Generational Association Studies of Dopaminergic Genes in Reward Deficiency Syndrome (RDS) Subjects: Selecting Appropriate Phenotypes for Reward Dependence Behaviors
Abstract
:1. Introduction
1.1. Background
1.2. Brief Description of Risk Alleles in a Number of Dopaminergic Reward Genes
| ADDICTIVE BEHAVIORS: Alcoholism; Drug Abuse; Smoking; Compulsive Eating and Obesity |
| IMPULSIVE BEHAVIORS: Attention Deficit Disorder; Attention Deficit Hyperactivity Disorder; Autistic Disorders; Tourette Syndrome |
| COMPULSIVE DISORDERS: Hypersexuality and Aberrant Sexual Behaviors; Pathological Gambling and Internet Gaming |
| PERSONALITY DISORDERS: Antisocial Personality Disorder; Conduct Disorder; Pathological Aggression; Generalized Anxiety Disorder |
2. Methodology
2.1. Subject Selection
2.2. Genotyping
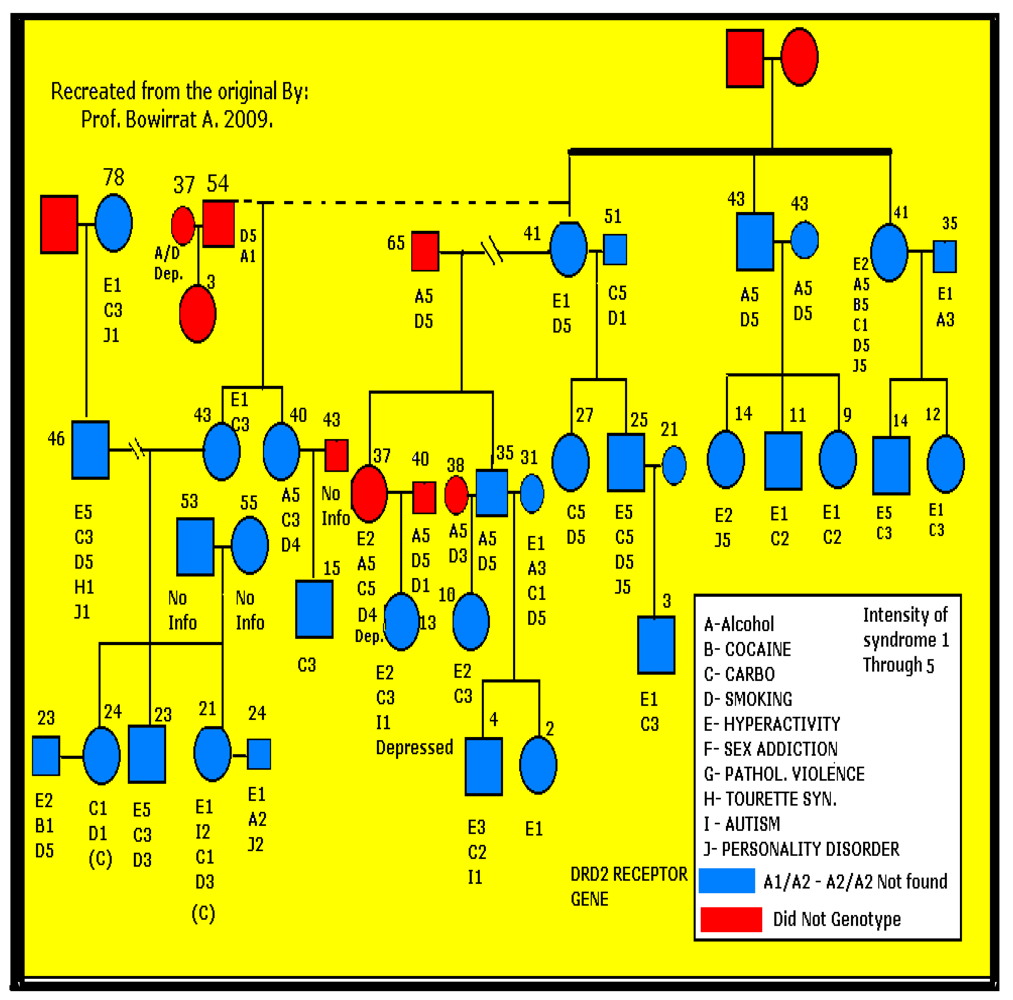
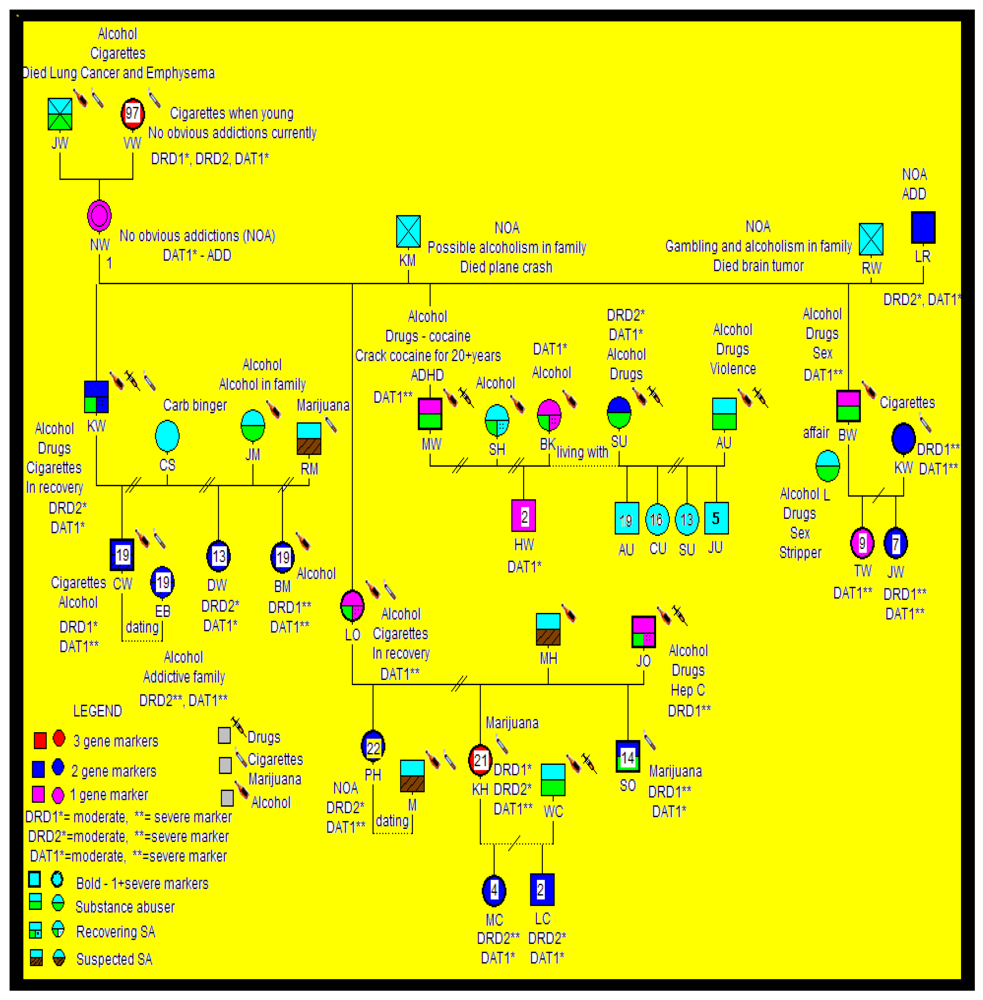
2.3. Statistical Analyses
3. Results
3.1. Genotyping
3.2. Findings and Their Implications
| Gene and Polymorphism | Percent Prevalence in Non-RDS Group | Percent Prevalence in Super Control Group | Percent Prevalence in RDS Group | Significance level p Value |
|---|---|---|---|---|
| DRD2-A1 Allele | 31.32 (n = 3,143) * | 3.3 (n = 30) ** | 78.2 (n = 55) | * p < 0.001 |
| Family A and B | ** p < 0.001 | |||
| DAT1-10/10 Allele | 37.4 (n = 91) * | Not Applicable | 58.18 (n = 55) | *p < 0.015 |
| Family A and B | ||||
| DBH-B1 Allele | 52.9 (n = 51) | Not Applicable | 65.0 (n = 32) | Not significant |
| Family A | ||||
| DRD1-A1/A1 | 65.2 (n = 61) | Not Applicable | 31.0 (n = 23) | Not significant |
| Family B |
4. Discussion
4.1. RDS, a Putative Endophenotype: Multiple Behaviors versus a Single Subset
| Reward Deficiency Syndrome or Related Disorder | Studies Demonstrating an Association with DRD2 Gene Polymorphism(s) |
|---|---|
| Pathological Gambling | [4,5,33,61,62,63,64,140] |
| Attention Deficit Hyperactivity Disorder | [2,4,24,99,154,196,207,208,209,210] |
| Post-Traumatic Stress Disorder | [4,176,177,196,212,213] |
| Eating; Obesity and Related Sequela | [46,133,134,170,178,179,181,182,197,198,200] |
| Energy Production | [134,180] |
| Hypertension | [181,182] |
| Schizophrenia | [12,19,49,112,183] |
| Early-Onset Sexual Intercourse; Hyper sexuality | [136,203] |
| Anti-Social Personality | [18,53] |
| Pathological Aggression | [6,105,171,186] |
| Schizoid-Avoidant Behavior | [143] |
| Novelty or Sensation Seeking | [26,27,36,83,109,113,144,161] |
| Substance Abuse | [12,13,20,22,29,46,65,71,90,103,110,114,117,172,173,174,175,193,199,201,202,205] |
| Heroin Addiction | [13,48,66,111,160] |
| Nicotine Dependence and Smoking Behavior | [13,17,22,81,91,104,184,185] |
| Personality Disorders and Crime | [100,186] |
| Parkinson’s Disease | [187,188] |
| Migraine | [189] |
| Tourette Syndrome | [5,99,190] |
| Huntington’s Disease | [191] |
| Cell Metabolism | [47] |
| Major Psychoses & Affective Disorder | [192,195,204] |
| Extraversion and Creativity | [21,23,145] |
4.2. “Super” Controls as a Phenotype: Exclusion of Multiple RDS Behaviors
4.3. Interactive Environmental and Genetic Roles in RDS Behaviors
4.4. From Bench to Bedside: Clinical Utility of RDS
5. Limitations, Caveats and Future Directions
6. Conclusions
Conflict of Interest
Acknowledgements
References
- Blum, K.; Cull, J.G.; Braverman, E.R.; Comings, D.E. Reward deficiency syndrome. Am. Sci. 1996, 84, 132–145. [Google Scholar]
- Blum, K.; Braverman, E.R. Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J. Psychoactive Drugs 2003, 32 (Suppl), 1–112. [Google Scholar]
- Blum, K.; Sheridan, P.J.; Wood, R.C.; Braverman, E.R.; Chen, T.J.; Cull, J.G.; Comings, D.E. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J. R. Soc. Med. 1996, 89, 396–400. [Google Scholar]
- Comings, D.E.; Blum, K. Reward deficiency syndrome: Genetic aspects of behavioral disorders. Prog. Brain Res. 2000, 126, 325–341. [Google Scholar]
- Comings, D.E.; Wu, S.; Chiu, C.; Ring, R.H.; Gade, R.; Ahn, C.; MacMurray, J.P.; Dietz, G.; Muhleman, D. Polygenic inheritance of Tourette syndrome, stuttering, attention deficit hyperactivity, conduct, and oppositional defiant disorder: The additive and subtractive effects of the three dopaminergic genes—DRD2, D beta H, and DAT1. Am. J. Med. Genet. 1996, 67, 264–288. [Google Scholar]
- Chen, T.J.H.; Blum, K.; Matthews, D.; Fisher, L.; Schnautz, N.; Braverman, E.R.; Schoolfield, J.; Downs, B.W.; Blum, S.H.; Mengucci, J.; et al. Preliminary association of both the Dopamine D2 Receptor (DRD2) [Taq1 A1 Allele] and the Dopamine Transporter (DAT1) [480 bp Allele] genes with pathological aggressive behavior, a clinical subtype of Reward Deficiency Syndrome (RDS) in adolescents. Gene Ther. Mol. Biol. 2007, 11, 93–112. [Google Scholar]
- Eisenberg, D.T.; Campbell, B.; Mackillop, J.; Lum, J.K.; Wilson, D.S. Season of birth and dopamine receptor gene associations with impulsivity, sensation seeking and reproductive behaviors. PLoS One 2007, 2. [Google Scholar]
- Berridge, K.C. The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology (Berl.) 2007, 191, 391–431. [Google Scholar]
- Blum, K.; Liu, Y.; Shriner, R.; Gold, M.S. Reward circuitry dopaminergic activation regulates food and drug craving behavior. Curr. Pharm. Des. 2011, 17, 1158–1167. [Google Scholar]
- Gardner, E.L. Addiction and brain reward and antireward pathways. Adv. Psychosom. Med. 2011, 30, 22–60. [Google Scholar]
- Blum, K.; Gold, M.S. Neuro-chemical activation of brain reward meso-limbic circuitry is associated with relapse prevention and drug hunger: A hypothesis. Med. Hypotheses 2011, 76, 576–584. [Google Scholar]
- Noble, E.P. The D2 dopamine receptor gene: A review of association studies in alcoholism and phenotypes. Alcohol 1998, 16, 33–45. [Google Scholar]
- Noble, E.P. The DRD2 gene in psychiatric and neurological disorder and its phenotypes. Pharmacogenomics 2000, 1, 309–333. [Google Scholar]
- Noble, E.P. D2 Dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. Am. J. Med. Genet. 2003, 116B, 103–125. [Google Scholar]
- Volkow, N.D.; Fowler, J.S.; Wang, G.J. Role of dopamine in drug reinforcement and addiction in humans: Results from imaging studies. Behav. Pharmacol. 2002, 13, 355–366. [Google Scholar]
- Downs, B.W.; Chen, A.L.C.; Chen, T.J.H.; Waite, R.L.; Braverman, E.R.; Kerner, M.; Braverman, D.; Rhoades, P.; Prihoda, T.J.; Palpmo, T.; et al. Nutrigenomic targeting of carbohydrate craving behavior: Can we manage obesity and aberrant craving behavior with neurochemical pathway manipulation by immunological compatible substances (nutrients) using a Genetic Positioning System (GPS) Map? Med. Hypotheses 2009, 73, 427–434. [Google Scholar]
- Vandenbergh, D.J.; O’Connor, R.J.; Grang, M.D.; Jefferson, A.L.; Vogler, G.P.; Strasser, A.A.; Kozlowski, L.T. Dopamine receptor genes (DRD2, DRD3, DRD4) and gene-gene interactions associated with smoking-related behaviors. Addict. Biol. 2007, 12, 106–116. [Google Scholar]
- Wang, T.J.; Huang, S.Y.; Lin, W.W.; Lo, H.Y.; Wu, P.L.; Wang, Y.S.; Wu, Y.S.; Ko, H.C.; Shih, J.C.; Lu, R.B. Possible interaction between MAOA and DRD2 genes associated with antisocial alcoholism among Han Chinese men in Taiwan. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 108–114. [Google Scholar]
- Arinami, T.; Itokawa, M.; Aoki, J.; Shibuya, H.; Ookubo, Y.; Iwawaki, A.; Ota, K.; Shimizu, H.; Hamaguchi, H.; Toru, M. Further association study on dopamine D2 receptor S311C in schizophrenia and affective disorders. Am. J. Med. Genet. 1996, 67, 133–138. [Google Scholar]
- Blum, K.; Noble, E.P.; Sheridan, P.J.; Montgomery, A.; Ritchie, T.; Jagadeeswaran, P.; Nogami, H.; Briggs, A.H.; Cohn, J.B. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA 1990, 263, 2055–2060. [Google Scholar]
- Cohen, M.X.; Young, J.; Baek, J.M.; Kessler, C.; Ranganath, C. Individual differences in extraversion and dopamine genetics predict neural reward responses. Brain Res. Cogn. Brain Res. 2005, 25, 851–861. [Google Scholar]
- Preuss, U.W.; Zill, P.; Koller, G.; Bondy, B.; Sokya, M. D2 dopamine receptor gene haplotypes and their influence on alcohol and tobacco consumption magnitude in alcohol-dependent individuals. Alcohol 2007, 42, 258–266. [Google Scholar]
- Reuter, M.; Roth, S.; Holve, K.; Hennig, J. Identification of first candidate genes for creativity: A pilot study. Brain Res. 2006, 1069, 190–197. [Google Scholar]
- Comings, D.E.; Gade-Andavilu, R.; Gonzalez, N.; Wu, S.; Muhlman, D.; Blake, H.; Chiu, F.; Wang, E.; Farwell, K.; Darakjy, S.; et al. Multivariate analysis of associations of 42 genes in ADHD, ODD and conduct disorder. Clin. Genet. 2000, 58, 31–40. [Google Scholar]
- Chen, C.K.; Chen, S.L.; Mill, J.; Huang, Y.S.; Lin, S.K.; Curran, S.; Purcell, S.; Sham, P.; Asherson, P. The dopamine transporter gene is associated with attention deficit hyperactivity disorder in a Taiwanese sample. Mol. Psychiatry 2003, 8, 393–396. [Google Scholar]
- Noble, E.P.; Ozkaragoz, T.Z.; Ritchie, T.; Zhang, X.; Bekin, T.R.; Belin, T.R.; Sparkes, R.S. D2 and D4 dopamine receptor polymorphisms and personality. Am. J. Med. Genet. 1998, 81, 257–267. [Google Scholar]
- Han, D.H.; Yoon, S.J.; Sung, Y.H.; Lee, Y.S.; Kee, B.S.; Lyoo, I.K.; Renshaw, P.F.; Cho, S.C. A preliminary study: Novelty seeking, frontal executive function, and dopamine receptor (D2) TaqI A gene polymorphism in patients with methamphetamine dependence. Compr. Phsyciatry 2008, 49, 387–392. [Google Scholar]
- Ratsma, J.E.; van der Stelt, O.; Schoffelmeer, A.N.M.; Westerveld And, A.; Boudewijn Gunning, W. P3 event-related potential, dopamine D2 receptor A1 allele, and sensation-seeking in adult children of alcoholics. Alcohol Clin.Exp. Res. 2001, 25, 960–967. [Google Scholar]
- Hill, S.Y.; Zezza, N.; Wipprecht, G.; Xu, J.; Neiswanger, K. Linkage studies of D2 and D4 receptor genes and alcoholism. Am. J. Med. Genet. 1999, 88, 676–685. [Google Scholar]
- van Holstein, M.; Aarts, E.; van der Schaaf, M.E.; Geurts, D.E.; Verkes, R.J.; Franke, B.; van Schouwenburg, M.R.; Cools, R. Human cognitive flexibility depends on dopamine D2 receptor signaling. Psychopharmacology (Berl.) 2011, 218, 567–578. [Google Scholar]
- Bau, C.H.D.; Almeida, S.; Hutz, M.H. The TaqI A1 allele of the dopamine D2 receptor gene and alcoholism in Brazil: Association and interaction with stress and harm avoidance on severity prediction. Am. J. Med. Genet. 2000, 96, 302–306. [Google Scholar]
- Comings, D.E.; Gade, R.; MacMurray, J.P.; Muhlleman, D.; Peters, W.R. Genetic variants of the human obesity (OB) gene: association with body mass index in young women psychiatric symptoms, and interaction with the dopamine D2 receptor gene. Mol. Psychiatry 1996, 1, 325–335. [Google Scholar]
- Comings, D.E.; Rosenthal, R.J.; Lesieur, H.R.; Rugle, L.J.; Muhleman, D.; Chiu, C.; Dietz, G.; Gade, R. A study of the dopamine D2 receptor gene in pathological gambling. Pharmacogenetics 1996, 6, 223–234. [Google Scholar]
- Koob, G.F. Neurobiology of addiction. Toward the development of new therapies. Ann. NY Acad. Sci. 2000, 909, 170–185. [Google Scholar]
- Epping-Jordan, M.P.; Markou, A.; Koob, G.F. The dopamine D-1 receptor antagonist SCH 23390 injected into the dorsolateral bed nucleus of the stria terminalis decreased cocaine reinforcement in the rat. Brain Res. 1998, 784, 105–115. [Google Scholar]
- Wightman, R.M.; Robinson, D.L. Transient changes in mesolimbic dopamine and their association with “reward”. J. Neurochem. 2002, 82, 721–735. [Google Scholar]
- Suhara, T.; Yasuno, F.; Sudo, Y.; Yamamoto, M.; Inoue, M.; Okubo, Y.; Suzuki, K. Dopamine D2 receptors in the insular cortex and the personality trait of novelty seeking. Neuroimage 2001, 13, 891–895. [Google Scholar]
- Hodge, C.W.; Chappelle, A.M.; Samson, H.H. Dopamine receptors in the medial prefrontal cortex influence ethanol and sucrose-reinforced responding. Alcohol Clin.Exp. Res. 1996, 20, 1631–1638. [Google Scholar]
- Hodge, C.W.; Cox, A.A. The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions. Psychopharmacology (Berl.) 1998, 139, 95–107. [Google Scholar]
- Grant, K.A. Emerging neurochemical concepts in the actions of ethanol at ligand-gated ion channels. Behav. Pharmacol. 1994, 5, 383–404. [Google Scholar]
- Althaus, M.; Groen, Y.; Wijers, A.A.; Mulder, L.J.; Minderaa, R.B.; Kema, I.P.; Dijck, J.D.; Hartman, C.A.; Hoekstra, P.J. Differential effects of 5-HTTLPR and DRD2/ANKKI1 polymorphisms on electrocortical measures of error and feedback processing in children. Clin. Neurophysiol. 2009, 120, 93–107. [Google Scholar]
- Rothman, R.B.; Blough, B.E.; Baumann, M.H. Duel dopamine/serotonin releasers as potential medications for stimulant and alcohol addictions. AAPS J. 2007, 9, E1–E10. [Google Scholar]
- Merlo, L.J.; Gold, M.S. Special report—Frontiers in psychiatric research: Addiction research: The state of the art in 2008. Psychiatr. Times 2008, 25, 52–57. [Google Scholar]
- Paczynski, R.P.; Gold, M.S. Cocaine and crack. In Lowinson and Ruiz’s Substance Abuse: A Comprehensive Textbook, 5th; Ruiz, P., Strain, E., Eds.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2011. [Google Scholar]
- Barnes, J.J.; Dean, A.J.; Nandam, L.S.; O'Connell, R.G.; Bellgrove, M.A. The molecular genetics of executive function: role of monoamine system genes. Biol. Psychiatry 2011, 69, e127–e143. [Google Scholar]
- Blum, K.; Braverman, E.R.; Wood, R.C.; Gill, J.; Li, C.; Chen, T.J.; Taub, M.; Montgomery, A.R.; Sheridan, P.J.; Cull, J.G. Increased prevalence of the Taq1 A1 allele of the dopamine receptor gene in obesity with comorbid substance use disorder. Pharmacogenetics 1996, 6, 297–305. [Google Scholar]
- Volkow, N.D.; Chang, L.; Wang, G.J.; Fowler, J.S.; Ding, Y.S.; Sedler, M.; Logan, J.; Franceschi, D.; Gatley, J.; Hitzemann, R.; et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: Associations with metabolism in the orbitofrontal cortex. Am. J. Psychiatry 2001, 158, 2015–2021. [Google Scholar]
- Perez de los Cobos, J.; Baiget, M.; Trujols, J.; Sinol, N.; Volpini, V.; Banuls, E.; Calafell, F.; Luquero, E.; del Rio, E.; Alvarez, E. Allelic and genotypic associations of DRD2 TaqI A polymorphism with heroin dependence in Spanish subjects: A case control study. Behav. Brain Funct. 2007, 3. [Google Scholar]
- Schindler, K.M.; Pato, M.T.; Dourado, A.; Macedo, A.; Azevedo, M.H.; Kennedy, J.L.; Pato, C.N. Association and linkage disequilibrium between a functional polymorphism of the dopamine-2 receptor gene and schizophrenia in a genetically homogeneous Portuguese population. Mol. Psychiatry 2002, 7, 1002–1005. [Google Scholar]
- Oscar-Berman, M.; Marinkovic, K. Alcohol: Effects on neurobehavioral functions and the brain. Neuropsychol. Rev. 2007, 17, 239–257. [Google Scholar]
- Dackis, C.A.; Gold, M.S.; Davies, R.K.; Sweeney, D.R. Bromocriptine treatment for cocaine abuse: The dopamine depletion hypothesis. Int. J. Psychiatry Med. 1985, 15, 125–135. [Google Scholar]
- Gold, M.S.; Graham, N.A.; Cocores, J.A.; Nixon, S.J. Editorial: Food addiction? J. Addict. Med. 2009, 3, 42–45. [Google Scholar]
- Rowe, D.C. Genetic and environmental components of antisocial behavior: A study of 265 twin pairs. Criminology 1986, 24, 513–532. [Google Scholar]
- Halbus, M.; Magnusson, T.; Magnusson, O. Influence of 5-HT1B/1D receptors on dopamine in the guinea pig NAc: A microdialysis study. Neurosci. Lett. 1997, 225, 57–60. [Google Scholar]
- Koob, G.F. Alcoholism: Allostasis and beyond. Alcohol Clin. Exp. Res. 2003, 27, 232–243. [Google Scholar]
- Hietata, J.; West, C.; Syvalahti, E.; Nagren, K.; Lehikoinen, P.; Sonninen, P.; Ruotsalainen, U. Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology (Berl.) 1994, 116, 285–290. [Google Scholar]
- Cools, A.R.; Gingras, M.A.; Nijmegen, J. High and low responders to novelty: A new tool in the search after the neurobiology of drug abuse liability. Pharmacol. Biochem. Behav. 1998, 60, 151–159. [Google Scholar]
- Kuikka, J.T.; Repo, E.; Bergstrom, K.A.; Tupala, E.; Tihonen, J. Specific binding and laterality of human extrastriatal dopamine D2/D3 receptors in the late onset type 1 alcoholic patients. Neurosci. Lett. 2000, 292, 57–59. [Google Scholar]
- Miller, W.B.; Pasta, D.J.; MacMurray, J.; Chiu, C.; Wu, H.; Comings, D.E. Dopamine receptor genes are associated with age at first sexual intercourse. J. Biosoc. Sci. 1999, 31, 43–54. [Google Scholar]
- Davis, C.; Levitan, R.D.; Kaplan, A.S.; Carter, J.; Reid, C.; Curtis, C.; Patte, K.; Hwang, R.; Kennedy, J.L. Reward sensitivity and the D2 dopamine receptor gene: A case-control study of binge eating disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008, 32, 620–628. [Google Scholar]
- Comings, D.E.; Gade-Andavolu, R.; Gonzalez, N.; Wu, S.; Muhleman, D.; Chen, C.; Koh, P.; Farwell, K.; Blake, H.; Dietz, G.; et al. The additive effect of neurotransmitter genes in pathological gambling. Clin. Genet. 2001, 60, 107–116. [Google Scholar]
- Ibanez, A.; Blanco, C.; Donahue, E.; Lesieur, H.R.; Perez de Castro, I.; Fernandez-Piqueras, J.; Saiz-Ruiz, J. Psychiatric comorbidity in pathological gamblers seeking treatment. Am. J. Psychiatry 2001, 158, 1733–1735. [Google Scholar]
- Reuter, J.; Raedler, T.; Rose, M.; Hand, I.; Glasher, J.; Buchel, C. Pathological gambling is linked to reduced activation of the mesolimbic system. Nat. Neurosci. 2005, 8, 147–148. [Google Scholar]
- Koepp, M.J.; Gunn, R.N.; Lawrence, A.D.; Cunningham, V.J.; Dagher, A.; Jones, T.; Brooks, D.J.; Bench, C.J.; Grasby, P.M. Evidence for striatal dopamine release during a video game. Nature 1998, 393, 266–268. [Google Scholar]
- Noble, E.P.; Syndilko, K.; Fitch, R.J.; Ritchie, T.; Bohlman, M.C.; Guth, P.; Sheridan, P.J.; Montgomery, A.; Heinzmann, C.; Sparkes, R.S.; et al. D2 dopamine receptor Taq1 A alleles in medically ill alcoholic and nonalcoholic patients. Alcohol Alcohol. 1994, 129, 729–744. [Google Scholar]
- Li, Y.; Shao, C.; Zhang, D.; Zhao, M.; Lin, L.; Yan, P.; Xie, Y.; Jiang, K.; Jin, L. The effect of dopamine D2, D5 receptor and transporter (SLC6A3) polymorphisms on the cue-elicited heroin craving in Chinese. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141B, 269–273. [Google Scholar]
- Little, K.Y.; Zang, L. Striatal dopaminergic abnormalities in human cocaine users. Am. J. Psychiatry 1999, 156, 238–245. [Google Scholar]
- Hutchinson, K.E.; McGeary, J.; Smolen, A.; Bryan, A.; Swift, R.M. The DRD4 VNTR polymorphism moderates craving after alcohol consumption. Health Psychol. 2002, 21, 139–146. [Google Scholar]
- Adler, C.M.; Elman, I.; Weisenfield, N.; Kestler, L.; Pickar, D.; Breier, A. Effects of acute metabolic stress on striatal dopamine release in healthy volunteers. Neuropsychopharmacology 2000, 22, 545–550. [Google Scholar]
- Blum, K.; Payne, J. Alcohol and the Addictive Brain; The Free Press (Simon and Schuster): New York, NY, USA, 1991. [Google Scholar]
- Blum, K.; Noble, E.P.; Sheridan, P.J.; Finley, O.; Montgomery, A.; Ritchie, T.; Ozkaragoz, T.; Fitch, R.J.; Sadlack, F.; Sheffield, D.; et al. Association of the A1 allele of the D2 dopamine receptor gene with severe alcoholism. Alcohol 1991, 8, 409–416. [Google Scholar]
- Carboni, E.; Silvagni, A.; Rolando, M.T.P.; Di Chiara, G. Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. J. Neurosci. 2000, 20, 1–5. [Google Scholar]
- Di Chiara, G. Drug addiction as dopamine-dependent associative learning disorder. Eur. J. Pharmacol. 1999, 375, 13–30. [Google Scholar]
- Di Chiara, G. NAc shell and core dopamine: Differential role in behavior and addiction. Behav. Res. 2002, 137, 75–114. [Google Scholar]
- Di Chiara, G.; Tanda, G.; Bassare, V.; Pontieri, F.; Acquas, E.; Fenu, S.; Cadoni, C.; Carboni, E. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdale dopamine. Ann. NY Acad. Sci. 1999, 877, 461–485. [Google Scholar]
- Di Chiara, G.; Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic systems of freely moving rats. Proc. Natl. Acad. Sci. USA 1988, 85, 5274–5278. [Google Scholar]
- Gessa, G.; Mutoni, F.; Coller, M.; Vargin, L.; Mercer, G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1986, 48, 201–203. [Google Scholar]
- Eshleman, A.J.; Henningsen, R.A.; Neve, K.A.; Janowsky, A. Release of dopamine via the human transporter. Mol. Pharmacol. 1994, 45, 312–316. [Google Scholar]
- Piechota, M.; Korostynski, M.; Solecki, W.; Gieryk, A.; Slezak, M.; Bilecki, W.; Ziolkowska, B.; Kostrzewa, E.; Cymerman, I.; Swiech, L.; et al. The dissection of transcriptional modules regulated by various drugs of abuse in the mouse striatum. Genome Biol. 2010, 11. [Google Scholar]
- Neville, M.J.; Johnstone, E.C.; Walton, R.T. Identification and characterization of ANKK1: A novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum. Mutat. 2004, 23, 540–545. [Google Scholar]
- Huang, W.; Payne, T.J.; Ma, J.Z.; Beuten, J.; Dupont, R.T.; Inohara, N.; Li, M.D. Significant association of ANKK1 and detection of a functional polymorphism with nicotine dependence in an African-American sample. Neuropsychopharmacology 2009, 34, 319–330. [Google Scholar]
- Noble, E.P.; Blum, K.; Ritchie, T.; Montgomery, A.; Sheridan, P. Allelic associations of the D2 dopamine receptor gene with receptor-binding characteristics. Arch. Gen. Psychiatry. 1991, 48, 648–654. [Google Scholar]
- Montag, C.; Markett, S.; Basten, U.; Stelzel, C.; Fiebach, C.; Canli, T.; Reuter, M. Epistasis of the DRD2/ANKK1 Taq Ia and the BDNF Val66Met polymorphism impacts novelty seeking and harm avoidance. Neuropsychopharmacology 2010, 35, 1860–1867. [Google Scholar]
- Jönsson, E.G.; Nöthen, M.M.; Grünhage, F.; Farde, L.; Nakashima, Y.; Propping, P.; Sedvall, G.C. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol. Psychiatry 1999, 4, 290–296. [Google Scholar]
- Duan, J.; Wainwright, M.S.; Comeron, J.M.; Saitou, N.; Sanders, A.R.; Gelernter, J.; Gejman, P.V. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum. Mol. Genet. 2003, 12, 205–216. [Google Scholar]
- Hirvonen, M.; Laakso, A.; Någren, K.; Rinne, J.O.; Pohjalainen, T.; Hietala, J. C957T polymorphism of the dopamine D2 receptor (DRD2) gene affects striatal DRD2 availability in vivo. Mol. Psychiatry 2004, 9, 1060–1061. [Google Scholar]
- Hill, S.Y.; Hoffman, E.K.; Zezza, N.; Thalamuthu, A.; Weeks, D.E.; Matthews, A.G.; Mukhopadhyay, I. Dopaminergic mutations: Within-family association and linkage in multiplex alcohol dependence families. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008, 147B, 517–526. [Google Scholar]
- Vandenbergh, D.J. Molecular cloning of neurotransmitter transporter genes: Beyond coding region of cDNA. Meth. Enzymol. 1998, 296, 498–514. [Google Scholar]
- Michelhaugh, S.K.; Fiskerstrand, C.; Lovejoy, E.; Bannon, M.J.; Quinn, J.P. The dopamine transporter gene (SLC6A3) variable number of tandem repeats domain enhances transcription in dopamine neurons. J. Neurochem. 2001, 79, 1033–1038. [Google Scholar]
- Guindalini, C.; Howard, M.; Haddley, K.; Laranjeira, R.; Collier, D.; Ammar, N.; Craig, I.; O’Gara, C.; Bubb, V.J.; Greenwood, T.; et al. A dopamine transporter gene functional variant associated with cocaine abuse in a Brazilian sample. Proc. Natl. Acad. Sci. USA 2006, 103, 4552–4557. [Google Scholar]
- Vandenbergh, D.J.; Bennett, C.J.; Grant, M.D.; Strasser, A.A.; O’Connor, R.; Stauffer, R.L.; Vogler, G.P.; Kozlowski, L.T. Smoking status and the human dopamine transporter variable number of tandem repeats (VNTR) polymorphism: Failure to replicate and finding that never-smokers may be different. Nicotine Tob.Res. 2002, 4, 333–340. [Google Scholar]
- Cook, E.H., Jr.; Stein, M.A.; Krasowski, M.D.; Cox, N.J.; Olkon, D.M.; Kieffer, J.E.; Leventhal, B.L. Association of attention-deficit disorder and the dopamine transporter gene. Am. J. Hum. Genet. 1995, 56, 993–998. [Google Scholar]
- Lee, S.S.; Lahey, B.B.; Waldman, I.; Van Hulle, C.A.; Rathouz, P.; Pelham, W.E.; Loney, J.; Cook, E.H. Association of dopamine transporter genotype with disruptive behavior disorders in an eight-year longitudinal study of children and adolescents. Am. J. Med. Genet. Neuropsychiatr. Genet. 2007, 144B, 310–317. [Google Scholar]
- Self, D.W. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology 2004, 47 (Suppl 1), 242–255. [Google Scholar]
- Comings, D.E.; Gade, R.; Wu, S.; Chiu, C.; Dietz, G.; Muhleman, D.; Saucier, G.; Ferry, L.; Rosenthal, R.J.; Lesieur, H.R.; et al. Studies of the potential role of the dopamine D1 receptor gene in addictive behaviors. Mol. Psychiatry 1997, 2, 44–56. [Google Scholar]
- Luo, Z.; Volkow, N.D.; Heintz, N.; Pan, Y.; Du, C. Acute cocaine induces fast activation of D1 receptor and progressive deactivation of D2 receptor striatal neurons: In vivo optical microprobe [Ca2+]i imaging. J. Neurosci. 2011, 31, 13180–13190. [Google Scholar]
- Lobo, M.K.; Covington, H.E., 3rd.; Chaudhury, D.; Friedman, A.K.; Sun, H.; Damez-Werno, D.; Dietz, D.M.; Zaman, S.; Koo, J.W.; Kennedy, P.J.; et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 2010, 330, 385–390. [Google Scholar]
- Egeland, J.A. A genetic study of manic-depressive disorder among the old order Amish of Pennsylvania. Pharmacopsychiatry 1988, 21, 74–75. [Google Scholar]
- Comings, D.E. Clinical and molecular genetics of ADHD and Tourette syndrome. Two related polygenic disorders. Ann. N. Y. Acad. Sci. 2001, 931, 50–83. [Google Scholar]
- Hess, C.; Reif, A.; Strobel, A.; Boreatti-Hümmer, A.; Heine, M.; Lesch, K.P.; Jacob, C.P. A functional dopamine-beta-hydroxylase gene promoter polymorphism is associated with impulsive personality styles, but not with affective disorders. J. Neural. Transm. 2009, 116, 121–130. [Google Scholar]
- McClernon, F.J.; Fuemmeler, B.F.; Kollins, S.H.; Kail, M.E.; Ashley-Koch, A.E. Interactions between genotype and retrospective ADHD symptoms predict lifetime smoking risk in a sample of young adults. Nicotine Tob. Res. 2008, 10, 117–127. [Google Scholar]
- Barkley, R.A.; Smith, K.M.; Fischer, M.; Navia, B. An examination of the behavioral and neuropsychological correlates of three ADHD candidate gene polymorphisms (DRD4 7+, DBH TaqI A2, and DAT1 40 bp VNTR) in hyperactive and normal children followed to adulthood. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141B, 487–498. [Google Scholar]
- Kirsch, P.; Reuter, M.; Mier, D.; Lonsdorf, T.; Stark, R.; Gallhofer, B.; Vaitl, D.; Hennig, J. Imaging gene-substance interactions: The effect of the DRD2 TaqIA polymorphism and the dopamine agonist bromocriptine on the brain activation during the anticipation of reward. Neurosci.Lett. 2006, 405, 196–201. [Google Scholar]
- McKinney, E.F.; Walton, R.T.; Yudkin, P.; Fuller, A.; Haldar, N.A.; Mant, D.; Murphy, M.; Welsh, K.I.; Marshall, S.E. Association between polymorphisms in dopamine metabolic enzymes and tobacco consumption in smokers. Pharmacogenetics 2000, 10, 483–491. [Google Scholar]
- Chen, T.J.; Blum, K.; Mathews, D.; Fisher, L.; Schnautz, N.; Braverman, E.R.; Schoolfield, J.; Downs, B.W.; Comings, D.E. Are dopamonergic genes involved in a predisposition to pathological aggression? Hypothesizing the importance of “super normal controls” in psychiatric genetic research of complex behavioral disorders. Med. Hypotheses 2005, 65, 703–707. [Google Scholar]
- Thompson, M.; Comings, D.E.; Feder, L.; George, S.R.; O’Dowd, B.F. Mutation screening of the dopamine D1 receptor region gene in Tourette’s syndrome and alcohol dependent patients. Am. J. Med. Genet. 1998, 81, 241–244. [Google Scholar]
- D’Amato, T.; Leboyer, M.; Malafosse, A.; Samolyk, D.; Lamouroux, A.; Junien, C.; Mallet, J. Two TaqI dimorphic sites at the human beta-hydroxylase locus. Nucleic Acids Res. 1989, 17, 5871. [Google Scholar]
- Siegel, S. Nonparametric Statistics for the Behavioral Sciences; McGraw Hill Book Co: New York, NY, USA, 1957; pp. 104–111. [Google Scholar]
- Blum, K.; Giordano, J.; Morse, S.; Liu, Y.; Tan, J.; Bowirrat, A.; Smolen, A.; Waite, R.; Downs, W.; Madigan, M.; et al. Genetic Addiction Risk Score (GARS) analysis: Exploratory development of polymorphic risk alleles in poly-drug addicted males. IIOAB J. 2010, 1, 1–14. [Google Scholar]
- Smith, L.; Watson, M.; Gates, S.; Ball, D.; Foxcroft, D. Meta-analysis of the association of the Taq1A polymorphism with the risk of alcohol dependency: A HuGE gene-disease association review. Am. J. Epidemiol. 2008, 167, 125–138. [Google Scholar]
- Lawford, B.R.; Young, R.M.; Noble, E.P.; Sargent, J.; Rowell, J.; Shadforth, S.; Zhang, X.; Ritchie, T. The D(2) dopamine receptor A(1) allele and opioid dependence: Association with heroin use and response to methadone treatment. Am. J. Med. Genet. 2000, 6, 592–598. [Google Scholar]
- Monakhov, M.; Golimbet, V.; Abramova, L.; Kaleda, V.; Karpov, V. Association study of three polymorphisms in the dopamine D2 receptor gene and schizophrenia in the Russian population. Schizophr. Res. 2008, 100, 302–307. [Google Scholar]
- López, J.; López, V.; Rojas, D.; Carrasco, X.; Rothhammer, P.; García, R.; Rothhammer, F.; Aboitiz, F. Effect of psychostimulants on distinct attentional parameters in attentional deficit/hyperactivity disorder. Biol. Res. 2004, 37, 461–468. [Google Scholar]
- Gorwood, P. Contribution of genetics to the concept of risk status for alcohol dependence. J. Soc. Biol. 2000, 194, 43–49. [Google Scholar]
- Shao, H.; Burrage, L.C.; Sinasac, D.S.; Hill, A.E.; Ernest, S.R.; O’Brien, W.; Courtland, H.W.; Jepsen, K.J.; Kirby, A.; Kulbokas, E.J.; Daly, M.J.; Broman, K.W.; Lander, E.S.; Nadeau, J.H. Genetic architecture of complex traits: Large phenotypic effects and pervasive epistasis. Proc. Natl. Acad. Sci. USA 2008, 105, 19910–19914. [Google Scholar]
- Hill, S.Y.; Neiswanger, K. The value of narrow psychiatric phenotypes and “Super” normal controls. In Handbook of Psychiatric Genetics; Blum, K., Noble, E.P., Eds.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Uhl, G.; Blum, K.; Noble, E.; Smith, S. Substance abuse vulnerability and D2 receptor genes. Trends Neurosci. 1993, 16, 83–88. [Google Scholar]
- Mash, D.C.; Staley, J.K.; Doepel, F.M.; Young, S.N.; Ervin, F.R.; Palmour, R.M. Altered dopamine transporter densities in alcohol-preferring vervet monkeys. Neuroreport 1996, 7, 457–462. [Google Scholar]
- Stice, E.; Spoor, S.; Bohon, C.; Small, D.M. Relation between obesity and blunted striatal response to food is moderated by Taq1A A1 allele. Science 2008, 322, 448–452. [Google Scholar]
- Tupala, E.; Hall, H.; Bergstrom, K.; Mantere, T.; Rasanen, P.; Sarkioja, T.; Tiihonen, J. Dopamine D2 receptors and transporter in type 1 and 2 alcoholics measured with human whole hemisphere autoradiography. Hum. Brain Mapp. 2003, 20, 91–102. [Google Scholar]
- Tupala, E.; Hall, H.; Bergstrom, K.; Sarkioja, T.; Rasanen, P.; Mantere, P.; Callaway, J.; Hiltunen, J.; Tiihonen, J. Dopamine D(2)/D(3)-receptor and transporter densities in NAc and amygdale of type 1 and type 2 alcoholics. Mol. Psychiatry 2001, 6, 261–267. [Google Scholar]
- Tupala, E.; Kuikka, J.T.; Hall, H.; Bergstrom, K.; Sarkioja, T.; Rasanen, P.; Mantere, T.; Hiltunen, J.; Vepsalainen, J.; Tiihonen, J. Measurement of the striatal dopamine transporter density and heterogeneity in type 1 alcoholics using human whole hemisphere autoradiography. Neuroimage 2001, 1, 87–94. [Google Scholar]
- Laakso, A.; Pohjalainen, T.; Bergman, J.; Kajander, J.; Haaparanta, M.; Solin, O.; Syvalahti, E.; Hietala, J. The A1 allele of the human D2 dopamine receptor gene is associated with increased activity of striatal L-amino acid decarboxylase in healthy subjects. Pharmacogenet. Genomics 2005, 15, 387–391. [Google Scholar]
- Sambataro, F.; Fazio, L.; Taurisano, P.; Gelao, B.; Porcelli, A.; Mancini, M.; Sinibaldi, L.; Ursini, G.; Masellis, R.; Caforio, G.; et al. DRD2 genotype-based variation of default mode network activity and of its relationship with striatal DAT binding. Schizophr Bull. 2011. [Google Scholar]
- Miller, N.S.; Gold, M.S. A hypothesis for a common neurochemical basis for alcohol and drug disorders. Psychiatr. Clin. North Am. 1993, 15, 105–117. [Google Scholar]
- Parsian, A.; Todd, R.D.; Devor, E.J.; O’Malley, K.L.; Suarez, B.K.; Reich, T.; Cloninger, C.R. Alcoholism and alleles of the human D2 dopamine receptor locus: Studies of Association and linkage. Arch. Gen. Psychiatry 1991, 48, 655–663. [Google Scholar]
- Repo, E.; Kuikka, J.T.; Bergstrom, K.A.; Karbu, J.; Hiltunen, J.; Tiihonen, J. Dopamine transporter and D2-receptor density in late-onset alcoholism. Psychopharmacology (Berl.) 1999, 147, 314–318. [Google Scholar]
- Oscar-Berman, M.; McNamara, P.; Freedman, M. Delayed-Response Tasks: Parallels between experimental ablation studies and finding in patients with frontal lesions. In Frontal Lobe Function and Injury; Levin, H.S., Eisenberg, H.M., Benton, A.L., Eds.; Oxford University Press: New York, NY, USA, 1991; pp. 231–255. [Google Scholar]
- Walsh, S.L.; Cunningham, K.A. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacology (Berl.) 1997, 130, 41–58. [Google Scholar]
- Pilla, M.; Perachon, S.; Sautel, F.; Garrido, F.; Mann, A.; Wermuth, C.G.; Schwartz, J.C.; Everitt, B.J.; Sokoloff, P. Selective inhibition of cocaine-seeking behavior by a partial dopamine D3 receptor agonist. Nature 1990, 400, 371–375. [Google Scholar]
- Myers, R.D.; Robinson, D.E. Mmu and D2 receptor antisense oligonucleotides injected in nucleus accumbens suppress high alcohol intake in genetic drinking HEP rats. Alcohol 1999, 18, 225–233. [Google Scholar]
- Xu, K.; Lichterman, D.; Kipsky, R.H.; Franke, P.; Liu, X.; Hu, Y.; Cao, L.; Schwab, S.G.; Wildenauer, D.B.; Bau, C.H.; et al. Association of specific haplotypes of D2 dopamine receptor gene with vulnerability to heroin dependence in distinct populations. Arch. Gen. Psychiatry 2004, 61, 567–606. [Google Scholar]
- Noble, E.P.; Noble, R.E.; Ritchie, T.; Grandy, D.K.; Sparkes, R.S. D1 receptor gene and obesity. Int. J. Eat. Disord. 1994, 15, 205–217. [Google Scholar]
- Epstein, L.H.; Temple, J.L.; Neaderhiser, B.J.; Salis, R.J.; Erbe, R.W.; Leddy, J.J. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behav. Neurosci. 2007, 121, 877–886. [Google Scholar]
- Meredith, J.M.; Moffatt, A.C.; Auger, A.P.; Snyder, G.L.; Greengard, P.; Blaustein, J.D. Mating-related stimulation induced phosphorylation of dopamine-and cyclic AMP-regulated phosphoprotein-32 in progestin receptor-containing areas in the female rat brain. J. Neurosci. 1998, 18, 10189–10195. [Google Scholar]
- Miller, W.B.; Pasta, D.J.; MacMurray, J.; Chiu, C.; Wu, H.; Comings, D.E. Dopamine receptor genes are associated with age at first intercourse. J. Biosoc. Sci. 1999, 31, 43–54. [Google Scholar]
- Pani, L.; Porcella, A.; Gessa, G.L. The role of stress in the pathophysiology of the dopaminergic system. Mol. Psychiatry 2000, 5, 14–21. [Google Scholar]
- Binczycka-Anholcer, M.N. Aggressive behavior and the public health condition. Wiad Lek. 2002, 55, 627–632. [Google Scholar]
- Pontius, A.A. Forensic significance of the limbic psychotic trigger reaction. Bull. Am. Acad. Psychiatry Law 1996, 24, 125–134. [Google Scholar]
- Kamarajan, C.; Rangaswamy, M.; Tang, Y.; Chorlian, D.B.; Pandey, A.K.; Roopesh, B.N.; Manz, N.; Saunders, R.; Stimus, A.T.; Porjesz, B. Dysfunctional reward processing in male alcoholics: An ERP study during a gambling task. J. Psychiatr. Res. 2010, 44, 576–590. [Google Scholar]
- Gebhardt, C.; Leisch, F.; Schussler, P.; Fuchs, K.; Stompe, T.; Sieghart, W.; Hornik, K.; Kasper, S.; Aschauer, H.N. Non-association of dopamine D4 and D2 receptor genes with personality in healthy individuals. Psychiatr. Genet. 2000, 10, 131–137. [Google Scholar]
- Sugiura, M.; Kawashima, R.; Nakagawa, M.; Okada, K.; Sato, T.; Goto, R.; Sato, K.; Ono, S.; Schormann, T.; Zilles, K.; et al. Correlation between human personality and neuronal activity in cerebral cortex. Neuroimage 2000, 11, 541–546. [Google Scholar]
- Blum, K.; Braverman, E.R.; Wu, S.; Cull, J.G.; Chen, T.J.; Gill, J.; Wood, R.; Eisenberg, A.; Sherman, M.; Davis, K.R.; et al. Association of polymorphisms of dopamine D2 receptor (DRD2) and dopamine transporter (DAT1) genes with schizoid/avoidant behavior (SAB). Mol. Psychiatry 1997, 2, 239–246. [Google Scholar]
- Keltikangas-Jarvinene, L.; Pulkki-Raback, L.; Eiovainio, M.; Raltakari, O.T.; Vilkari, J.; Lehtimaki, T. DRD2 C32806T modifies the effect of child-rearing environment on adulthood novelty seeking. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009, 150B, 389–394. [Google Scholar]
- Golimbet, V.E.; Alfimova, M.V.; Gritsenko, I.K.; Ebstein, R.P. Relationship between dopamine system genes and extraversion and novelty seeking. Neurosci. Behav. Physiol. 2007, 37, 601–606. [Google Scholar]
- Emanuele, E.; Brondino, N.; Pesenti, S.; Re, S.; Geroldi, D. Genetic loading on human loving styles. Neuro Endocrionl. Lett. 2007, 28, 815–821. [Google Scholar]
- Parsons, L.H.; Weiss, F.; Koob, G.F. Serotonin1b receptor stimulation enhances dopamine-mediated reinforcement. Psychopharmacology (Berl.) 1996, 128, 150–160. [Google Scholar]
- Wise, R.A. Brain reward circuitry: Insight from unsensed incentives. Neuron 2002, 36, 229–240. [Google Scholar]
- Yadid, G.; Pacak, K.; Kopin, I.J.; Goldstein, D.S. Endogenous serotonin stimulates striatal dopamine release in conscious rats. J. Pharmacol. Exp. Ther. 1994, 270, 1158–1165. [Google Scholar]
- Hill, S.Y. Alternative strategies for uncovering genes contributing to alcoholism risk: Unpredictable findings in a genetic wonderland. Alcohol 1998, 16, 53–59. [Google Scholar]
- Bouchard, T.J. Genes, environment, and personality. Science 1994, 9, 415–421. [Google Scholar]
- Thut, G.; Schultz, W.; Roelcke, U.; Nienhusmeier, M.; Missimer, J.; Maguire, R.P.; Leenders, K.L. Activation of the human brain by monetary reward. Neuroreport 1997, 8, 1225–1228. [Google Scholar]
- Clark, W.R.; Grunstein, M. Are we hardwired? The role of genes. In Human Behavior; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Archer, T.; Oscar-Berman, M.; Blum, K. Epigenetics in developmental disorder: ADHD and endophenotypes. J. Genet. Syndr. Gene Ther. 2011, 2. [Google Scholar]
- van den Bree, M.B.; Johnson, E.O.; Neale, M.C.; Pickens, R.W. Genetic and environmental influences on drug use and abuse/dependence in male and female twins. DrugAlcohol Depend. 1998, 52, 231–241. [Google Scholar]
- Johnson, E.O.; van den Bree, M.B.; Gupman, A.E.; Pickens, R.W. Extension of a typology of alcohol dependence based on relative genetic and environmental loading. Alcohol Clin. Exp. Res. 1998, 22, 1421–1429. [Google Scholar]
- Gerasimov, M.R.; Schiffer, W.K.; Gardner, E.L.; Marsteller, D.A.; Lennon, I.C.; Taylor, S.J.; Brodie, J.D.; Ashby, C.R., Jr.; Dewey, S.L. GABAergic blockade of cocaine-associated cue-induced increases in nucleus accumbens dopamine. Eur. J. Pharmacol. 2001, 414, 205–209. [Google Scholar]
- Smith, J.E.; Co, C.; Freeman, M.E.; Lane, J.D. Brain neurotransmitter turnover correlated with morphine-seeking behavior of rats. Pharmacol. Biochem. Behav. 1982, 16, 509–519. [Google Scholar]
- Blum, K.; Sheridan, P.J.; Wood, R.C.; Braverman, E.R.; Chen, T.J.; Comings, D.E. Dopamine D2 receptor gene variants: Association and linkage studies in impulsive-addictive-compulsive behaviour. Pharmacogenetics 1995, 5, 121–141. [Google Scholar]
- The, L.K.; Izzuddin, A.F.; Fazleen Haslinda, M.H.; Zakaria, Z.A.; Salleh, M.Z. Tridimensional personalities and polymorphism of dopamine D2 receptor among heroin addicts. Biol. Res. Nurs. 2011. [Google Scholar]
- Kazantseva, A.; Gaysina, D.; Malykh, S.; Khusnutdinova, E. The role of dopamine transporter (SLC6A3) and dopamine D2 receptor/ankyrin repeat and kinase domain containing 1 (DRD2/ANKK1) gene polymorphisms in personality traits. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1033–1040. [Google Scholar]
- Enoch, M.A. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl.) 2011, 214, 17–31. [Google Scholar]
- Raz, S.; Berger, B.D. Social isolation increases morphine intake: Behavioral and psychopharmacological aspects. Behav. Pharmacol. 2010, 21, 39–46. [Google Scholar]
- Chauvet, C.; Lardeux, V.; Goldberg, S.R.; Jaber, M.; Solinas, M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology 2009, 34, 2767–2778. [Google Scholar]
- Rhodes, T. Risk environments and drug harms: A social science for harm reduction approach. Int. J. Drug Policy 2009, 20, 193–201. [Google Scholar]
- Perry, J.L.; Carroll, M.E. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl.) 2008, 200, 1–26. [Google Scholar]
- Kippin, T.E.; Szumlinski, K.K.; Kapasova, Z.; Rezner, B.; See, R.E. Prenatal stress enhances responsiveness to cocaine. Neuropsychopharmacology 2008, 33, 769–782. [Google Scholar]
- Abreu-Villaça, Y.; Queiroz-Gomes Fdo, E.; Dal Monte, A.P.; Filgueiras, C.C.; Manhães, A.C. Individual differences in novelty-seeking behavior but not in anxiety response to a new environment can predict nicotine consumption in adolescent C57BL/6 mice. Behav. Brain Res. 2006, 167, 175–182. [Google Scholar]
- Lu, L.; Xu, N.J.; Ge, X.; Yue, W.; Su, W.J.; Pei, G.; Ma, L. Reactivation of morphine conditioned place preference by drug priming: Role of environmental cues and sensitization. Psychopharmacology (Berl.) 2002, 59, 125–132. [Google Scholar]
- Blum, K.; Chen, A.L.C.; Chen, T.J.H.; Rhoades, P.; Prihoda, T.J.; Downs, B.W.; Waite, R.L.; Williams, L.; Braverman, E.R.; Braverman, D.; et al. LG839: Anti-obesity effects and polymorphic gene correlates of reward deficiency syndrome. Adv. Ther. 2008, 25, 894–913. [Google Scholar]
- Suzuki, H.; Han, S.D.; Lucas, L.R. Chronic passive exposure to aggression decreases D(2) and 5-HT(1B) receptor densities. Physiol. Behav. 2010, 99, 562–570. [Google Scholar]
- Noble, E.P. Addiction and its reward process through polymorphisms of the D2 dopamine receptor gene: A review. Eur. Psychiatry 2000, 15, 79–89. [Google Scholar]
- Lohoff, F.W.; Bloch, P.J.; Hodge, R.; Nall, A.H.; Ferraro, T.N.; Kampman, K.M.; Dackis, G.A.; O’Brien, C.P.; Pettinati, H.M.; Oslin, D.W. Association analysis between polymorphisms in dopamine D2 receptor (DRD2) and dopamine transporter (DAT1) genes with cocaine dependence. Neurosci.Lett. 2010, 473, 87–91. [Google Scholar]
- Yang, B.Z.; Kranzler, H.R.; Zhao, H.; Gruen, J.R.; Luo, X.; Gelernter, J. Haplotypic variants in DRD2, ANKK1, TTc12, and NCAM1 are associated with comorbid alcohol and drug dependence. Alcohol Clin. Exp. Res. 2008, 32, 2117–2127. [Google Scholar]
- Volkow, N.D.; Wang, G.J.; Begleiter, H.G.; Porjesz, B.; Fowler, J.S.; Telang, F.; Wong, C.; Ma, Y.; Logan, J.; Goldstein, R.; et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families. Arch. Gen. Psychiatry 2006, 63, 999–1008. [Google Scholar]
- Young, R.M.; Lawford, B.R.; Noble, E.P.; Kann, B.; Wilkie, A.; Ritchie, T.; Arnold, L.; Shadforth, S. Harmful drinking in military veterans with post-traumatic stress disorder: Association with the D2 dopamine receptor A1 allele. Alcohol Alcohol. 2002, 37, 451–456. [Google Scholar]
- Lawford, B.R.; McD Young, R.; Noble, E.P.; Kann, B.; Arnold, L.; Rowell, J.; Ritchie, T.L. D2 dopamine receptor gene polymorphism: Paroxetine and social functioning in posttraumatic stress disorder. Eur. Neuropsychopharmacol. 2003, 13, 313–320. [Google Scholar]
- Comings, D.E.; Flanagan, S.D.; Dietz, G.; Muhleman, D.; Knell, E.; Grysin, R. The dopamine D2 receptor (DRD2) as a major gene in obesity and height. Biochem. Med. Metab. Biol. 1993, 50, 176–185. [Google Scholar]
- Jenkinson, C.P.; Hanson, R.; Cray, K.; Wiedrich, C.; Knowler, W.C.; Bogardus, C.; Baier, L. Associations of dopamine D2 receptor polymorphisms Ser311Cys and TaqIA with obesity or type 2 diabetes mellitus in Pima Indians. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1233–1238. [Google Scholar]
- Tataranni, P.A.; Baier, L.; Jenkinson, C.; Harper, I.; Del parigi, A.; Bogardus, C. A Ser311Cys mutation in the human dopamine receptor D2 gene is associated with reduced energy expenditure. Diabetes 2001, 50, 901–904. [Google Scholar]
- Thomas, G.N.; Tomlinson, B.; Critchley, J.A. Modulation of blood pressure and obesity with the dopamine D2 receptor gene TaqI polymorphism. Hypertension 2000, 36, 177–182. [Google Scholar]
- Thomas, G.N.; Critchley, J.A.; Tomlinson, B.; Cockram, C.S.; Chan, J.C. Relationships between the TaqI polymorphism of the dopamine D2 receptor and blood pressure in hyperglycaemic and normoglycaemic Chinese subjects. Clin. Endocrinol. (Oxf.) 2001, 55, 605–611. [Google Scholar]
- de Haan, L.; van Bruggen, M.; Lavalaye, J.; Booij, J.; Dingemans, P.M.; Linszen, D. Subjective experience and D2 receptor occupancy in patients with recent-onset schizophrenia treated with low-dose olanzapine or haloperidol: A randomized, double-blind study. Am. J. Psychiatry 2003, 160, 303–309. [Google Scholar]
- Spitz, M.R.; Shi, H.; Yang, F.; Hudmon, K.S.; Jiang, H.; Chamberlain, R.M.; Amos, C.I.; Wan, Y.; Cinciripini, P.; Hong, W.K.; et al. Case-control study of the D2 dopamine receptor gene and smoking status in lung cancer patients. J. Natl. Cancer Inst. 1998, 90, 358–363. [Google Scholar]
- Vandenbergh, D.J.; O’Connor, R.J.; Grant, M.D.; Jefferson, A.L.; Vogler, G.P.; Strasser, A.A.; Kozlowski, L.T. Dopamine receptor genes (DRD2, DRD3, and DRD4) and gene-gene interactions associated with smoking-related behaviors. Addict. Biol. 2007, 12, 106–116. [Google Scholar]
- Guo, G.; Roettger, M.E.; Shih, J.C. Contributions of the DAT1 and DRD2 genes to serious and violent delinquency among adolescents and young adults. Hum. Genet. 2006, 121, 125–136. [Google Scholar]
- Nakajima, T.; Nimura, T.; Yamaguchi, K.; Ando, T.; Itoh, M.; Yoshimoto, T.; Shirane, R. The impact of stereotactic pallidal surgery on the dopamine D2 receptor in Parkinson disease: A positron emission tomography study. J. Neurosurg. 2003, 98, 57–63. [Google Scholar]
- Stoessl, A.J.; de la Fuente-Fernandez, R. Dopamine receptors in Parkinson’s disease: Imaging studies. Adv. Neurol. 2003, 91, 65–71. [Google Scholar]
- Peroutka, S.J.; Price, S.C.; Wilhoit, T.L.; Jones, K.W. Comorbid migraine with aura, anxiety, and depression is associated with dopamine D2 receptor (DRD 2) Ncol alleles. Mol. Med. 1998, 4, 14–21. [Google Scholar]
- Lee, C.C.; Chou, I.C.; Tsai, C.H.; Wang, T.R.; Li, T.C.; Tsai, F.J. Dopamine receptor D2 gene polymorphisms are associated in Taiwanese children with Tourette syndrome. Pediatr. Neurol. 2005, 33, 272–276. [Google Scholar]
- Vetter, J.M.; Jehle, T.; Heinemeyer, J.; Franz, P.; Behrens, P.F.; Jackisch, R.; Landwehrmeyer, G.B.; Feuerstein, T.J. Mice transgenic for exon 1 of Huntington’s disease: Properties of cholinergic and dopaminergic pre-synaptic function in the striatum. J. Neurochem. 2003, 85, 1054–1063. [Google Scholar]
- Serretti, A.; Lattuada, E.; Lorenzi, C.; Lilli, R.; Smeraldi, E. Dopamine receptor D2 Ser/Cys 311 variant is associated with delusion and disorganization symptomatology in major psychoses. Mol. Psychiatry 2000, 5, 270–274. [Google Scholar]
- Lowinson, J.; Ruiz, P.; Millman, R.; Langrod, J. Substance Abuse: A Comprehensive Textbook, 3rd ed; William & Wilkins: Philadelphia, PA, USA, 1997. [Google Scholar]
- Panagis, G.; Nomikos, G.G.; Miliaressis, E.; Chergui, K.; Kastellakis, A.; Svensson, T.H.; Spyraki, C. Ventral pallidum self-stimulation induces stimulus dependent increase in c-fos expression in reward-related brain regions. Neuroscience 1997, 77, 175–186. [Google Scholar]
- Smith, M.; Wasmuth, J.; McPherson, J.D. Cosegregation of an 11q22.3–9p22 translocation with affective disorder: Proximity of the dopamine D2 receptor gene relative to the translocation breakpoint. Am. J. Hum. Genet. 1989, 45, A220. [Google Scholar]
- Comings, D.E.; Comings, B.G.; Muhleman, D.; Dietz, G.; Shahbahrami, B.; Tast, D.; Knell, E.; Kocsis, P.; Baumgarten, R.; Kovacs, B.W.; et al. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. JAMA 1991, 266, 1793–1800. [Google Scholar]
- Joranby, L.; Frost Peneda, K.; Gold, M.S. Addiction to food and brain reward systems. Sex. Addict. Compul. 2005, 12, 201–217. [Google Scholar]
- Hoebel, B.G.; Avena, N.M.; Bocarsly, M.E.; Rada, P. Natural addiction: A behavioral and circuit model based on sugar addiction in rats. J. Addict. Med. 2009, 3, 33–41. [Google Scholar]
- Hammond, J.C.; Gold, M.S. Caffeine dependence, withdrawal, overdose and treatment: A review. Direct. Psychiatry 2008, 28, 177–189. [Google Scholar]
- Volkow, N.D.; Wise, R.A. How can drug addiction help us understand obesity? Nat. Neurosci. 2005, 8, 555–560. [Google Scholar]
- Kleiner, K.D.; Gold, M.S.; Frost-Pineda, K.; Lenz-Brunsman, B.; Perri, M.G.; Jabocs, W.S. Body mass index and alcohol use. J. Addict. Dis. 2004, 23, 105–118. [Google Scholar]
- Warren, M.; Forst-Pineda, K.; Gold, M. Body mass index and marijuana use. J. Addict. Dis. 2005, 24, 95–100. [Google Scholar]
- Merlo, L.L.; Carnes, S.; Carnes, P.J.; Gold, M.S. Hypersexuality disorders: Addiction, compulsion, or impulse behaviors? Bio. Psychiatry 2008, 63, S1–S301. [Google Scholar]
- Bruijnzeel, A.W.; Repetto, M.; Gold, M.S. Neurobiological mechanisms in addictive and psychiatric disorders. Psychiatr. Clin. North Am. 2004, 27, 661–674. [Google Scholar]
- Bruijnzeel, T.; Gold, M.S. The role of corticotrophin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res. Rev. 2005, 49, 505–528. [Google Scholar]
- Gold, M.S. Dual Diagnosis: Nosology, Diagnosis, and Treatment Confusion—Chicken or Egg? Haworth Press: New York, NY, USA, 2007. [Google Scholar]
- Volkow, N.D.; Wang, G.J.; Kollins, S.H.; Wigal, T.L.; Newcorn, J.H.; Telang, F.; Fowler, J.S.; Zhu, W.; Logan, J.; Ma, Y.; et al. Evaluating dopamine reward pathway in ADHD: Clinical implications. JAMA 2009, 302, 1084–1091. [Google Scholar]
- Nyman, E.S.; Ogdie, M.N.; Loukola, A.; Varilo, T.; Taanila, A.; Hurtig, T.; Moilanen, I.K.; Loo, S.K.; McGough, J.J.; Järvelin, M.R.; et al. ADHD candidate gene study in a population-based birth cohort: Association with DBH and DRD2. J. Am. Acad. Child. Adolesc. Psychiatry 2007, 46, 1614–1621. [Google Scholar]
- Faraone, S.V.; Perlis, R.H.; Doyle, A.E.; Smoller, J.W.; Goralnick, J.J.; Holmgren, M.A.; Sklar, P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol. Psychiatry 2005, 57, 1313–1323. [Google Scholar]
- Gizer, I.R.; Ficks, C.; Waldman, I.D. Candidate gene studies of ADHD: A meta-analytic review. Hum. Genet. 2009, 126, 51–90. [Google Scholar]
- Lu, R.B.; Ko, H.C.; Chang, F.M.; Yin, S.J.; Pakstis, A.J.; Kidd, J.R.; Kidd, K.K. No association between alcoholism and multiple polymorphism at the dopamine D2 receptor gene (DRD2) in three distinct Taiwanese populations. Biol. Psychiatry 1996, 39, 419–429. [Google Scholar]
- Comings, D.E.; Muhleman, D.; Gysin, R. Dopamine D2 receptor (DRD2) gene and susceptibility to posttraumatic stress disorder: A study and replication. Biol. Psychiatry 1996, 40, 368–372. [Google Scholar]
- Bowirrat, A.; Chen, T.J.; Blum, K.; Madigan, M.; Bailey, J.A.; Chuan Chen, A.L.; Downs, B.W.; Braverman, E.R.; Radi, S.; Waite, R.L.; et al. Neuro-psychopharmacogenetics and neurological antecedents of posttraumatic stress disorder: Unlocking the mysteries of resilience and vulnerability. Curr. Neuropharmacol. 2010, 8, 335–358. [Google Scholar]
- Lee, D.M. ASAM Patient Placement Criteria for the Treatment of Substance Related Disorders, 2nd ed; American Society of Addiction Medicine: Chevy Chase, MD, USA, 2001; (revised). [Google Scholar]
- Sussman, S.; Leventhal, A.; Bluthenthal, R.N.; Freimuth, M.; Forster, M.; Ames, S.L. A framework for the specificity of addictions. Int. J. Environ. Res. Public Health. 2011, 8, 3399–3415. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Blum, K.; Chen, A.L.C.; Oscar-Berman, M.; Chen, T.J.H.; Lubar, J.; White, N.; Lubar, J.; Bowirrat, A.; Braverman, E.; Schoolfield, J.; et al. Generational Association Studies of Dopaminergic Genes in Reward Deficiency Syndrome (RDS) Subjects: Selecting Appropriate Phenotypes for Reward Dependence Behaviors. Int. J. Environ. Res. Public Health 2011, 8, 4425-4459. https://doi.org/10.3390/ijerph8124425
Blum K, Chen ALC, Oscar-Berman M, Chen TJH, Lubar J, White N, Lubar J, Bowirrat A, Braverman E, Schoolfield J, et al. Generational Association Studies of Dopaminergic Genes in Reward Deficiency Syndrome (RDS) Subjects: Selecting Appropriate Phenotypes for Reward Dependence Behaviors. International Journal of Environmental Research and Public Health. 2011; 8(12):4425-4459. https://doi.org/10.3390/ijerph8124425
Chicago/Turabian StyleBlum, Kenneth, Amanda L. C. Chen, Marlene Oscar-Berman, Thomas J. H. Chen, Joel Lubar, Nancy White, Judith Lubar, Abdalla Bowirrat, Eric Braverman, John Schoolfield, and et al. 2011. "Generational Association Studies of Dopaminergic Genes in Reward Deficiency Syndrome (RDS) Subjects: Selecting Appropriate Phenotypes for Reward Dependence Behaviors" International Journal of Environmental Research and Public Health 8, no. 12: 4425-4459. https://doi.org/10.3390/ijerph8124425
APA StyleBlum, K., Chen, A. L. C., Oscar-Berman, M., Chen, T. J. H., Lubar, J., White, N., Lubar, J., Bowirrat, A., Braverman, E., Schoolfield, J., Waite, R. L., Downs, B. W., Madigan, M., Comings, D. E., Davis, C., Kerner, M. M., Knopf, J., Palomo, T., Giordano, J. J., ... Bailey, J. A. (2011). Generational Association Studies of Dopaminergic Genes in Reward Deficiency Syndrome (RDS) Subjects: Selecting Appropriate Phenotypes for Reward Dependence Behaviors. International Journal of Environmental Research and Public Health, 8(12), 4425-4459. https://doi.org/10.3390/ijerph8124425





