Strengthening Jordan’s Laboratory Capacity for Communicable Diseases: A Comprehensive Multi-Method Mapping Toward Harmonized National Laboratories and Evidence-Informed Public Health Planning
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Team Formation and Stakeholder Engagement
2.3. Tools Development and Data Collection
2.4. Analysis and Evaluation
2.5. Validation Workshop and Reporting
2.6. Ethical Considerations
3. Results
3.1. Policy and Regulatory Landscape
3.2. Availability of Laboratory SOPs and Guidelines for Communicable Diseases
3.3. Quality Assurance and Laboratory Quality Management
3.4. Workforce Capacity and Training
4. Discussion
4.1. Contextualizing Jordan’s Laboratory System Within Public Health Infrastructure
4.2. Alignment with International Standards and Global Benchmarks
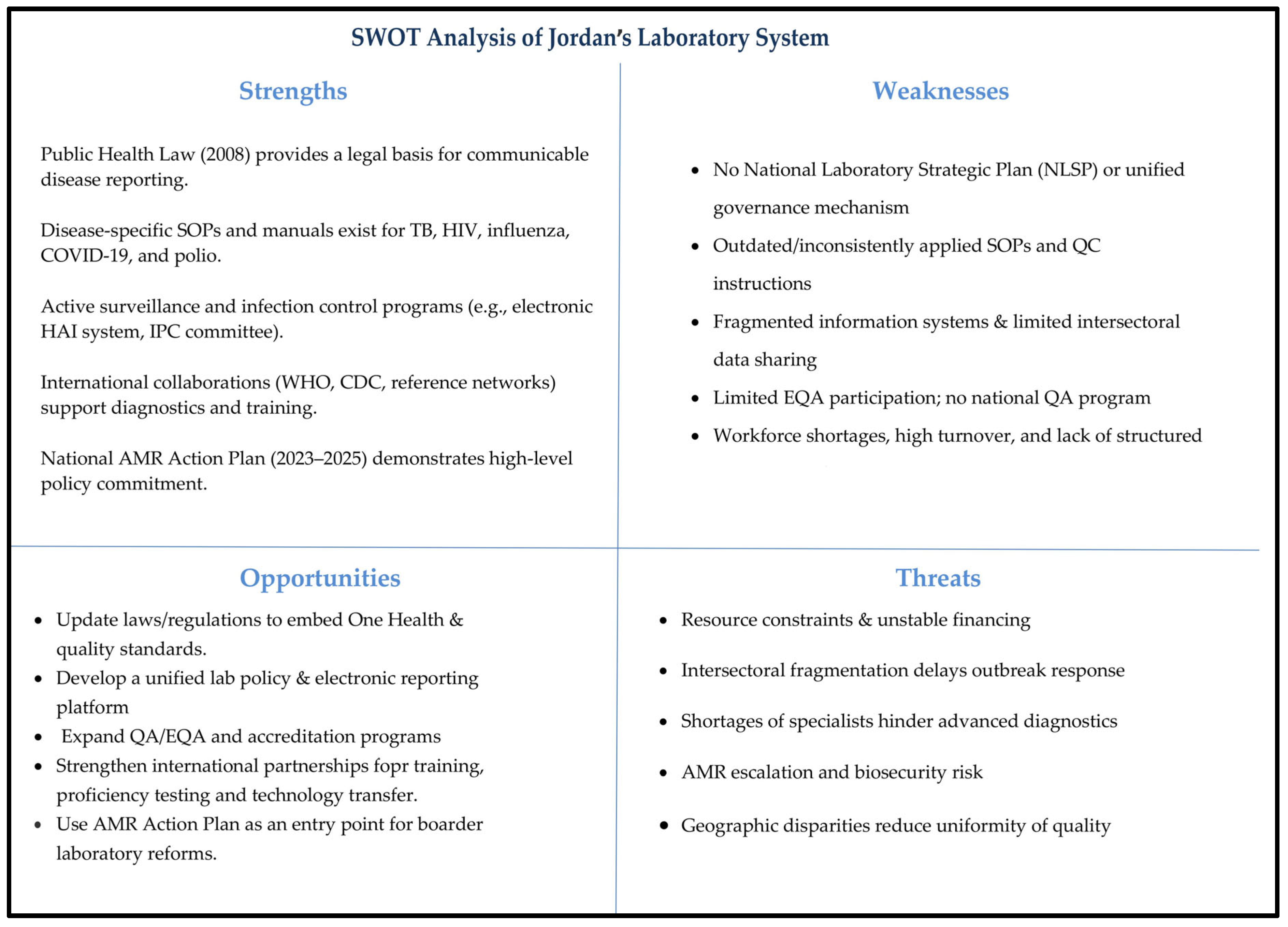
4.3. Gaps, Strengths, and Implications for Health Security and One Health
4.4. Regional and International Comparision
4.5. Recommendations and Way Forward
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial Resistance |
| BSL | Biosafety Level |
| CPHL | Central Public Health Laboratory |
| COVID-19 | Coronavirus Disease of 2019 |
| DAKKS | Deutsche Akkreditierungsstelle (German Accreditation) |
| EMPHNET | The Eastern Mediterranean Public Health Network |
| EQC | External Quality Control |
| FAO | Food and Agriculture Organization of the United Nations |
| HIV | Human Immunodeficiency Virus |
| HCAC | Health Care Accreditation Council |
| IATA | International Air Transport Association |
| ICAO | International Civil Aviation Organization |
| IHR | International Health Regulations |
| IOM | International Organization for Migration |
| IQC | Internal Quality Control |
| JCDC | Jordan Center for Disease Control |
| JEE | Joint External Evaluation |
| JFDA | Jordan Food and Drug Administration |
| KII | Key Informant Interview |
| MoA | Ministry of Agriculture |
| MoEnv | Ministry of Environment |
| MoH | Ministry of Health |
| NGO | Non-Governmental Organization |
| NLSP | National Laboratory Strategic Plan |
| PPE | Personal Protective Equipment |
| PCR | Polymerase Chain Reaction |
| QMS | Quality Management System |
| QC | Quality Control |
| RMS | Royal Medical Services |
| SOP | Standard Operating Procedure |
| TB | Tuberculosis |
| USAID | United States Agency for International Development |
| WAJ | Water Authority of Jordan |
| WEEC | Water, Energy and Environment Center |
| WHO | World Health Organization |
| CDC | U.S Centers for Disease Control and Prevention |
| GHSA | Global Health Security Agenda |
| LMICs | Low- and Middle-Income Countries |
| FDA | U.S Food and Drug Administration |
| NHS | National Health Service |
| IOS | International Organization for Standardization |
| UNICEF | United Nations Children’s Fund |
| UKAS | The United Kingdom Accreditation Service |
| WHO EMRO | The WHO Regional Office for the Eastern Mediterranean |
References
- Boutayeb, A. The Burden of Communicable and Non-Communicable Diseases in Developing Countries. In Handbook of Disease Burdens and Quality of Life Measures; Preedy, V.R., Watson, R.R., Eds.; Springer: New York, NY, USA, 2010; pp. 531–546. ISBN 978-0-387-78665-0. [Google Scholar]
- Burstein, R.; Henry, N.J.; Collison, M.L.; Marczak, L.B.; Sligar, A.; Watson, S.; Marquez, N.; Abbasalizad-Farhangi, M.; Abbasi, M.; Abd-Allah, F.; et al. Mapping 123 Million Neonatal, Infant and Child Deaths between 2000 and 2017. Nature 2019, 574, 353–358. [Google Scholar] [CrossRef]
- Setyawati, R.; Astuti, A.; Utami, T.P.; Adiwijaya, A.; Hasyim, D.M. The Importance of Early Detection in Disease Management. J. World Future Med. Health Nurs. 2024, 2, 51–63. [Google Scholar]
- McCauley, J.; Van Kerkhove, M.; Abu Raddad, L.J.; Meredith, L.; Brennan, R.; Abubakar, A.; Barakat, A. Expansion of Laboratory Capacity in the Eastern Mediterranean Region During the COVID-19 Pandemic: Lessons Learned and Future Strategies for Sustainability. Influenza Other Respir. Viruses 2024, 18, e70030. [Google Scholar] [CrossRef]
- Koopmans, M. Surveillance Strategy for Early Detection of Unusual Infectious Disease Events. Curr. Opin. Virol. 2013, 3, 185–191. [Google Scholar] [CrossRef]
- Bradford, A.K. The Role of the Laboratory in Disease Surveillance. East. Mediterr. Health J. 1996, 2, 68–72. [Google Scholar]
- Kan, B. Performing Laboratory Network Surveillance to Monitor the Emergence and Spread of Infectious Diseases. China CDC Wkly. 2022, 4, 233. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Over 280,000 Children Immunized in First Week of Measles Campaign; World Health Organization: Geneva, Switzerland, 2025; Available online: https://www.emro.who.int/jor/jordan-news/who-jor-measles-campaign.html (accessed on 31 July 2025).
- Momani, S.; Alyahya, M.S.; Zayed, D.K.; Tarif, A.B.; Nimri, O.F.; Alshaikh, S.; Belbiesi, A.; Al-Tammemi, A.B. Evaluating Jordan’s Antimicrobial Resistance National Action Plan (2018–2022) Implementation: Progress and Recommendations. BMC Public Health 2025, 25, 2718. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y. Types and Frequencies of Pre-Analytical Errors in the Clinical Laboratory at the University Hospital of Korea. Clin. Lab. 2019, 65, 1735–1743. [Google Scholar] [CrossRef]
- Qaqish, A.; Al-Omari, M.; Abbas, M.M.; Said, R.; Al Tamimi, M.; Ghazo, M. Decentralization of COVID-19 Molecular Diagnosis, a Success Story from Jordan. J. Glob. Health 2022, 12, 03045. [Google Scholar] [CrossRef]
- Sorrell, E.M.; El Azhari, M.; Maswdeh, N.; Kornblet, S.; Standley, C.J.; Katz, R.L.; Ablan, I.; Fischer, J.E. Mapping of Networks to Detect Priority Zoonoses in Jordan. Front. Public Health 2015, 3, 219. [Google Scholar] [CrossRef] [PubMed]
- Barbé, B.; Verdonck, K.; Mukendi, D.; Lejon, V.; Lilo Kalo, J.-R.; Alirol, E.; Gillet, P.; Horié, N.; Ravinetto, R.; Bottieau, E.; et al. The Art of Writing and Implementing Standard Operating Procedures (SOPs) for Laboratories in Low-Resource Settings: Review of Guidelines and Best Practices. PLoS Negl. Trop. Dis. 2016, 10, e0005053. [Google Scholar] [CrossRef]
- Zayed, D.K.; Momani, S.; Horabi, M.; Alquran, A.; Al-Nawaiseh, F.K.; Tarif, A.B.; Nimri, O.F.; Alyahya, M.S.; Madi, T.; Shatat, A.; et al. Exploring Policies, Strategies, and Legislations Related to the One Health Approach to Zoonoses, Antimicrobial Stewardship, and Climate Change in Jordan: A Multimethod Study with SWOT Analysis. Int. J. Environ. Res. Public Health 2025, 22, 749. [Google Scholar] [CrossRef]
- Clarke, A.E.; Friese, C.; Washburn, R.S. Situational Analysis: Grounded Theory After the Interpretive Turn. Res. Ethics Qual. Res. 2018, 19, 35. [Google Scholar] [CrossRef]
- Ondoa, P.; Datema, T.; Isadore, J.; Oskam, L.; Keita-Sow, M.-S.; Ndihokubwayo, J.-B.; Lewis, K.; Nkengasong, J. A New Matrix for Scoring the Functionality of National Laboratory Networks in Africa-Introducing the LABNET Scorecard. Afr. J. Lab. Med. 2016, 5, a498. [Google Scholar] [CrossRef]
- World Health Organization (WHO). JEE Report Jordan; World Health Organization: Geneva, Switzerland, 2016; Available online: https://extranet.who.int/sph/jee-report-jordan-2016 (accessed on 2 August 2025).
- Kheirallah, K.A.; Al-Nusair, M.; Aljabeiti, S.; Sheikali, N.; Alzoubi, A.; Alsulaiman, J.W.; Al-Mistarehi, A.H.; Alzoubi, H.; Mousa, A.A.B.; Allouh, M.Z. Jordan’s Pandemic Influenza Preparedness (PIP): A Reflection on COVID-19 Response. Int. J. Environ. Res. Public Health 2022, 19, 7200. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (U.S. CDC) Laboratories. Available online: https://www.cdc.gov/global-health-protection/php/programs-and-institutes/laboratories.html#:~:text=Accurately%20diagnosing%20diseases%20and%20identifying,quality%2C%20effective%20laboratory%20systems (accessed on 2 August 2025).
- Masanza, M.M.; Nqobile, N.; Mukanga, D.; Gitta, S.N. Laboratory Capacity Building for the International Health Regulations (IHR[2005]) in Resource-Poor Countries: The Experience of the African Field Epidemiology Network (AFENET). BMC Public Health 2010, 10, S8. [Google Scholar] [CrossRef]
- Ondoa, P.; van der Broek, A.; Jansen, C.; de Bruijn, H.; Schultsz, C. National Laboratory Policies and Plans in Sub-Saharan African Countries: Gaps and Opportunities. Afr. J. Lab. Med. 2017, 6, 578. [Google Scholar] [CrossRef]
- Qutishat, A.S. Medical Laboratory Quality and Accreditation in Jordan. Clin. Biochem. 2009, 42, 256–258. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Regional Office for Africa Guidance for Establishing a National Health Laboratory System; World Health Organization: Geneva, Switzerland, 2024; Available online: https://iris.who.int/handle/10665/148351 (accessed on 2 August 2025).
- Food and Agriculture Organization of the United Nations (FAO). Pandemic Readiness Enhancement Programme for Jordan; Food and Agriculture Organization of the United Nations: Rome, Italy, 2025; Available online: https://www.fao.org/one-health/partners/projects/pf-jordan/en#:~:text=Jordan%2C%20at%20the%20crossroads%20of,sectoral%20response%20to%20health%20emergencies (accessed on 2 August 2025).
- World Health Organization (WHO). Zoonotic Disease: Emerging Public Health Threats in the Region; World Health Organization: Geneva, Switzerland, 2025; Available online: https://www.emro.who.int/about-who/rc61/zoonotic-diseases.html (accessed on 2 August 2025).
- World Health Organization (WHO). Health Emergency Preparedness and International Health Regulations—One Health; World Health Organization: Geneva, Switzerland, 2025; Available online: https://www.emro.who.int/cpi/programmes/one-health.html#:~:text=legislation%2C%20and%20conduct%20of%20research (accessed on 2 August 2025).
- Ndoungué, V.F.; Bello, D.; Kameni, J.M.F.; Lamtoing, A.D.; Epee, C.E.D.; Abdou, S.; Mouliom, M.M.M.; Njajou, O.T.; Tieblé, T.; Wango, R.K.; et al. IHR-PVS National Bridging Workshop in Cameroon: An Interactive and Participatory Approach to Engage Stakeholders in the Development of a One Health Road Map. One Health 2023, 16, 100552. [Google Scholar] [CrossRef] [PubMed]
- Mahrous, H.; Redi, N.; Nguyen, T.M.N.; Al Awaidy, S.; Mostafavi, E.; Samhouri, D. One Health Operational Framework for Action for the Eastern Mediterranean Region, Focusing on Zoonotic Diseases. East. Mediterr. Health J. 2020, 26, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Bansal, D.; Jaffrey, S.; Al-Emadi, N.A.; Hassan, M.; Islam, M.M.; Al-Baker, W.A.A.; Radwan, E.; Hamdani, D.; Haroun, M.I.; Enan, K.; et al. A New One Health Framework in Qatar for Future Emerging and Re-Emerging Zoonotic Diseases Preparedness and Response. One Health 2023, 16, 100487. [Google Scholar] [CrossRef] [PubMed]
- Farag, E.A.B.; Nour, M.; El Idrissi, A.; Berrada, J.; Moustafa, A.; Mehmood, M.; Mahmoud, M.; El-Sayed, A.; Alhajri, F.; Al-Hajri, M.; et al. Survey on Implementation of One Health Approach for MERS-CoV Preparedness and Control in Gulf Cooperation Council and Middle East Countries. Emerg. Infect. Dis. J. 2019, 25, E1–E6. [Google Scholar] [CrossRef]
- Al-Ghadeer, H.; Chu, D.K.W.; Rihan, E.A.; Abd-Allah, E.; Gu, H.; Chin, A.W.H.; Qasim, I.; Aldoweriej, A.; Alharbi, S.; Al-Aqil, M.; et al. Circulation of Influenza A(H5N8) Virus, Saudi Arabia. Emerg. Infect. Dis. J. 2018, 24, 1961. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.Y.; Saidouni, A.; Wambua, L.W.; Mahrous, H.; Malik, S.M.M.R.; Lubogo, M.; Van de Weerdt, R.; Adam, A.H.; Mohamed, H.H.; Al Makhzoumi, K.; et al. IHR-PVS National Bridging Workshop for Somalia: An Interactive and Participatory Approach for Operationalizing the One Health Roadmap. One Health 2024, 19, 100858. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization—Geneva; World Health Organization Regional Office for Africa; United States Centers for Disease Control and Prevention; Association of Public Health Laboratories. Guidance for Development of National Laboratory Strategic Plans. Available online: https://www.aphl.org/programs/global_health/Documents/GH_2010Aug13_GuidanceNLStrategicPlans.pdf (accessed on 2 August 2025).

| No. | Core Capacity | Sub Components |
|---|---|---|
| 1 | Political, Legal, Regulatory and Financial Framework | Legislation Governance National policies and plans Finances |
| 2 | Structure, Organization, and Coverage | Tiered laboratory network Coverage Multisectoral Collaboration |
| 3 | Standardization of Testing | Guidelines and protocols Diagnostic algorithms |
| 4 | Infrastructure and Biosafety | Infrastructure Biosafety and Biosecurity |
| 5 | Equipment and Supplies | Equipment Supply Chain Management |
| 6 | Workforce | Workforce Human Resource Strategies and Management Education and Training |
| 7 | Information Management Systems | Surveillance and Epidemiology Reporting |
| 8 | Quality Management System | Quality Assurance (QC, and EQA) Certification and Accreditation |
| 9 | Priority Diseases | Prioritization Antimicrobial resistance |
| 10 | Research | Research and Development |
| Policy/Regulation | Year | Scope and Coverage | Observations from Mapping |
|---|---|---|---|
| Public Health Law No. 47 (Chapter V) | 2008 | Communicable disease reporting, outbreak response, and some One Health principles (human–animal health) | Provides legal basis for disease notification. Not updated since 2008; does not explicitly mandate lab quality standards or modern One Health coordination. |
| Licensing of Private Medical Laboratories Regulation | 2003 (amended 2018) | Licensure requirements for private clinical laboratories (staff qualifications, equipment, safety, etc.) | Applies uniform criteria to all private labs. Lacks risk-based categorization of labs by testing complexity. Limited provisions for ongoing quality monitoring (no mandatory proficiency testing or accreditation). |
| MoH Internal Quality Control Instructions | 2006 | Guidelines for internal quality control procedures in medical laboratories. | Outdated—not aligned with current quality management best practices (e.g., ISO 15189). No updates or enforcement mechanism; implementation varies by lab. |
| Infectious Substance Transport Guidelines | N/A (in use) | Instructions for packaging and transporting infectious samples between labs (domestic referrals). | Not aligned with IATA/ICAO international transport standards. Missing detailed requirements for certified packaging, labeling, and shipper training. Poses compliance and safety risks. |
| Biosafety/Biosecurity Guidelines | N/A (various) | Basic lab safety rules and biohazard handling procedures (internal to MoH and some institutes). | No unified biosafety laws. Existing guidelines are fragmented and not consistently enforced. Biosafety level standards (e.g., for BSL-3) not formally adopted at national level. |
| National Laboratory Policy/Strategy | Not present | (Would outline national vision, roles, and development plans for labs) | No official national lab policy or strategic plan exists, leaving a strategic gap in laboratory system development. |
| Disease/Condition | Laboratory SOP/Guideline Availability | Notes (Source and Last Update) |
|---|---|---|
| Tuberculosis (TB) | Yes—Present. National TB Laboratory Manual available. | NTP lab manual in place (updated ~2018; aligned with WHO TB guidelines). Widely used in MoH TB labs. |
| HIV/AIDS | Yes—Present. HIV testing algorithm and guidelines. | National HIV testing guidelines (covering ELISA/rapid testing and confirmatory strategies). Updated in recent years via National AIDS Program. |
| Influenza (seasonal and avian) | Yes—Present. SOPs for sample collection and PCR testing. | Implemented at CPHL/National Influenza Center. Follows WHO protocols for influenza surveillance; includes avian influenza testing procedures. |
| COVID-19 | Yes—Present. SARS-CoV-2 testing SOPs (PCR). | Developed in 2020; incorporated into MoH lab practices. Includes specimen handling, PCR protocols, and safety measures. |
| Poliomyelitis | Yes—Present. Polio laboratory testing protocol. | Jordan uses WHO polio laboratory network protocols. Stool specimen referral to regional reference lab; national lab follows standard WHO polio SOPs for sample handling. |
| Brucellosis | No dedicated SOP. Uses general microbiological methods. | Culture and serological tests performed at central labs, but no single national guideline document; labs refer to WHO/FAO manuals as needed. |
| Rabies (Human) | No in-country testing SOP. Clinical diagnosis; samples sent abroad. | No routine lab confirmation for human rabies in Jordan. Suspect cases managed clinically or confirmed via external reference (e.g., CDC) if at all. |
| Rabies (Animal) | No capacity/SOP. Not performed domestically. | Veterinary labs lack facility for rabies testing (no fluorescence antibody test in-country). Suspected animal cases are euthanized without lab confirmation. |
| Hemorrhagic Fevers (e.g., CCHF) | No dedicated SOP. Ad hoc approach for diagnostics. | Rare cases would rely on external reference lab testing or use of WHO guidelines. No local SOP due to infrequent occurrence. |
| Foodborne Pathogens (e.g., Salmonella) | Partial. Standard lab methods followed; no national guideline. | Public health labs follow ISO or WHO standard methods for culture and identification of common foodborne bacteria, but no unified national document specific to foodborne outbreak lab response. |
| Quality Component | Status in Jordan | Comments |
|---|---|---|
| Internal Quality Control (IQC) | Practiced in most labs, but no updated national guidelines or standardization. | Basic IQC (controls with tests) is common, especially in larger labs. However, the only official guidance is the 2006 instruction, which is outdated. Smaller labs have variable compliance. |
| External Quality Assurance (Proficiency Testing) | Limited participation; not systematized or mandatory. | Only specific programs (TB, HIV, influenza, etc.) engage in EQA via international networks. Majority of labs do not undergo regular proficiency testing. No national EQA program or requirement in place. |
| Laboratory-specific, non-laboratory-specific, animal, environmental and food safety accreditation standards | Examples on Laboratory-specific accreditations:
| |
| Quality Management System (QMS) | Absent or ad hoc in most labs. Quality activities not formally coordinated. | No lab has a complete QMS according to ISO or SLIPTA framework. However, Jordan offers several laboratory accreditations programs at both national and international levels, including:
Quality managers are not designated in most labs. Any QMS elements (SOPs, document control, audits) are implemented locally in an inconsistent manner. |
| National QA Coordination | Not established. No central QA unit or oversight body for lab quality. | Each lab or program handles quality on its own. There is no national reference laboratory or committee sending EQA panels or monitoring quality across labs. |
| Biosafety/Biosecurity | Basic measures in place; lacks comprehensive standards and enforcement. | Labs use PPE and biosafety cabinets where available. No updated biosafety guidelines or training program nationally. BSL-3 capacity very limited (only at CPHL for TB). Biosecurity controls are minimal outside top-tier labs. |
| Domain | Key Gap (Findings) | Recommendation | Supporting Evidence/International Best Practice |
|---|---|---|---|
| Strategic Planning | No NLSP; fragmented vision across sectors | Develop and implement an NLSP with One Health focus | WHO and APHL guidance [23,33]; Uganda/Ethiopia experience with SLMTA and ISO accreditation [20] |
| Legal and Regulatory Framework | Outdated Public Health Law (2008); uniform licensing; outdated QC (2006) | Revise laws to mandate QA/EQA, risk-based licensing, and updated biosafety/transport standards | WHO EMRO One Health framework [26]; IHR–PVS bridging workshops (Cameroon, Kenya) [27] |
| Governance and Coordination | No empowered national coordinating mechanism; siloed MoH/MoA/private labs | Establish a National Laboratory Coordination Committee under JCDC/Epidemics Committee | WHO EMRO unified governance [26]; Cameroon/Kenya coordinating committees [27] |
| Quality Assurance and Workforce | No national EQA program; uneven accreditation; workforce shortages and turnover | Establish EQA reference center; adopt SLIPTA/SLMTA; expand FETP-lab track; strengthen CPD and incentives | WHO/AFRO stepwise quality improvement [20,23]; AFENET workforce strengthening |
| One Health Surveillance | Weak human–animal lab integration; no unified zoonotic reporting | Create shared One Health laboratory database; institutionalize JRAs and simulation exercises | FAO Pandemic Readiness Programme (Jordan) [24]; regional models (Qatar, Saudi Arabia) [28,29,30,31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayed, D.K.; Al-Smadi, R.A.; Almaayteh, M.; Al-Hjouj, T.; Hamdan, O.; Ghalyoun, A.A.; Alsaleh, O.; Abu Touk, T.; Almaseidin, S.N.; Madi, T.; et al. Strengthening Jordan’s Laboratory Capacity for Communicable Diseases: A Comprehensive Multi-Method Mapping Toward Harmonized National Laboratories and Evidence-Informed Public Health Planning. Int. J. Environ. Res. Public Health 2025, 22, 1459. https://doi.org/10.3390/ijerph22091459
Zayed DK, Al-Smadi RA, Almaayteh M, Al-Hjouj T, Hamdan O, Ghalyoun AA, Alsaleh O, Abu Touk T, Almaseidin SN, Madi T, et al. Strengthening Jordan’s Laboratory Capacity for Communicable Diseases: A Comprehensive Multi-Method Mapping Toward Harmonized National Laboratories and Evidence-Informed Public Health Planning. International Journal of Environmental Research and Public Health. 2025; 22(9):1459. https://doi.org/10.3390/ijerph22091459
Chicago/Turabian StyleZayed, Dalia Kashef, Ruba A. Al-Smadi, Mohammad Almaayteh, Thekryat Al-Hjouj, Ola Hamdan, Ammar Abu Ghalyoun, Omar Alsaleh, Tariq Abu Touk, Saddam Nawaf Almaseidin, Thaira Madi, and et al. 2025. "Strengthening Jordan’s Laboratory Capacity for Communicable Diseases: A Comprehensive Multi-Method Mapping Toward Harmonized National Laboratories and Evidence-Informed Public Health Planning" International Journal of Environmental Research and Public Health 22, no. 9: 1459. https://doi.org/10.3390/ijerph22091459
APA StyleZayed, D. K., Al-Smadi, R. A., Almaayteh, M., Al-Hjouj, T., Hamdan, O., Ghalyoun, A. A., Alsaleh, O., Abu Touk, T., Almaseidin, S. N., Madi, T., Hassan, S. K., Horabi, M., Belbiesi, A., Mukattash, T. L., & Al-Tammemi, A. B. (2025). Strengthening Jordan’s Laboratory Capacity for Communicable Diseases: A Comprehensive Multi-Method Mapping Toward Harmonized National Laboratories and Evidence-Informed Public Health Planning. International Journal of Environmental Research and Public Health, 22(9), 1459. https://doi.org/10.3390/ijerph22091459








