda, S.; Verma, I.M. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 12662–12667. [Google Scholar] [CrossRef]
- Zhang, E.; Liu, Y.; Dentin, R.; Pongsawakul, P.Y.; Liu, A.C.; Hirota, T.; A Nusinow, D.; Sun, X.; Landais, S.; Kodama, Y.; et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat. Med. 2010, 16, 1152–1156. [Google Scholar] [CrossRef]
- Shephard, R.J. Adhesion molecules, catecholamines and leucocyte redistribution during and following exercise. Sports Med. 2003, 33, 261–284. [Google Scholar] [CrossRef]
- Dimitrov, S.; Benedict, C.; Heutling, D.; Westermann, J.; Born, J.; Lange, T. Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood 2009, 113, 5134–5143. [Google Scholar] [CrossRef]
- Atanackovic, D.; Schnee, B.; Schuch, G.; Faltz, C.; Schulze, J.; Weber, C.S.; Schafhausen, P.; Bartels, K.; Bokemeyer, C.; Brunner-Weinzierl, M.C.; et al. Acute psychological stress alerts the adaptive immune response: Stress-induced mobilization of effector T cells. J. Neuroimmunol. 2006, 176, 141–152. [Google Scholar] [CrossRef]
- Atanackovic, D.; Brunner-Weinzierl, M.C.; Kröger, H.; Serke, S.; Deter, H.C. Acute psychological stress simultaneously alters hormone levels, recruitment of lymphocyte subsets, and production of reactive oxygen species. Immunol. Investig. 2002, 31, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Ginsberg, D.I.; Huerta, P.; Citton, M.; Drapeau, C. Consumption of Aphanizomenon flos-aquae has rapid effects on the circulation and function of immune cells in humans. J. Am. Nutraceutical Assoc. 2000, 2, 50–58. [Google Scholar]
- Jensen, G.S.; Redman, K.A.; Benson, K.F.; Carter, S.G.; Mitzner, M.A.; Reeves, S.; Robinson, L. Antioxidant bioavailability and rapid immune-modulating effects after consumption of a single acute dose of a high-metabolite yeast immunogen: Results of a placebo-controlled double-blinded crossover pilot study. J. Med. Food 2011, 14, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Clausen, J.; Vergeiner, B.; Enk, M.; Petzer, A.L.; Gastl, G.; Gunsilius, E. Functional significance of the activation-associated receptors CD25 and CD69 on human NK-cells and NK-like T-cells. Immunobiology 2003, 207, 85–93. [Google Scholar] [CrossRef]
- Rudnicka, K.; Matusiak, A.; Chmiela, M. CD25 (IL-2R) expression correlates with the target cell induced cytotoxic activity and cytokine secretion in human natural killer cells. Acta Biochim. Pol. 2015, 62, 885–894. [Google Scholar] [CrossRef]
- Pahl, J.H.W.; Koch, J.; Götz, J.J.; Arnold, A.; Reusch, U.; Gantke, T.; Rajkovic, E.; Treder, M.; Cerwenka, A. CD16A Activation of NK Cells Promotes NK Cell Proliferation and Memory-Like Cytotoxicity against Cancer Cells. Cancer Immunol. Res. 2018, 6, 517–527. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakashima, A.; Igarashi, M.; Saito, K.; Konno, M.; Yamazaki, N.; Takimoto, H. Euglena gracilis Z and its carbohydrate storage substance relieve arthritis symptoms by modulating Th17 immunity. PLoS ONE 2018, 13, e0191462. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Hata, S.; Suzuki, K.; Yoshida, E.; Nakano, R.; Mitra, S.; Arashida, R.; Asayama, Y.; Yabuta, Y.; Takeuchi, T. Oral administration of paramylon, a beta-1,3-D-glucan isolated from Euglena gracilis Z inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. J. Vet. Med. Sci. 2010, 72, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.M.; Mills, K.H.G.; Basdeo, S.A. The Effects of Trained Innate Immunity on T Cell Responses; Clinical Implications and Knowledge Gaps for Future Research. Front. Immunol. 2021, 12, 706583. [Google Scholar] [CrossRef] [PubMed]
- De Marco Castro, E.; Calder, P.C.; Roche, H.M. β-1,3/1,6-Glucans and Immunity: State of the Art and Future Directions. Mol. Nutr. Food Res. 2021, 65, e1901071. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).