Effects of Various Doses of Selenite on Stinging Nettle (Urtica dioica L.)
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Plant Material and Cultivation
2.3. Sample Preparation for Fresh and Dry Weight Analysis
2.4. Sample Preparation for Selenium Determination
2.5. Determination of Selenium Using Graphite Furnace Atomic Absorption Spectrometry
2.6. Preparation of Plant Tissues for Thiol Determinations
2.7. Determination of Thiols
2.8. Descriptive Statistics
3. Results and Discussion
3.1. Selenium Influence on Plants
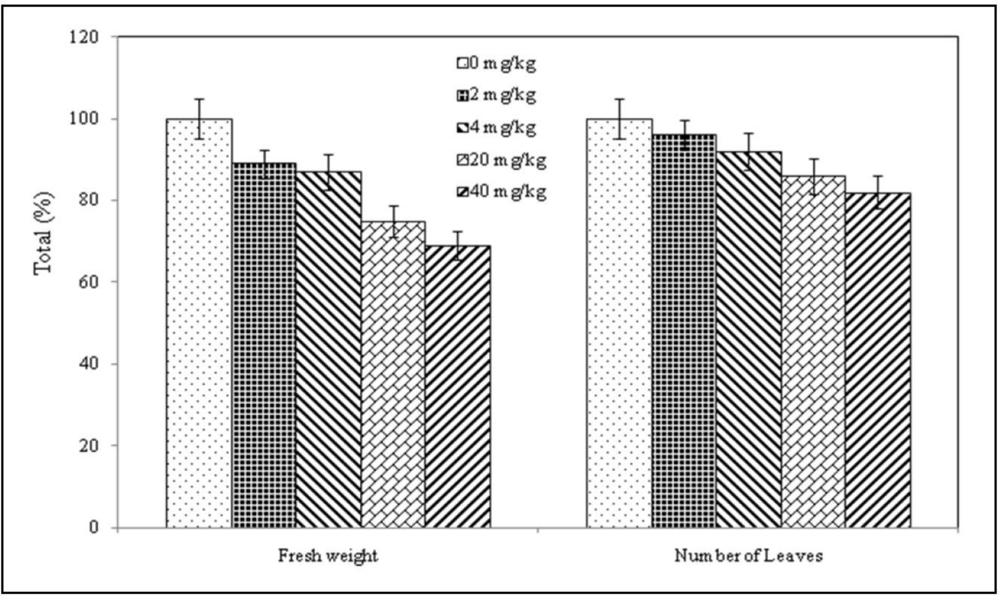
3.2. Plant Behavior
3.3. Root System of Plants
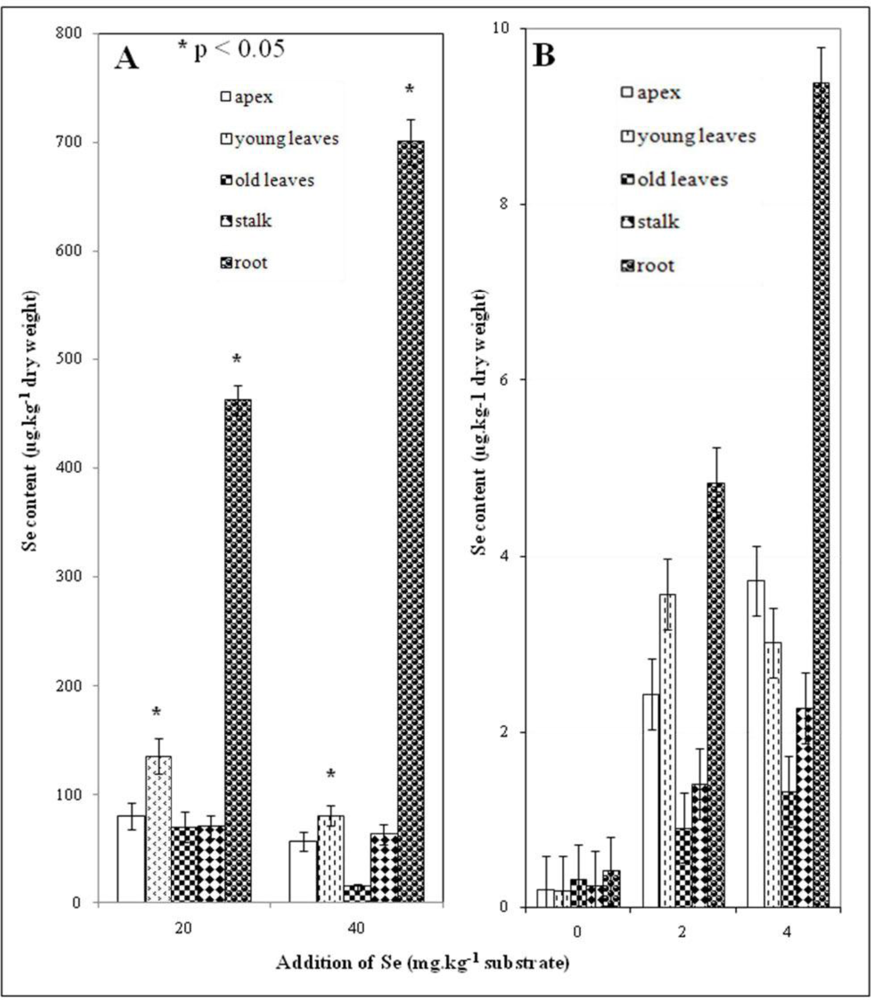
3.4. Selenium Content in Plant
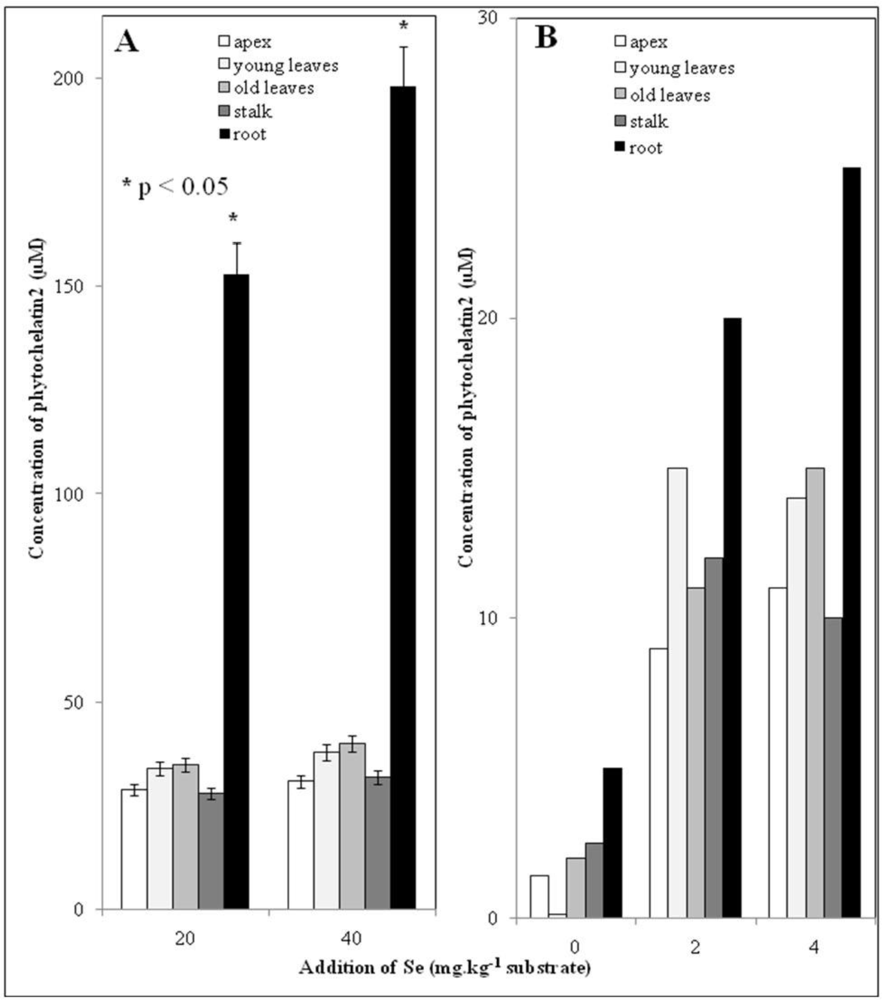
3.5. Low Molecular Mass Thiols–phytochelatins
4. Conclusions
Acknowledgments
References and Notes
- Rayman, MP. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [Green Version]
- Mozaffarian, D. Fish, Mercury, Selenium and Cardiovascular risk: Current evidence and unanswered questions. Int. J. Environ. Res. Public Health 2009, 6, 1894–1916. [Google Scholar]
- Pickering, IJ; Prince, RC; Salt, DE; George, GN. Quantitative, chemically specific imaging of selenium transformation in plants. Proc. Natl. Acad. Sci. U.S.A 2000, 97, 10717–10722. [Google Scholar]
- Terry, N; Zayed, AM; de Souza, MP; Tarun, AS. Selenium in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol 2000, 51, 401–432. [Google Scholar]
- Lauchli, A. Selenium in plants: Uptake, functions, and environmental toxicity. Bot. Acta 1993, 106, 455–468. [Google Scholar]
- Smith, FW; Hawkesford, MJ; Prosser, IM; Clarkson, DT. Isolation of cDNA from Saccharomyces cerevisiae that encodes a high affinity sulphate transporter at the plasma membrane. Mol. Gen. Genet 1995, 247, 709–715. [Google Scholar]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol 2005, 56, 15–39. [Google Scholar]
- Pilon-Smits, EAH; Leduc, DL. Phytoremediation of selenium using transgenic plants. Curr. Opin. Biotechnol 2009, 20, 207–212. [Google Scholar]
- Zhu, YG; Pilon-Smits, EAH; Zhao, FJ; Williams, PN; Meharg, AA. Selenium in higher plants: Understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 2009, 14, 436–442. [Google Scholar]
- Audet, P; Charest, C. Allocation plasticity and plant-metal partitioning: Meta-analytical perspectives in phytoremediation. Environ. Pollut 2008, 156, 290–296. [Google Scholar]
- Kopsell, DA; Randle, WM; Mills, HA. Nutrient accumulation in leaf tissue of rapid-cycling Brassuca oleracea responds to increasing sodium selenate concentrations. J. Plant. Nutr 2000, 23, 927–935. [Google Scholar]
- Hawrylak, B; Szymanska, M. Selenium as a sulphydrylic group inductor in plants. Cell. Mol. Biol. Lett 2004, 9, 329–336. [Google Scholar]
- Meister, A; Anderson, ME. Glutathione. Annu. Rev. Biochem 1983, 52, 711–760. [Google Scholar]
- Rotruck, JT; Pope, AL; Ganther, HE; Swanson, AB; Hafeman, DG; Hoekstra, WG. Selenium–Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar]
- Cobbett, C; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol 2002, 53, 159–182. [Google Scholar]
- Cobbett, CS. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 2000, 123, 825–832. [Google Scholar]
- Babula, P; Adam, V; Opatrilova, R; Zehnalek, J; Havel, L; Kizek, R. Uncommon heavy metals, metalloids and their plant toxicity: a review. Environ. Chem. Lett 2008, 6, 189–213. [Google Scholar]
- Supalkova, V; Huska, D; Diopan, V; Hanustiak, P; Zitka, O; Stejskal, K; Baloun, J; Pikula, J; Havel, L; Zehnalek, J; Adam, V; Trnkova, L; Beklova, M; Kizek, R. Electroanalysis of plant thiols. Sensors 2007, 7, 932–959. [Google Scholar]
- Adam, V; Fabrik, I; Kohoutkova, V; Babula, P; Hubalek, J; Vrba, R; Trnkova, L; Kizek, R. Automated electrochemical analyzer as a new tool for detection of thiols. Int. J. Electrochem. Sci 2010, 5, 429–447. [Google Scholar]
- Meharg, AA. Variation in arsenic accumulation–hyperaccumulation in ferns and their allies. New Phytol 2003, 157, 25–31. [Google Scholar]
- De Clercq, E. Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med. Res. Rev 2000, 20, 323–349. [Google Scholar]
- Illek, J; Akbay, P; Basaran, AA; Undeger, U; Basaran, N. In vitro immunomodulatory activity of flavonoid glycosides from Urtica dioica L. Phytother. Res 2003, 17, 34–37. [Google Scholar]
- Koch, E. Extracts from fruits of saw palmetto (Sabal serrulata) and roots of stinging nettle (Urtica dioica): Viable alternatives in the medical treatment of benign prostatic hyperplasia and associated lower urinary tracts symptoms. Planta Med 2001, 67, 489–500. [Google Scholar]
- Supalkova, V; Petrek, J; Baloun, J; Adam, V; Bartusek, K; Trnkova, L; Beklova, M; Diopan, V; Havel, L; Kizek, R. Multi-instrumental investigation of affecting of early somatic embryos of spruce by cadmium(II) and lead(II) ions. Sensors 2007, 7, 743–759. [Google Scholar]
- Diopan, V; Shestivska, V; Zitka, O; Galiova, M; Adam, V; Kaiser, J; Horna, A; Novotny, K; Liska, M; Havel, L; Zehnalek, J; Kizek, R. Determination of plant thiols by liquid chromatography coupled with coulometric and amperometric detection in lettuce treated by Lead(II) ions. Electroanalysis 2010, 22, 1248–1259. [Google Scholar]
- Koutnik, V; Docekalova, H. Selenium distribution oat plants sown during ontogenesis. Rostlinna Vyroba 1994, 40, 163–172. [Google Scholar]
- Uden, PC. Modern trends in the speciation of selenium by hyphenated techniques. Anal. Bioanal. Chem 2002, 373, 422–431. [Google Scholar]
- Polatajko, A; Jakubowski, N; Szpunar, J. State of the art report of selenium speciation in biological samples. J. Anal. Atom. Spectrom 2006, 21, 639–654. [Google Scholar]
- Petrlova, J; Mikelova, R; Stejskal, K; Kleckerova, A; Zitka, O; Petrek, J; Havel, L; Zehnalek, J; Adam, V; Trnkova, L; Kizek, R. Simultaneous determination of eight biologically active thiol compounds using gradient elution-liquid chromatography with Coul-Array detection. J. Sep. Sci 2006, 29, 1166–1173. [Google Scholar]
- Potesil, D; Petrlova, J; Adam, V; Vacek, J; Klejdus, B; Zehnalek, J; Trnkova, L; Havel, L; Kizek, R. Simultaneous femtomole determination of cysteine, reduced and oxidized glutathione, and phytochelatin in maize (Zea mays L.) kernels using high-performance liquid chromatography with electrochemical detection. J. Chromatogr. A 2005, 1084, 134–144. [Google Scholar]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar]
- Kotrba, P; Najmanova, J; Macek, T; Ruml, T; Mackova, M. Genetically modified plants in phytoremediation of heavy metal and metalloid soil and sediment pollution. Biotechnol. Adv 2009, 27, 799–810. [Google Scholar]
- Pickering, IJ; Prince, RC; George, MJ; Smith, RD; George, GN; Salt, DE. Reduction and coordination of arsenic in Indian mustard. Plant Physiol 2000, 122, 1171–1177. [Google Scholar]
- Zitka, O; Stejskal, K; Kleckerova, A; Adam, V; Beklova, M; Horna, A; Supalkova, V; Havel, L; Kizek, R. Utilizing electrochemical techniques for detection of biological samples. Chem. Listy 2007, 101, 225–231. [Google Scholar]
| Sample ID | Applied dose [mg Se/kg soil] | Number of doses | Total Se addition [mg Se/kg soil] | |
|---|---|---|---|---|
| A | 1 | 0.0 | 0 | 0.0 |
| 2 | 2.0 | 1 | 2.0 | |
| 3 | 4.0 | 1 | 4.0 | |
| B | 4 | 2.0 | 10 | 20.0 |
| 5 | 4.0 | 10 | 40.0 | |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Krystofova, O.; Adam, V.; Babula, P.; Zehnalek, J.; Beklova, M.; Havel, L.; Kizek, R. Effects of Various Doses of Selenite on Stinging Nettle (Urtica dioica L.). Int. J. Environ. Res. Public Health 2010, 7, 3804-3815. https://doi.org/10.3390/ijerph7103804
Krystofova O, Adam V, Babula P, Zehnalek J, Beklova M, Havel L, Kizek R. Effects of Various Doses of Selenite on Stinging Nettle (Urtica dioica L.). International Journal of Environmental Research and Public Health. 2010; 7(10):3804-3815. https://doi.org/10.3390/ijerph7103804
Chicago/Turabian StyleKrystofova, Olga, Vojtech Adam, Petr Babula, Josef Zehnalek, Miroslava Beklova, Ladislav Havel, and Rene Kizek. 2010. "Effects of Various Doses of Selenite on Stinging Nettle (Urtica dioica L.)" International Journal of Environmental Research and Public Health 7, no. 10: 3804-3815. https://doi.org/10.3390/ijerph7103804
APA StyleKrystofova, O., Adam, V., Babula, P., Zehnalek, J., Beklova, M., Havel, L., & Kizek, R. (2010). Effects of Various Doses of Selenite on Stinging Nettle (Urtica dioica L.). International Journal of Environmental Research and Public Health, 7(10), 3804-3815. https://doi.org/10.3390/ijerph7103804






