A Summary of Pain Locations and Neuropathic Patterns Extracted Automatically from Patient Self-Reported Sensation Drawings
Abstract
1. Introduction
2. Methods
2.1. Cohort Description
2.2. Data Acquisition
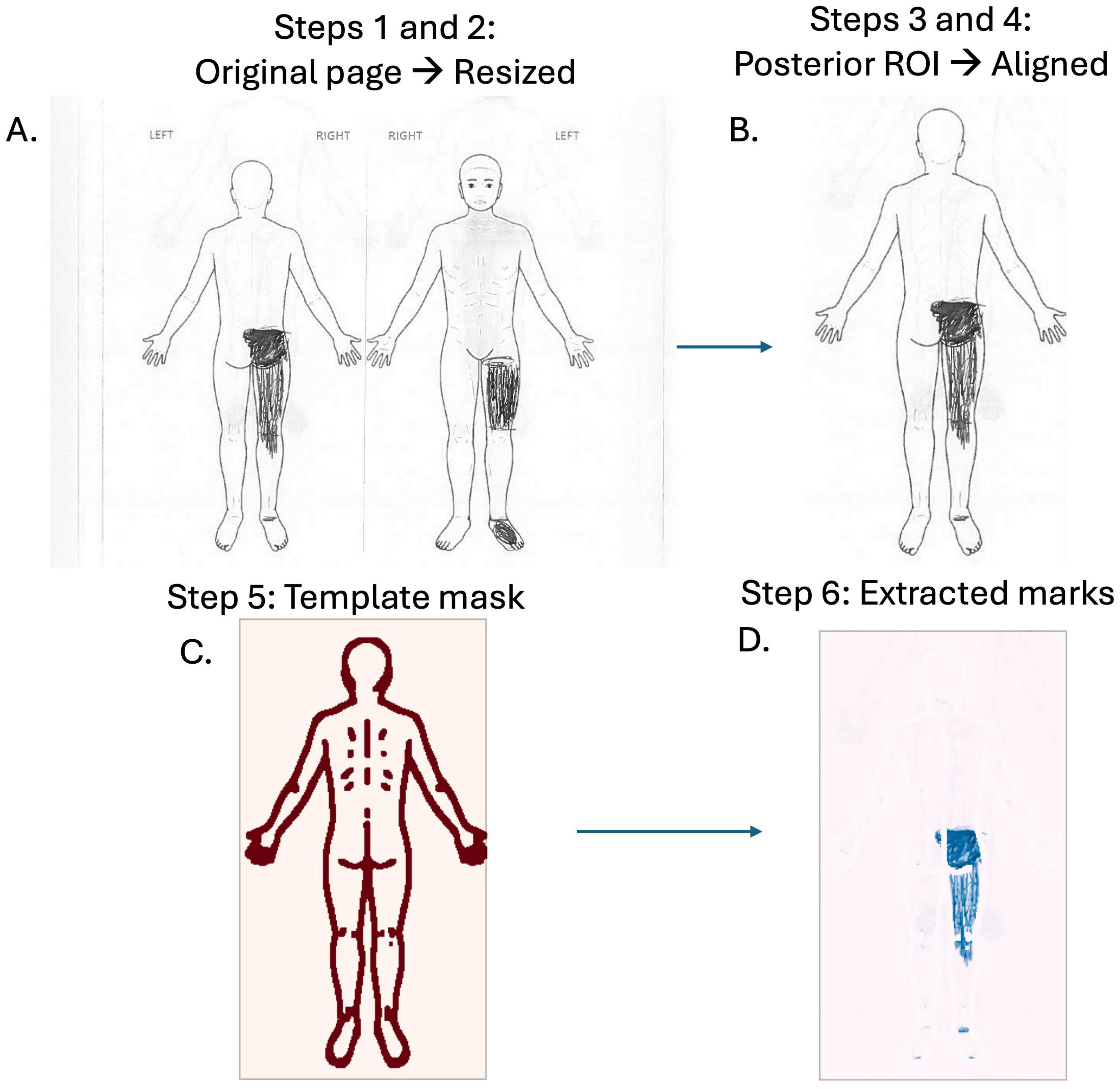
2.3. Image Conversion
2.4. Pre-Processing and Registration
2.5. Template Subtraction
2.6. Quality Control
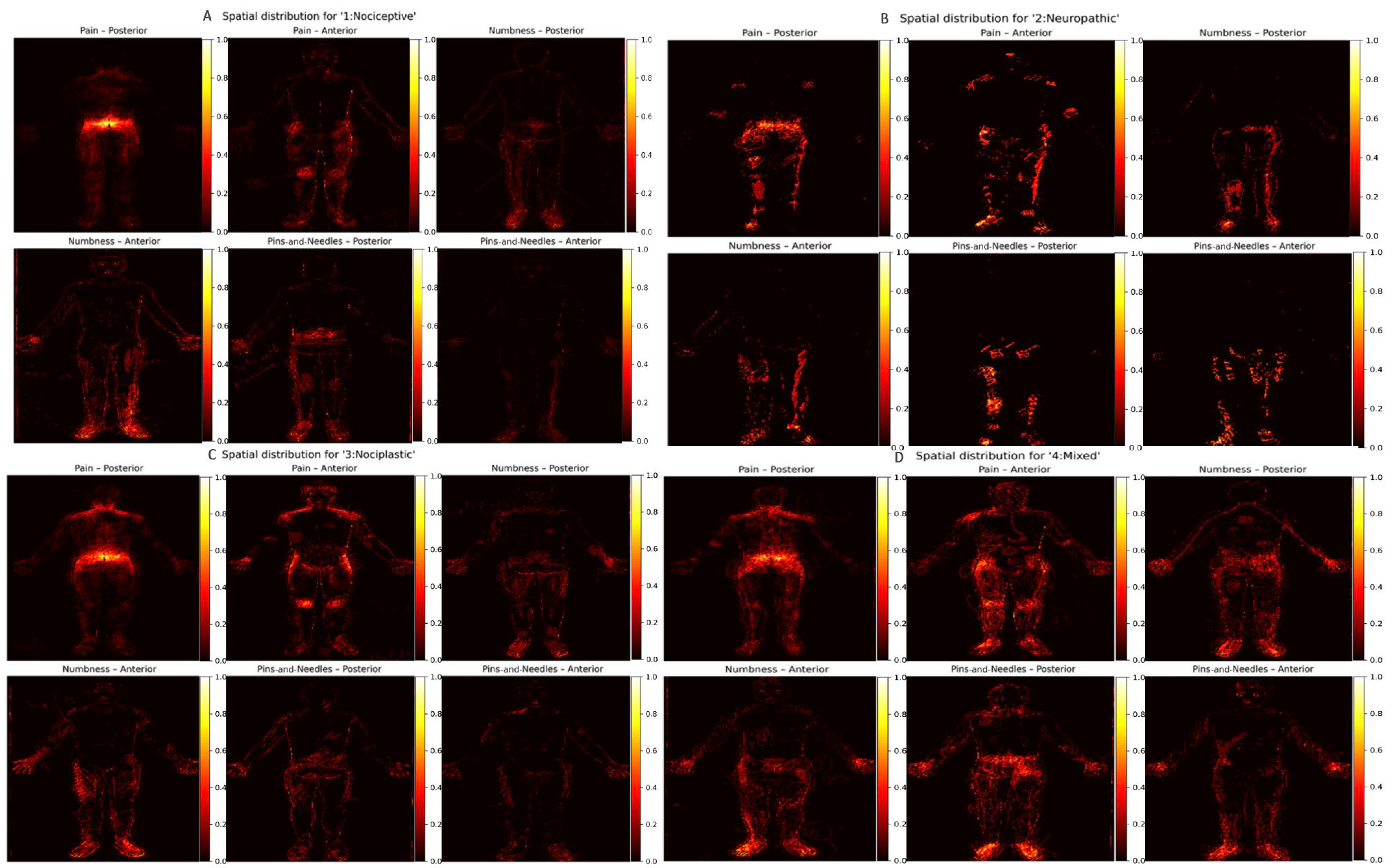
2.7. Symptom Descriptors and Pain Phenotype Categorization
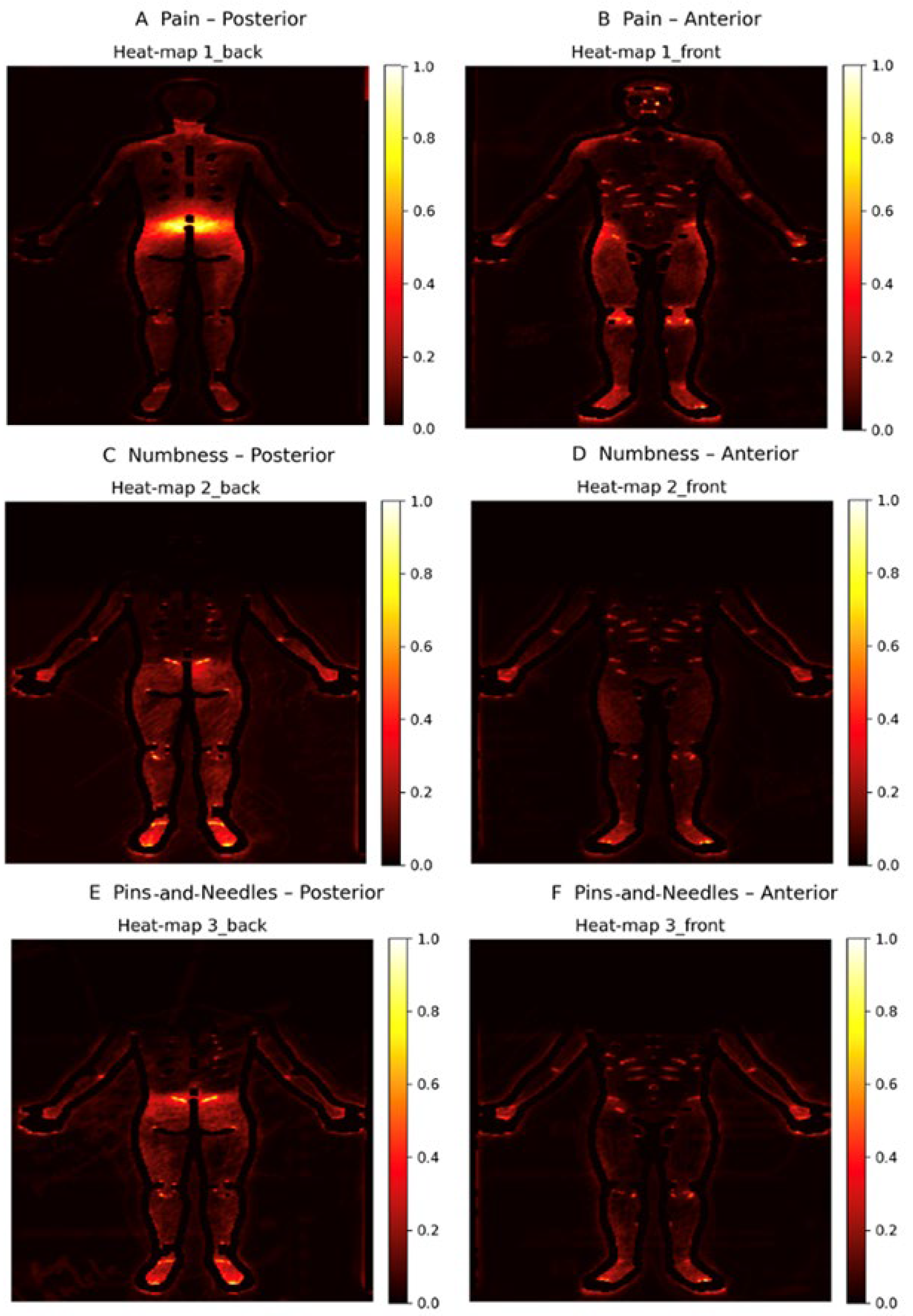
2.8. Heat Map Construction
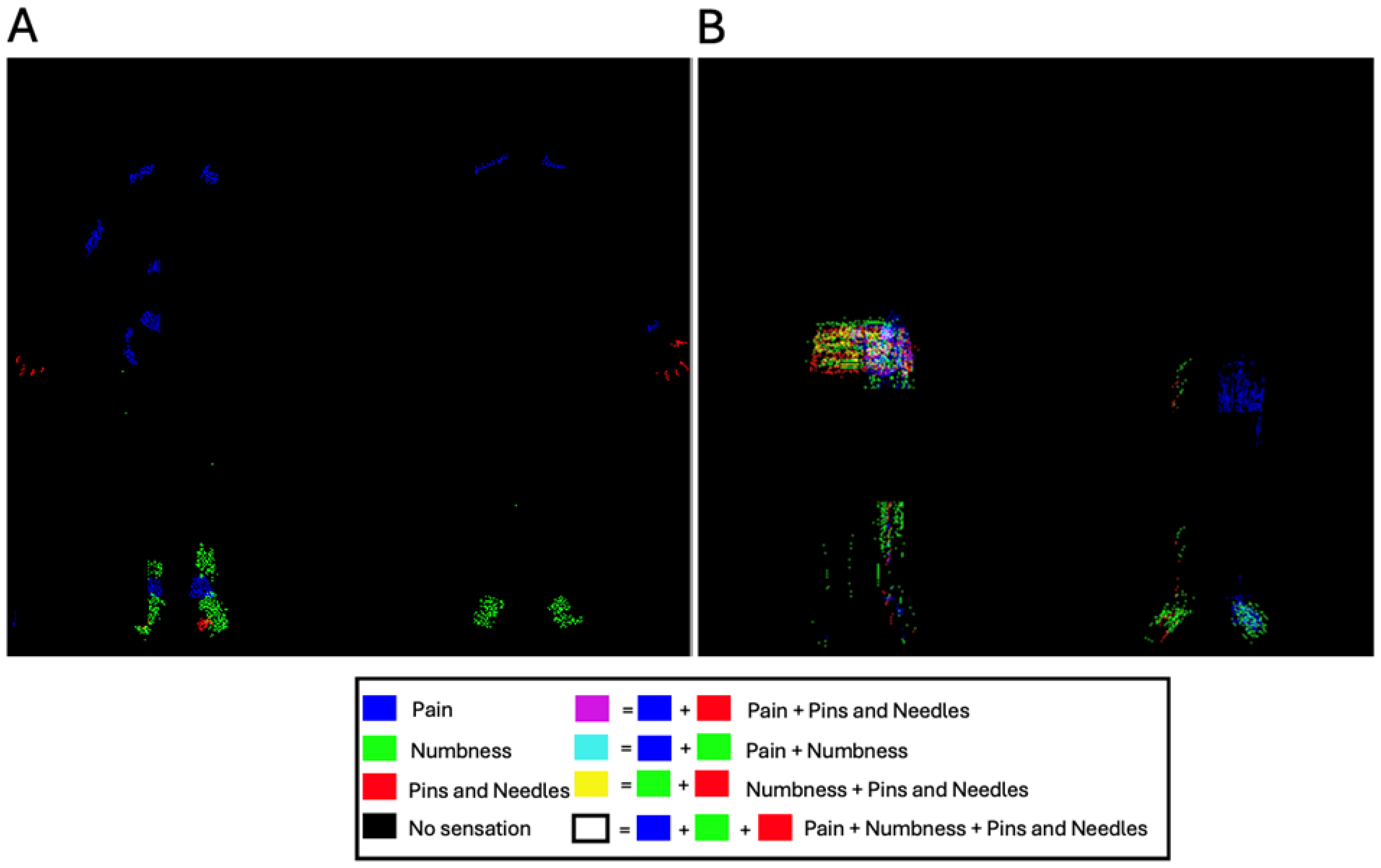
2.9. Composite Color Maps
2.10. Software Environment
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Fillingim, R.B.; Loeser, J.D.; Baron, R.; Edwards, R.R. Assessment of Chronic Pain: Domains, Methods, and Mechanisms. J. Pain 2016, 17, T10–T20. [Google Scholar] [CrossRef] [PubMed]
- Brummett, C.M.; Bakshi, R.R.; Goesling, J.; Leung, D.; Moser, S.E.; Zollars, J.W.; Williams, D.A.; Clauw, D.J.; Hassett, A.L. Preliminary validation of the Michigan Body Map. Pain 2016, 157, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Schott, G.D. The cartography of pain: The evolving contribution of pain maps. Eur. J. Pain 2010, 14, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, J.M.; George, T.K.; Aiyer, R.; Schmidt, K.; Halamka, J.; D’Souza, R.S. Artificial Intelligence and Pain Medicine: An Introduction. J. Pain Res. 2024, 17, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Ojala, J.; Suvilehto, J.T.; Nummenmaa, L.; Kalso, E. Bodily maps of emotions and pain: Tactile and hedonic sensitivity in healthy controls and patients experiencing chronic pain. Pain 2023, 164, 2665–2674. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.; Niburski, K.; Stoopler, M.; Ingelmo, P. Telehealth and Chronic Pain Management from Rapid Adaptation to Long-Term Implementation in Pain Medicine: A Narrative Review. Pain Rep. 2021, 6, e912. [Google Scholar] [CrossRef] [PubMed]
- Clemens, J.Q.; Locke, K., Jr.; Landis, J.R.; Kreder, K.; Rodriguez, L.V.; Yang, C.C.; Tu, F.F.; Harte, S.E.; Schrepf, A.; Farrar, J.T.; et al. Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. Validation of a simple body map to measure widespread pain in urologic chronic pelvic pain syndrome: A MAPP Research Network study. Neurourol. Urodyn. 2024, 43, 727–737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dixit, A.; Lee, M. Quantification of Digital Body Maps for Pain: Development and Application of an Algorithm for Generating Pain Frequency Maps. JMIR Form. Res. 2022, 6, e36687. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.G.; Palsson, T.S.; Royo, A.C.; Bjarkam, C.R.; Boudreau, S.A. Digital Pain Mapping and Tracking in Patients With Chronic Pain: Longitudinal Study. J. Med. Internet Res. 2020, 22, e21475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hue, T.F.; Lotz, J.C.; Zheng, P.; Black, D.M.; Bailey, J.; Ewing, S.K.; Fields, A.J.; Mehling, W.; Scheffler, A.; Strigo, I.; et al. Design of the COMEBACK and BACKHOME Studies, Longitudinal Cohorts for Comprehensive Deep Phenotyping of Adults with Chronic Low-Back Pain (cLBP): A part of the BACPAC Research Program. medRxiv 2024. medRxiv:24305574. [Google Scholar] [CrossRef]
- University of Michigan Mechanistic Research Center—The Back Pain Consortium Research Program. 28 April 2021. Available online: https://clinicaltrials.gov/study/NCT04870957 (accessed on 9 June 2021).
- Fairbank, J.C.T.; Pynsent, P.B. The Oswestry Disability Index. Spine 2000, 25, 2940–2952. Available online: https://journals.lww.com/spinejournal/fulltext/2000/11150/the_oswestry_disability_index.17.aspx (accessed on 1 September 2025). [CrossRef] [PubMed]
- Cella, D.; Riley, W.; Stone, A.; Rothrock, N.; Reeve, B.; Yount, S.; Amtmann, D.; Bode, R.; Buysse, D.; Choi, S.; et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol. 2010, 63, 1179–1194. [Google Scholar] [CrossRef]
- Rublee, E.; Rabaud, V.; Konolige, K.; Bradski, G. ORB: An Efficient Alternative to SIFT or SURF. In Proceedings of the 2011 International Conference on Computer Vision, Barcelona, Spain, 6–13 November 2011; IEEE: New York, NY, USA, 2011; pp. 2564–2571. [Google Scholar] [CrossRef]
- Hartley, R.; Zisserman, A. Multiple View Geometry in Computer Vision, 2nd ed.; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Bradski, G. The OpenCV library. Dr Dobb’s J. 2000, 25, 120–125. [Google Scholar]
- Freynhagen, R.; Baron, R.; Gockel, U.; Tölle, T.R. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 2006, 22, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Cappelleri, J.C.; Bienen, E.J.; Koduru, V.; Sadosky, A. Measurement properties of painDETECT by average pain severity. Clin. Outcomes Res. 2014, 6, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Shirvalkar, P.; Prosky, J.; Chin, G.; Ahmadipour, P.; Sani, O.G.; Desai, M.; Schmitgen, A.; Dawes, H.; Shanechi, M.M.; Starr, P.A.; et al. First-in-human prediction of chronic pain state using intracranial neural biomarkers. Nat. Neurosci. 2023, 26, 1090–1099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Full Analysis Cohort | |
|---|---|
| N | 332 |
| Age, years (mean ± SD) | 54.17 ± 16.26 |
| Body Mass Index (BMI), kg/m2 (mean ± SD) | 26.64 ± 4.94 |
| Sex (%) | |
| 40.06 |
| 56.33 |
| 3.61 |
| Race (%) | |
| 10.84 |
| 71.39 |
| 6.33 |
| 0.90 |
| 6.93 |
| 3.61 |
| Education Level (%) | |
| 39.46 |
| 39.76 |
| 9.04 |
| 7.83 |
| 0.30 |
| 3.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bishara, A.; de Rinaldis, E.; Hue, T.F.; Peterson, T.; Cummings, J.; Torres-Espin, A.; Bailey, J.F.; Lotz, J.C.; REACH Investigators. A Summary of Pain Locations and Neuropathic Patterns Extracted Automatically from Patient Self-Reported Sensation Drawings. Int. J. Environ. Res. Public Health 2025, 22, 1456. https://doi.org/10.3390/ijerph22091456
Bishara A, de Rinaldis E, Hue TF, Peterson T, Cummings J, Torres-Espin A, Bailey JF, Lotz JC, REACH Investigators. A Summary of Pain Locations and Neuropathic Patterns Extracted Automatically from Patient Self-Reported Sensation Drawings. International Journal of Environmental Research and Public Health. 2025; 22(9):1456. https://doi.org/10.3390/ijerph22091456
Chicago/Turabian StyleBishara, Andrew, Elisabetta de Rinaldis, Trisha F. Hue, Thomas Peterson, Jennifer Cummings, Abel Torres-Espin, Jeannie F. Bailey, Jeffrey C. Lotz, and REACH Investigators. 2025. "A Summary of Pain Locations and Neuropathic Patterns Extracted Automatically from Patient Self-Reported Sensation Drawings" International Journal of Environmental Research and Public Health 22, no. 9: 1456. https://doi.org/10.3390/ijerph22091456
APA StyleBishara, A., de Rinaldis, E., Hue, T. F., Peterson, T., Cummings, J., Torres-Espin, A., Bailey, J. F., Lotz, J. C., & REACH Investigators. (2025). A Summary of Pain Locations and Neuropathic Patterns Extracted Automatically from Patient Self-Reported Sensation Drawings. International Journal of Environmental Research and Public Health, 22(9), 1456. https://doi.org/10.3390/ijerph22091456






