Where Inequities Emerge: Racial and Ethnic Differences Across the COVID-19 Hospitalization Continuum
Highlights
- Racial and ethnic inequities in severe COVID-19 outcomes persisted among Medicaid beneficiaries despite uniform insurance coverage.
- Disparities were most evident at hospital admission, underscoring upstream social and structural determinants that shape exposure risk and severity at presentation.
- By examining the hospitalization continuum (COVID-19 diagnosis at admission, excess in-hospital mortality, and mortality within COVID-19 hospitalizations), this study clarifies where inequities are most pronounced.
- Findings show that insurance coverage alone is insufficient to prevent inequities in severe infectious disease-related hospitalization burden and hospital outcomes.
- Equity-focused strategies should prioritize prevention, early diagnosis, and timely outpatient evaluation and treatment access to reduce avoidable hospitalizations in Medicaid and similarly vulnerable populations.
- Continuum-based evaluation of inequities can guide more targeted preparedness and health system responses for the COVID-19 endemic phase and future public health emergencies.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Source
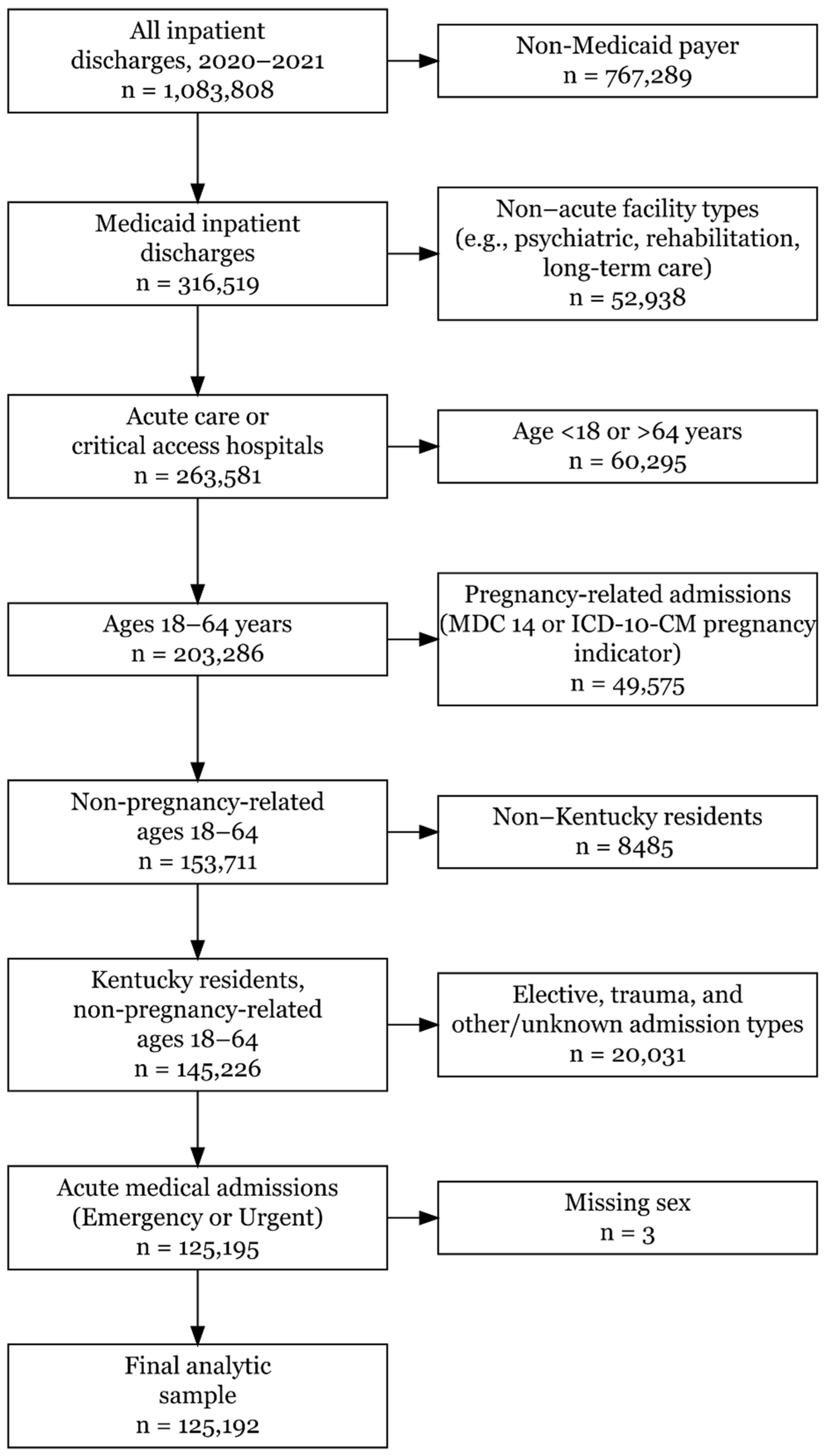
2.3. Study Sample
2.4. Outcome and Exposure
2.5. Covariates
2.6. Statistical Analysis
2.7. Sensitivity Analyses
3. Results
3.1. Study Sample and Cohort Derivation
3.2. Descriptive Characteristics by COVID-19 Diagnosis
3.3. Adjusted Odds of COVID-19 Diagnosis at Hospitalization
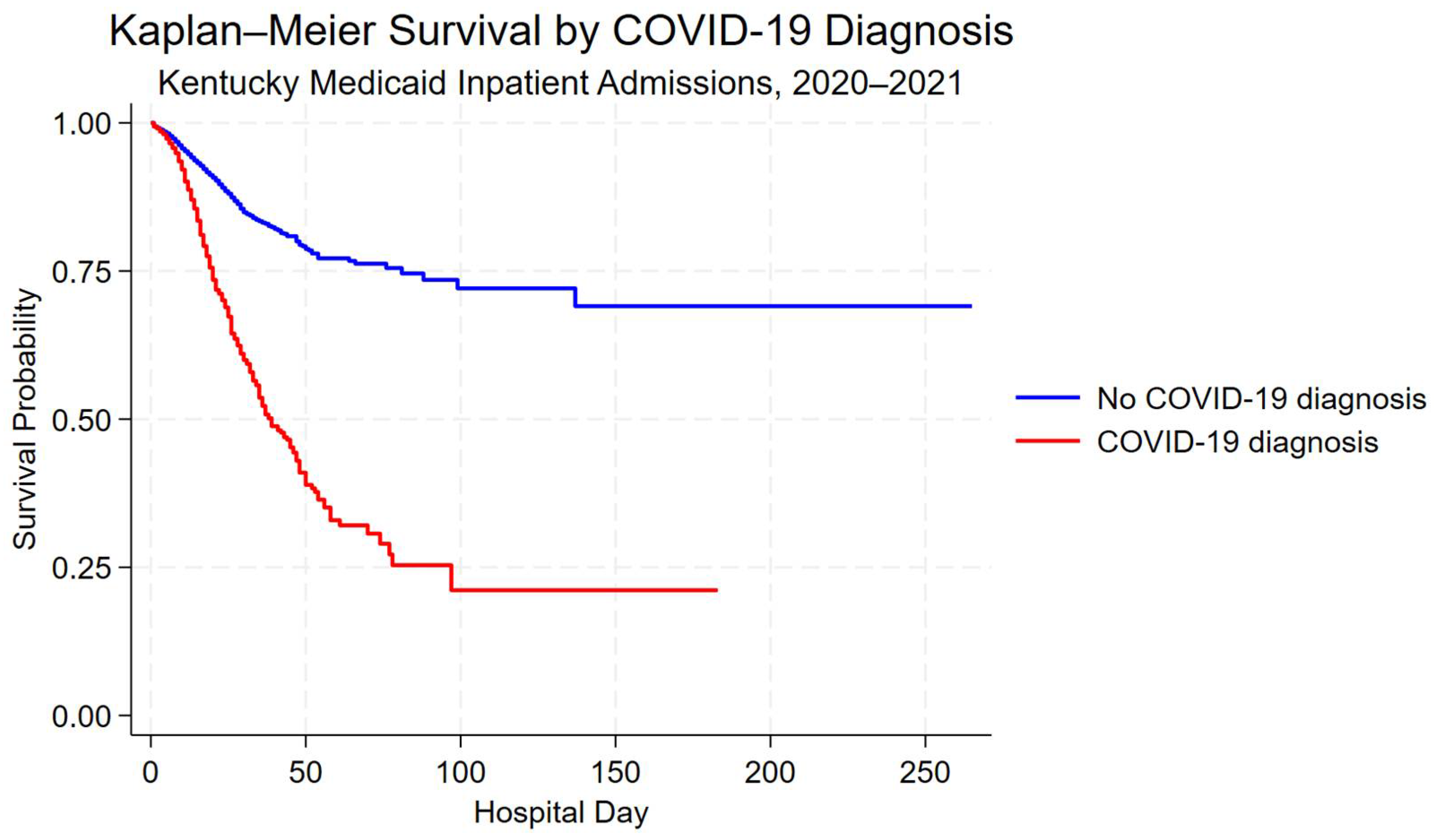
3.4. Unadjusted Survival by COVID-19 Diagnosis
3.5. Adjusted In-Hospital Mortality: COVID-19 vs. Non-COVID-19 Hospitalizations
3.6. Racial Inequities in In-Hospital Mortality Among COVID-19 Hospitalizations
3.7. Sensitivity Analyses
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Adjusted HR 1 | 95% CI 2 | p-Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age Group (ref = 18–20) | ||||
| 21–24 | 1.29 | 0.41 | 4.00 | 0.664 |
| 25–29 | 0.94 | 0.32 | 2.79 | 0.911 |
| 30–34 | 1.86 | 0.59 | 5.84 | 0.289 |
| 35–39 | 1.44 | 0.47 | 4.42 | 0.522 |
| 40–44 | 2.15 | 0.74 | 6.27 | 0.162 |
| 45–49 | 2.25 | 0.77 | 6.59 | 0.141 |
| 50–54 | 2.38 | 0.78 | 7.26 | 0.129 |
| 55–59 | 2.57 | 0.86 | 7.70 | 0.093 |
| 60–64 | 2.92 | 0.96 | 8.86 | 0.058 |
| Sex (ref = Male) | ||||
| Female | 0.81 | 0.68 | 0.95 | 0.009 |
| Race/Ethnicity (ref = NH White) 3 | ||||
| NH Black | 0.89 | 0.70 | 1.13 | 0.326 |
| NH Asian | 0.84 | 0.34 | 2.12 | 0.717 |
| NH AI/AN | 1.58 | 0.10 | 24.52 | 0.744 |
| NH NH/PI | 0.93 | 0.23 | 3.69 | 0.914 |
| Hispanic | 0.91 | 0.68 | 1.21 | 0.507 |
| CCI Score (ref = 0) 4 | ||||
| 1–2 | 1.28 | 1.04 | 1.58 | 0.018 |
| 3–4 | 1.06 | 0.81 | 1.40 | 0.652 |
| ≥5 | 1.40 | 1.09 | 1.79 | 0.008 |
| Hospital Type (ref = Critical Access) 5 | ||||
| Acute Care | 0.72 | 0.58 | 0.90 | 0.004 |
| Hospital Bed Size (ref = <1000 beds) | ||||
| 1000–2999 beds | 0.84 | 0.43 | 1.64 | 0.611 |
| 3000–5999 beds | 1.03 | 0.51 | 2.08 | 0.928 |
| 6000–9999 beds | 1.18 | 0.60 | 2.33 | 0.633 |
| Over 9999 beds | 1.15 | 0.62 | 2.11 | 0.665 |
| Hospital Ownership (ref = Non-Profit) | ||||
| For-Profit | 0.97 | 0.72 | 1.33 | 0.869 |
| Discharge Quarter (ref = 2020 Q1) | ||||
| 2020 Q2 | 0.41 | 0.12 | 1.47 | 0.171 |
| 2020 Q3 | 0.35 | 0.13 | 0.98 | 0.045 |
| 2020 Q4 | 0.55 | 0.22 | 1.41 | 0.213 |
| 2021 Q1 | 0.36 | 0.14 | 0.92 | 0.033 |
| 2021 Q2 | 0.43 | 0.15 | 1.21 | 0.110 |
| 2021 Q3 | 0.93 | 0.33 | 2.61 | 0.885 |
| 2021 Q4 | 0.63 | 0.23 | 1.74 | 0.369 |
| Panel A. Matching Summary | ||
| NH White 1 | NH Black 1 | |
| Unmatched sample (COVID-19-positive inpatients) | 6504 | 1253 |
| Matched 1:1 analytic sample | 636 | 1253 |
| Total matched sample | 1889 | — |
| Panel B. Balance Statistics Before and After Matching (Values shown are proportions in NH White vs. NH Black groups; SD = Absolute Standardized Difference) | ||
| Characteristic | Before Matching (NH White vs. NH Black, SD 4) | After Matching (NH White vs. NH Black, SD 4) |
| Death (in-hospital) | 0.064 vs. 0.088, SD = 0.084 | 0.064 vs. 0.069, SD = 0.015 |
| Age Group (ref = 18–20) | ||
| 21–24 | 0.026 vs. 0.040, SD = 0.077 | 0.047 vs. 0.040, SD = 0.036 |
| 25–29 | 0.055 vs. 0.083, SD = 0.110 | 0.097 vs. 0.083, SD = 0.051 |
| 30–34 | 0.066 vs. 0.087, SD = 0.077 | 0.085 vs. 0.087, SD = 0.007 |
| 35–39 | 0.088 vs. 0.081, SD = 0.023 | 0.091 vs. 0.081, SD = 0.035 |
| 40–44 | 0.104 vs. 0.117, SD = 0.043 | 0.113 vs. 0.117, SD = 0.013 |
| 45–49 | 0.117 vs. 0.120, SD = 0.008 | 0.112 vs. 0.120, SD = 0.025 |
| 50–54 | 0.160 vs. 0.148, SD = 0.034 | 0.148 vs. 0.148, SD = 0.000 |
| 55–59 | 0.181 vs. 0.152, SD = 0.076 | 0.151 vs. 0.152, SD = 0.004 |
| 60–64 | 0.188 vs. 0.150, SD = 0.101 | 0.146 vs. 0.150, SD = 0.011 |
| Sex (ref = Male) | ||
| Female | 0.517 vs. 0.516, SD = 0.002 | 0.498 vs. 0.516, SD = 0.036 |
| CCI Score (ref = 0) 2 | ||
| 1–2 | 0.318 vs. 0.308, SD = 0.022 | 0.318 vs. 0.308, SD = 0.021 |
| 3–4 | 0.132 vs. 0.153, SD = 0.062 | 0.154 vs. 0.153, SD = 0.002 |
| ≥5 | 0.236 vs. 0.247, SD = 0.024 | 0.237 vs. 0.247, SD = 0.021 |
| Hospital Type (ref = Critical Access) 3 | ||
| Acute Care | 0.463 vs. 0.113, SD = 0.840 | 0.197 vs. 0.113, SD = 0.234 |
| Hospital Bed Size (ref = <1000 beds) | ||
| 1000–2999 beds | 0.106 vs. 0.028, SD = 0.315 | 0.035 vs. 0.028, SD = 0.038 |
| 3000–5999 beds | 0.154 vs. 0.089, SD = 0.202 | 0.126 vs. 0.089, SD = 0.120 |
| 6000–9999 beds | 0.169 vs. 0.161, SD = 0.022 | 0.223 vs. 0.161, SD = 0.158 |
| Over 9999 beds | 0.541 vs. 0.720, SD = 0.378 | 0.612 vs. 0.720, SD = 0.231 |
| Hospital Ownership (ref = Non-Profit) | ||
| For-Profit | 0.106 vs. 0.052, SD = 0.201 | 0.080 vs. 0.052, SD = 0.114 |
| Discharge Quarter (ref = 2020 Q1) | ||
| 2020 Q2 | 0.026 vs. 0.071, SD = 0.208 | 0.072 vs. 0.071, SD = 0.005 |
| 2020 Q3 | 0.046 vs. 0.104, SD = 0.223 | 0.101 vs. 0.104, SD = 0.010 |
| 2020 Q4 | 0.148 vs. 0.171, SD = 0.061 | 0.176 vs. 0.171, SD = 0.014 |
| 2021 Q1 | 0.160 vs. 0.148, SD = 0.033 | 0.148 vs. 0.148, SD = 0.000 |
| 2021 Q2 | 0.065 vs. 0.073, SD = 0.035 | 0.090 vs. 0.073, SD = 0.059 |
| 2021 Q3 | 0.329 vs. 0.241, SD = 0.197 | 0.217 vs. 0.241, SD = 0.057 |
| 2021 Q4 | 0.221 vs. 0.187, SD = 0.085 | 0.195 vs. 0.187, SD = 0.021 |
| Panel C. Propensity Score-Matched Cox Proportional Hazards Model | ||
| Group Comparison | Adjusted HR (95% CI [Lower—Upper]) | p-value |
| NH Black (ref = NH White) | 1.1 (0.69–1.74) | 0.692 |
| Outcome | Sample (LOS Restricted to 1–60 Days) | Key Exposure/Comparison | Adjusted Effect (95% CI) | p-Value |
|---|---|---|---|---|
| In-hospital mortality (Cox model) | All Medicaid inpatient stays (n = 124,957; 3257 deaths) | COVID-19-positive vs. non-COVID-19 inpatient stays | HR = 2.35 (95% CI: 2.08–2.65) | <0.001 |
| In-hospital mortality (Cox model) | COVID-19-positive inpatients (n = 8371; 689 deaths) | NH Black vs. NH White | HR = 0.90 (95% CI: 0.71–1.14) | 0.390 |
| Outcome | Sample | Key Exposure/Comparison | Adjusted Effect (95% CI) | p-Value |
|---|---|---|---|---|
| COVID-19 diagnosis among hospitalized patients (logistic regression) | All Medicaid inpatient stays (n = 125,192) | NH Black vs. NH White | OR = 1.40 (95% CI: 1.25–1.58) | <0.001 |
| In-hospital mortality (Cox model) | All Medicaid inpatient stays (n = 125,192) | COVID-19-positive vs. non-COVID-19 inpatient stays | HR = 2.40 (95% CI: 2.12–2.71) | <0.001 |
| Outcome | Sample | Matching Specification | Key Exposure/Comparison | Adjusted Effect (95% CI) | p-Value |
|---|---|---|---|---|---|
| In-hospital mortality (Cox model) | COVID-19-positive NH Black and NH White inpatients (n = 2455; 164 deaths) | 1:2 nearest-neighbor propensity score matching (caliper = 0.10) on age group, sex, CCI category, hospital class, bed size, ownership, and discharge quarter | NH Black vs. NH White | HR = 0.86 (95% CI: 0.63–1.17) | 0.330 |
References
- Truman, B.I.; Chang, M.-H.; Moonesinghe, R. Provisional COVID-19 Age-Adjusted Death Rates, by Race and Ethnicity—United States, 2020–2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 601–605. [Google Scholar] [CrossRef]
- Abidin, Z.U.; Thirumalareddy, J.; Gupta, J.S.; Abdul Jabbar, A.B. Disparities in COVID-19 Mortality in the United States, 2020–2023. BMC Public Health 2025, 25, 3257. [Google Scholar] [CrossRef]
- Artiga, S.; Corallo, B.; Pham, O. Racial Disparities in COVID-19: Key Findings from Available Data and Analysis; Kaiser Family Foundation: San Francisco, CA, USA, 2020. [Google Scholar]
- Parolin, Z.; Lee, E.K. The Role of Poverty and Racial Discrimination in Exacerbating the Health Consequences of COVID-19. Lancet Reg. Health-Am. 2022, 7, 100178. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Buhr, R.G.; Fonarow, G.C.; Hsu, J.J.; Brown, A.F.; Ziaeian, B. Racial and Ethnic Disparities and the National Burden of COVID-19 on Inpatient Hospitalizations: A Retrospective Study in the United States in the Year 2020. J. Racial Ethn. Health Disparities 2025, 12, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Haro-Ramos, A.Y.; Brown, T.T.; Deardorff, J.; Aguilera, A.; Pollack Porter, K.M.; Rodriguez, H.P. Frontline Work and Racial Disparities in Social and Economic Pandemic Stressors during the First COVID-19 Surge. Health Serv. Res. 2023, 58, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.; Roll, S.; Miller, S.; Lee, H.; Larimore, S.; Grinstein-Weiss, M. Racial and Ethnic Disparities in Housing Instability During the COVID-19 Pandemic: The Role of Assets and Income Shocks. J. Econ. Race Policy 2023, 6, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Boehmer, T.K.; Koumans, E.H.; Skillen, E.L.; Kappelman, M.D.; Carton, T.W.; Patel, A.; August, E.M.; Bernstein, R.; Denson, J.L.; Draper, C.; et al. Racial and Ethnic Disparities in Outpatient Treatment of COVID-19—United States, January-July 2022. MMWR Morb. Mortal Wkly. Rep. 2022, 71, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Kompaniyets, L.; Pennington, A.F.; Goodman, A.B.; Rosenblum, H.G.; Belay, B.; Ko, J.Y.; Chevinsky, J.R.; Schieber, L.Z.; Summers, A.D.; Lavery, A.M.; et al. Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized With COVID-19, March 2020-March 2021. Prev. Chronic Dis. 2021, 18, E66. [Google Scholar] [CrossRef] [PubMed]
- Shakib, S.H.; Little, B.B.; Karimi, S.M.; Goldsby, M. COVID-19 Mortality Among Hospitalized Medicaid Patients in Kentucky (2020–2021): A Geospatial Study of Social, Medical, and Environmental Risk Factors. Atmosphere 2025, 16, 684. [Google Scholar] [CrossRef]
- Magesh, S.; John, D.; Li, W.T.; Li, Y.; Mattingly-App, A.; Jain, S.; Chang, E.Y.; Ongkeko, W.M. Disparities in COVID-19 Outcomes by Race, Ethnicity, and Socioeconomic Status: A Systematic-Review and Meta-Analysis. JAMA Netw. Open 2021, 4, e2134147. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Health Facilities and Services Data—Kentucky Cabinet for Health and Family Services. Available online: https://www.chfs.ky.gov/agencies/ohda/Pages/hfsd.aspx (accessed on 12 December 2025).
- Kadri, S.S.; Gundrum, J.; Warner, S.; Cao, Z.; Babiker, A.; Klompas, M.; Rosenthal, N. Uptake and Accuracy of the Diagnosis Code for COVID-19 Among US Hospitalizations. JAMA 2020, 324, 2553–2554. [Google Scholar] [CrossRef] [PubMed]
- Glasheen, W.P.; Cordier, T.; Gumpina, R.; Haugh, G.; Davis, J.; Renda, A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am. Health Drug Benefits 2019, 12, 188–197. [Google Scholar] [PubMed]
- Thielke, A.; Curtis, P.; King, V. Addressing COVID-19 Health Disparities: Opportunities for Medicaid Programs; Issue Brief; Milbank Memorial Fund: New York, NY, USA, 2021. [Google Scholar]
- Acosta, A.M.; Garg, S.; Pham, H.; Whitaker, M.; Anglin, O.; O’Halloran, A.; Milucky, J.; Patel, K.; Taylor, C.; Wortham, J.; et al. Racial and Ethnic Disparities in Rates of COVID-19-Associated Hospitalization, Intensive Care Unit Admission, and In-Hospital Death in the United States From March 2020 to February 2021. JAMA Netw. Open 2021, 4, e2130479. [Google Scholar] [CrossRef] [PubMed]
- Shakib, S.H.; Little, B.B.; Karimi, S.; McKinney, W.P.; Goldsby, M.; Kong, M. Mediating Effect of the Stay-at-Home Order on the Association between Mobility, Weather, and COVID-19 Infection and Mortality in Indiana and Kentucky: March to May 2020. Atmosphere 2024, 15, 1100. [Google Scholar] [CrossRef]
- Xie, Y.; Bowe, B.; Maddukuri, G.; Al-Aly, Z. Comparative Evaluation of Clinical Manifestations and Risk of Death in Patients Admitted to Hospital with Covid-19 and Seasonal Influenza: Cohort Study. BMJ 2020, 371, m4677. [Google Scholar] [CrossRef]
- Shakib, S.; Karimi, S.; McGeeney, J.; Parh, M.; Zarei, H.; Chen, Y.; Goldman, B.; Novario, D.; Schurfranz, M.; Warren, C.; et al. Local Health Department COVID-19 Vaccination Efforts and Associated Outcomes: Evidence from Jefferson County, Kentucky. Vaccines 2025, 13, 901. [Google Scholar] [CrossRef] [PubMed]
- Shakib, S.H.; Antimisiaris, D. Analysis of Polypharmacy in Older Adults Pre- and Post-COVID-19. Expert Rev. Clin. Pharmacol. 2025, 18, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Lazic, A.; Tilford, J.M.; Martin, B.C.; Rezaeiahari, M.; Goudie, A.; Baghal, A.; Greer, M. In-Hospital Mortality by Race and Ethnicity Among Hospitalized COVID-19 Patients Using Data From the US National COVID Cohort Collaborative. Am. J. Med. Open 2024, 12, 100070. [Google Scholar] [CrossRef]
- Richardson, S.; Martinez, J.; Hirsch, J.S.; Cerise, J.; Lesser, M.; Roswell, R.O.; Davidson, K.W.; Northwell Health COVID-19 Research Consortium. Association of Race/Ethnicity with Mortality in Patients Hospitalized with COVID-19. PLoS ONE 2022, 17, e0267505. [Google Scholar] [CrossRef] [PubMed]
| Overall (n = 125,192) | Non-COVID-19 (n = 116,783) | COVID-19 (n = 8409) | |
|---|---|---|---|
| n (%) 1 | n (%) 1 | n (%) 1 | |
| Age Group | |||
| 18–20 | 3118 (2.49%) | 2988 (2.56%) | 130 (1.55%) |
| 21–24 | 4895 (3.91%) | 4666 (4.00%) | 229 (2.72%) |
| 25–29 | 9168 (7.32%) | 8673 (7.43%) | 495 (5.89%) |
| 30–34 | 11,485 (9.17%) | 10,870 (9.31%) | 615 (7.31%) |
| 35–39 | 12,914 (10.32%) | 12,141 (10.40%) | 773 (9.19%) |
| 40–44 | 14,899 (11.90%) | 13,981 (11.97%) | 918 (10.92%) |
| 45–49 | 15,000 (11.98%) | 13,985 (11.98%) | 1015 (12.07%) |
| 50–54 | 17,656 (14.10%) | 16,342 (13.99%) | 1314 (15.63%) |
| 55–59 | 18,834 (15.04%) | 17,406 (14.90%) | 1428 (16.98%) |
| 60–64 | 17,223 (13.76%) | 15,731 (13.47%) | 1492 (17.74%) |
| Sex | |||
| Male | 63,514 (50.73%) | 59,390 (50.86%) | 4124 (49.04%) |
| Female | 61,678 (49.27%) | 57,393 (49.14%) | 4285 (50.96%) |
| Race/Ethnicity 2 | |||
| NH White | 104,988 (83.86%) | 98,484 (84.33%) | 6504 (77.35%) |
| NH Black | 16,442 (13.13%) | 15,189 (13.01%) | 1253 (14.90%) |
| NH Asian | 553 (0.44%) | 477 (0.41%) | 76 (0.90%) |
| NH AI/AN | 122 (0.10%) | 116 (0.10%) | 6 (0.07%) |
| NH NH/PI | 79 (0.06%) | 65 (0.06%) | 14 (0.17%) |
| Hispanic | 3008 (2.40%) | 2452 (2.10%) | 556 (6.61%) |
| Length of stay, days, median (IQR) 3 | - | 3 (2–6) | 5 (3–9) |
| CCI Score 4 | |||
| 0 | 38,788 (30.98%) | 36,074 (30.89%) | 2714 (32.27%) |
| 1–2 | 34,375 (27.46%) | 31,725 (27.17%) | 2650 (31.51%) |
| 3–4 | 15,685 (12.53%) | 14,581 (12.49%) | 1104 (13.13%) |
| ≥5 | 36,344 (29.03%) | 34,403 (29.46%) | 1941 (23.08%) |
| Hospital Type | |||
| Critical Access 5 | 43,760 (34.95%) | 40,545 (34.72%) | 3215 (38.23%) |
| Acute Care | 81,432 (65.05%) | 76,238 (65.28%) | 5194 (61.77%) |
| Hospital Bed Size | |||
| Under 1000 beds | 1694 (1.35%) | 1494 (1.28%) | 200 (2.38%) |
| 1000–2999 beds | 8577 (6.85%) | 7845 (6.72%) | 732 (8.70%) |
| 3000–5999 beds | 16,947 (13.54%) | 15,760 (13.50%) | 1187 (14.12%) |
| 6000–9999 beds | 19,498 (15.57%) | 18,080 (15.48%) | 1418 (16.86%) |
| Over 9999 beds | 78,476 (62.68%) | 73,604 (63.03%) | 4872 (57.94%) |
| Hospital Ownership | |||
| Non-Profit | 111,777 (89.28%) | 104,166 (89.20%) | 7611 (90.51%) |
| For-Profit | 13,415 (10.72%) | 12,617 (10.80%) | 798 (9.49%) |
| Discharge Quarter | |||
| 2020 Q1 | 14,913 (11.91%) | 14,869 (12.73%) | 44 (0.52%) |
| 2020 Q2 | 15,033 (12.01%) | 14,633 (12.53%) | 400 (4.76%) |
| 2020 Q3 | 16,461 (13.15%) | 15,929 (13.64%) | 532 (6.33%) |
| 2020 Q4 | 14,964 (11.95%) | 13,673 (11.71%) | 1291 (15.35%) |
| 2021 Q1 | 15,644 (12.50%) | 14,338 (12.28%) | 1306 (15.53%) |
| 2021 Q2 | 16,413 (13.11%) | 15,865 (13.59%) | 548 (6.52%) |
| 2021 Q3 | 16,156 (12.90%) | 13,618 (11.66%) | 2538 (30.18%) |
| 2021 Q4 | 15,608 (12.47%) | 13,858 (11.87%) | 1750 (20.81%) |
| Adjusted OR 1 | 95% CI 2 | p-Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age Group (ref = 18–20) | ||||
| 21–24 | 1.18 | 0.9 | 1.56 | 0.237 |
| 25–29 | 1.43 | 1.11 | 1.83 | 0.005 |
| 30–34 | 1.42 | 1.13 | 1.77 | 0.002 |
| 35–39 | 1.64 | 1.31 | 2.05 | <0.001 |
| 40–44 | 1.72 | 1.37 | 2.17 | <0.001 |
| 45–49 | 2.01 | 1.6 | 2.53 | <0.001 |
| 50–54 | 2.34 | 1.86 | 2.93 | <0.001 |
| 55–59 | 2.46 | 1.95 | 3.12 | <0.001 |
| 60–64 | 2.91 | 2.29 | 3.69 | <0.001 |
| Sex (ref = Male) | ||||
| Female | 1.1 | 1.05 | 1.16 | <0.001 |
| Race/Ethnicity (ref = NH White) 3 | ||||
| NH Black | 1.41 | 1.26 | 1.59 | <0.001 |
| NH Asian | 2.59 | 1.98 | 3.38 | <0.001 |
| NH AI/AN | 0.76 | 0.34 | 1.69 | 0.501 |
| NH NH/PI | 4.07 | 2.52 | 6.57 | <0.001 |
| Hispanic | 4.14 | 3.38 | 5.08 | <0.001 |
| CCI Score (ref = 0) 4 | ||||
| 1–2 | 0.98 | 0.88 | 1.09 | 0.705 |
| 3–4 | 0.82 | 0.72 | 0.94 | 0.004 |
| ≥5 | 0.59 | 0.52 | 0.68 | <0.001 |
| Hospital Type (ref = Critical Access) 5 | ||||
| Acute Care | 0.86 | 0.67 | 1.1 | 0.235 |
| Hospital Bed Size (ref = <1000 beds) | ||||
| 1000–2999 beds | 0.97 | 0.73 | 1.28 | 0.821 |
| 3000–5999 beds | 0.73 | 0.55 | 0.96 | 0.026 |
| 6000–9999 beds | 0.71 | 0.44 | 1.14 | 0.152 |
| Over 9999 beds | 0.61 | 0.46 | 0.82 | 0.001 |
| Hospital Ownership (ref = Non-Profit) | ||||
| For-Profit | 0.71 | 0.54 | 0.94 | 0.017 |
| Discharge Quarter (ref = 2020 Q1) | ||||
| 2020 Q2 | 8.99 | 5.5 | 14.68 | <0.001 |
| 2020 Q3 | 11.13 | 8.1 | 15.3 | <0.001 |
| 2020 Q4 | 31.28 | 22.04 | 44.4 | <0.001 |
| 2021 Q1 | 30.53 | 21.39 | 43.57 | <0.001 |
| 2021 Q2 | 11.42 | 8.12 | 16.05 | <0.001 |
| 2021 Q3 | 62.57 | 41.94 | 93.35 | <0.001 |
| 2021 Q4 | 41.76 | 28.62 | 60.94 | <0.001 |
| Adjusted HR 1 | 95% CI 2 | p-Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Exposure (ref = Non-COVID-19) | ||||
| COVID-19 | 2.38 | 2.09 | 2.7 | <0.001 |
| Age Group (ref = 18–20) | ||||
| 21–24 | 0.87 | 0.6 | 1.25 | 0.449 |
| 25–29 | 1.03 | 0.72 | 1.47 | 0.888 |
| 30–34 | 1.35 | 0.88 | 2.07 | 0.171 |
| 35–39 | 1.39 | 0.86 | 2.25 | 0.177 |
| 40–44 | 1.43 | 0.92 | 2.21 | 0.11 |
| 45–49 | 1.62 | 1.06 | 2.48 | 0.026 |
| 50–54 | 1.62 | 1.00 | 2.63 | 0.052 |
| 55–59 | 1.88 | 1.16 | 3.05 | 0.011 |
| 60–64 | 2.15 | 1.34 | 3.45 | 0.001 |
| Sex (ref = Male) | ||||
| Female | 0.90 | 0.84 | 0.97 | 0.006 |
| Race/Ethnicity (ref = NH White) 3 | ||||
| NH Black | 0.81 | 0.70 | 0.94 | 0.006 |
| NH Asian | 0.79 | 0.49 | 1.29 | 0.348 |
| NH AI/AN | 0.94 | 0.33 | 2.69 | 0.903 |
| NH NH/PI | 1.02 | 0.34 | 3.07 | 0.969 |
| Hispanic | 0.90 | 0.72 | 1.12 | 0.339 |
| CCI Score (ref = 0) 4 | ||||
| 1–2 | 1.45 | 1.17 | 1.81 | 0.001 |
| 3–4 | 1.59 | 1.20 | 2.10 | 0.001 |
| ≥5 | 2.61 | 2.01 | 3.38 | <0.001 |
| Hospital Type (ref = Critical Access) 5 | ||||
| Acute Care | 0.92 | 0.71 | 1.19 | 0.519 |
| Hospital Bed Size (ref = <1000 beds) | ||||
| 1000–2999 beds | 0.78 | 0.42 | 1.45 | 0.435 |
| 3000–5999 beds | 0.98 | 0.55 | 1.77 | 0.957 |
| 6000–9999 beds | 0.95 | 0.51 | 1.79 | 0.883 |
| Over 9999 beds | 1.19 | 0.67 | 2.13 | 0.554 |
| Hospital Ownership (ref = Non-Profit) | ||||
| For-Profit | 0.94 | 0.75 | 1.19 | 0.609 |
| Discharge Quarter (ref = 2020 Q1) | ||||
| 2020 Q2 | 1.24 | 1.04 | 1.47 | 0.018 |
| 2020 Q3 | 1.16 | 1.00 | 1.35 | 0.05 |
| 2020 Q4 | 1.19 | 1.02 | 1.40 | 0.025 |
| 2021 Q1 | 1.02 | 0.90 | 1.16 | 0.759 |
| 2021 Q2 | 1.11 | 0.96 | 1.3 | 0.162 |
| 2021 Q3 | 1.40 | 1.20 | 1.63 | <0.001 |
| 2021 Q4 | 1.27 | 1.09 | 1.48 | 0.002 |
| Adjusted HR 1 | 95% CI 2 | p-Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Adjusted Cox Model 3 (n = 7757; deaths = 650) | ||||
| NH Black (ref = NH White) 4 | 0.89 | 0.70 | 1.13 | 0.326 |
| Propensity Score-Matched (PSM) Cox Model 5 (n = 1889; deaths = 114) | ||||
| NH Black (ref = NH White) 4 | 1.10 | 0.69 | 1.74 | 0.692 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Shakib, S.H.; Goldsby, M.; Karimi, S.M.; Siddique, F.; Kanwal, F.N.; Little, B.B. Where Inequities Emerge: Racial and Ethnic Differences Across the COVID-19 Hospitalization Continuum. Int. J. Environ. Res. Public Health 2026, 23, 181. https://doi.org/10.3390/ijerph23020181
Shakib SH, Goldsby M, Karimi SM, Siddique F, Kanwal FN, Little BB. Where Inequities Emerge: Racial and Ethnic Differences Across the COVID-19 Hospitalization Continuum. International Journal of Environmental Research and Public Health. 2026; 23(2):181. https://doi.org/10.3390/ijerph23020181
Chicago/Turabian StyleShakib, Shaminul H., Michael Goldsby, Seyed M. Karimi, Farzana Siddique, Farah N. Kanwal, and Bert B. Little. 2026. "Where Inequities Emerge: Racial and Ethnic Differences Across the COVID-19 Hospitalization Continuum" International Journal of Environmental Research and Public Health 23, no. 2: 181. https://doi.org/10.3390/ijerph23020181
APA StyleShakib, S. H., Goldsby, M., Karimi, S. M., Siddique, F., Kanwal, F. N., & Little, B. B. (2026). Where Inequities Emerge: Racial and Ethnic Differences Across the COVID-19 Hospitalization Continuum. International Journal of Environmental Research and Public Health, 23(2), 181. https://doi.org/10.3390/ijerph23020181








