Bacterial Contamination in Dental Unit Water Lines at Primary Health Care Centers (2022–2023): A Nationwide Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Prevalence of Bacteriological Contamination (DUWLs)
3.2. Multivariable Logistic Regression Model
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| DUWLs | Dental Unit Water Lines, used in dental units to supply water to handpieces and cup fillers. |
| CFU | Colony Forming Unit, a standard used to measure bacterial concentration in water sample lab results. |
| IWR | Independent Water Reservoir, an attachable bottle to the dental chair. |
| WHO | World Health Organization |
| CDC | Center for Disease Control and Prevention |
| HPC | Hetero plate count |
| TPC | Total Plate Count |
| MOH | Ministry of Health |
| PHA | Public Health Administration |
| EHD | Environmental Health Department |
| ADA | American Dental Association |
| EPS | Extracellular polymeric substances |
| GCC | Gulf Cooperation Countries |
| Stata 17 | A statistical software used to analyze data for descriptive and regression analysis. |
References
- Odonnell, M.J.; Boyle, M.A.; Russell, R.J.; Coleman, D.C. Management of dental unit waterline biofilms in the 21st century. Future Microbiol. 2011, 6, 1209–1226. [Google Scholar] [CrossRef]
- Chen, P.; Zeng, J.; Hong, F.; Li, C.; Wang, H.; Yu, X. The importance of biofilm contamination control for dental unit waterlines: A multicenter assessment of the microbiota diversity of biofilm in dental unit waterlines. J. Oral Microbiol. 2023, 16, 2299496. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Wirthlin, M.R.; Marshall, G.W.; Rowland, R.W. Formation and Decontamination of Biofilms in Dental Unit Waterlines. J. Periodontol. 2003, 74, 1595–1609. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.C.; O’Donnell, M.J.; Shore, A.C.; Russell, R.J. Biofilm problems in dental unit water systems and its practical control. J. Appl. Microbiol. 2009, 106, 1424–1437. [Google Scholar] [CrossRef] [PubMed]
- Palenik, C.J.; Miller, C.H. The effect of distillation and line cleaning on the quality of water emitted from dental units. Am. J. Dent. 2003, 16, 385–389. [Google Scholar]
- Shuai, T.; Shao, T.; Yi, L.; Han, S.; Jiménez-Herrera, M.F.; Wang, Z.; Li, X. The effect of different types of water sources on dental unit waterline contamination: A systematic review and meta analysis. Heliyon 2024, 10, e35745. [Google Scholar] [CrossRef]
- Sauer, K.; Camper, A.K.; Ehrlich, G.D.; William Costerton, J.; Davies, D.G. Pseudomonas aeruginosa Displays Multiple Phenotypes during Development as a Biofilm. J. Bacteriol. 2002, 184, 1140–1154. [Google Scholar] [CrossRef]
- Spagnolo, A.M.; Sartini, M.; Cristina, M.L. Microbial Contamination of Dental Unit Waterlines and Potential Risk of Infection: A Narrative Review. Pathogens 2020, 9, 651. [Google Scholar] [CrossRef]
- Kohn, W.G.; Collins, A.S.; Cleveland, J.L.; Harte, J.A.; Eklund, K.J.; Malvitz, D.M.; Centers for Disease, C. Guidelines for infection control in dental health-care settings—2003. Recomm. Rep. Morb. Mortal. Wkly. Rep. 2003, 52, 1–61. [Google Scholar]
- Marino, F.; Mazzotta, M.; Pascale, M.R.; Derelitto, C.; Girolamini, L.; Cristino, S. First water safety plan approach applied to a Dental Clinic complex: Identification of new risk factors associated with Legionella and P. aeruginosa contamination, using a novel sampling, maintenance and management program. J. Oral Microbiol. 2023, 15, 2223477. [Google Scholar] [CrossRef]
- European Council. D. 93/42/EEC Medical Devices. In Official Journal European Communities; European Council: Paris, France, 1993. [Google Scholar]
- Enivronment Public Authority, K. Executive Lists. Available online: https://epa.gov.kw/ExecutiveLists (accessed on 1 September 2025).
- Ma’ayeh, S.Y.; Al-Hiyasat, A.S.; Hindiyeh, M.Y.; Khader, Y.S. Legionella pneumophila contamination of a dental unit water line system in dental teaching centre. Int. J. Dent. Hyg. 2008, 6, 48–55. [Google Scholar] [CrossRef]
- Lal, B.; Ravindra, K.; Biswal, M. Appraisal of microbial contamination of dental unit water systems and practices of general dental practitioners for risk reduction. Environ. Sci. Pollut. Res. 2018, 25, 33566–33572. [Google Scholar] [CrossRef]
- Hussain Akbar, J.; Behbehani, J.; Karched, M. Biofilm growth and microbial contamination of dental unit waterlines at Kuwait University dental center. Front. Oral Health 2023, 3, 1071018. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Ito, T.; Shiota, Y.; Kawata, Y.; Yamamoto, T.; Takashiba, S. Effectiveness and safety of low-concentrated ozonized water for the reduction of contamination in dental unit water lines. Heliyon 2019, 5, e02306. [Google Scholar] [CrossRef] [PubMed]
- Khajezadeh, M.; Mohseni, F.; Khaledi, A.; Firoozeh, A. Contamination of dental unit water lines (DUWL) with Legionella pneumophila and Pseudomonas aeruginosa; A Middle East systematic review and meta-analysis. Eur. J. Microbiol. Immunol. 2023, 12, 93–99. [Google Scholar] [CrossRef]
- Vinh, R.; Azzolin, K.A.; Stream, S.E.; Carsten, D.; Eldridge, L.A.; Estrich, C.G.; Lipman, R.D. Dental unit waterline infection control practice and knowledge gaps. J. Am. Dent. Assoc. 2024, 155, 515–525.e511. [Google Scholar] [CrossRef] [PubMed]
- Hoogenkamp, M.A.; Brandt, B.W.; Laheij, A.M.G.A.; de Soet, J.J.; Crielaard, W. The microbiological load and microbiome of the Dutch dental unit; ‘please, hold your breath’. Water Res. 2021, 200, 117205. [Google Scholar] [CrossRef]
- Gawish, S.; Abbass, A.; Abaza, A. Occurrence and biofilm forming ability of Pseudomonas aeruginosa in the water output of dental unit waterlines in a dental center in Alexandria, Egypt. Germs 2019, 9, 71. [Google Scholar] [CrossRef]
- Dang, Y.; Zhang, Q.; Wang, J.; Wang, Q.; Han, M.; Niu, Y.; Li, H.; Li, X. Assessment of microbiota diversity in dental unit waterline contamination. PeerJ 2022, 10, e12723. [Google Scholar] [CrossRef]
- Arvand, M.; Hack, A. Microbial contamination of dental unit waterlines in dental practices in Hesse, Germany: A cross-sectional study. Eur. J. Microbiol. Immunol. 2013, 3, 49. [Google Scholar] [CrossRef]
- Barbot, V.; Robert, A.; Rodier, M.-H.; Imbert, C. Update on infectious risks associated with dental unit waterlines. FEMS Immunol. Med. Microbiol. 2012, 65, 196–204. [Google Scholar] [CrossRef]
- Costa, D.; Mercier, A.; Gravouil, K.; Lesobre, J.; Delafont, V.; Bousseau, A.; Verdon, J.; Imbert, C. Pyrosequencing analysis of bacterial diversity in dental unit waterlines. Water Res. 2015, 81, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Buitrago, J.M.; Kolbe, R.J.; Siqueira, M.F. Dental unit waterline testing practices: An 11-Year retrospective study. BMC Oral Health 2023, 23, 867. [Google Scholar] [CrossRef]
- Lizzadro, J.; Mazzotta, M.; Girolamini, L.; Dormi, A.; Pellati, T.; Cristino, S. Comparison between Two Types of Dental Unit Waterlines: How Evaluation of Microbiological Contamination Can Support Risk Containment. Int. J. Environ. Res. Public Health 2019, 16, 328. [Google Scholar] [CrossRef]
- Hikal, W.M.; Kačániova, M.; Hussein, D.E.E.; Ghit, A.; Smaoui, S.; Aleem, M.T.; Čmiková, N.; Said-Al Ahl, H.A.H. Dental unit waterlines and health risks of pathogenic microbial contamination: An update review. J. Biol. Stud. 2023, 6, 282–298. [Google Scholar] [CrossRef]
- Kettering, J.D.; Muñoz-Viveros, C.A.; Stephens, J.A.; Patrick Naylor, W.; Zhang, W. Reducing Bacterial Counts in Dental Unit Waterlines: Distilled Water vs. Antimicrobial Agents. J. Calif. Dent. Assoc. 2002, 30, 735–740. [Google Scholar] [CrossRef]
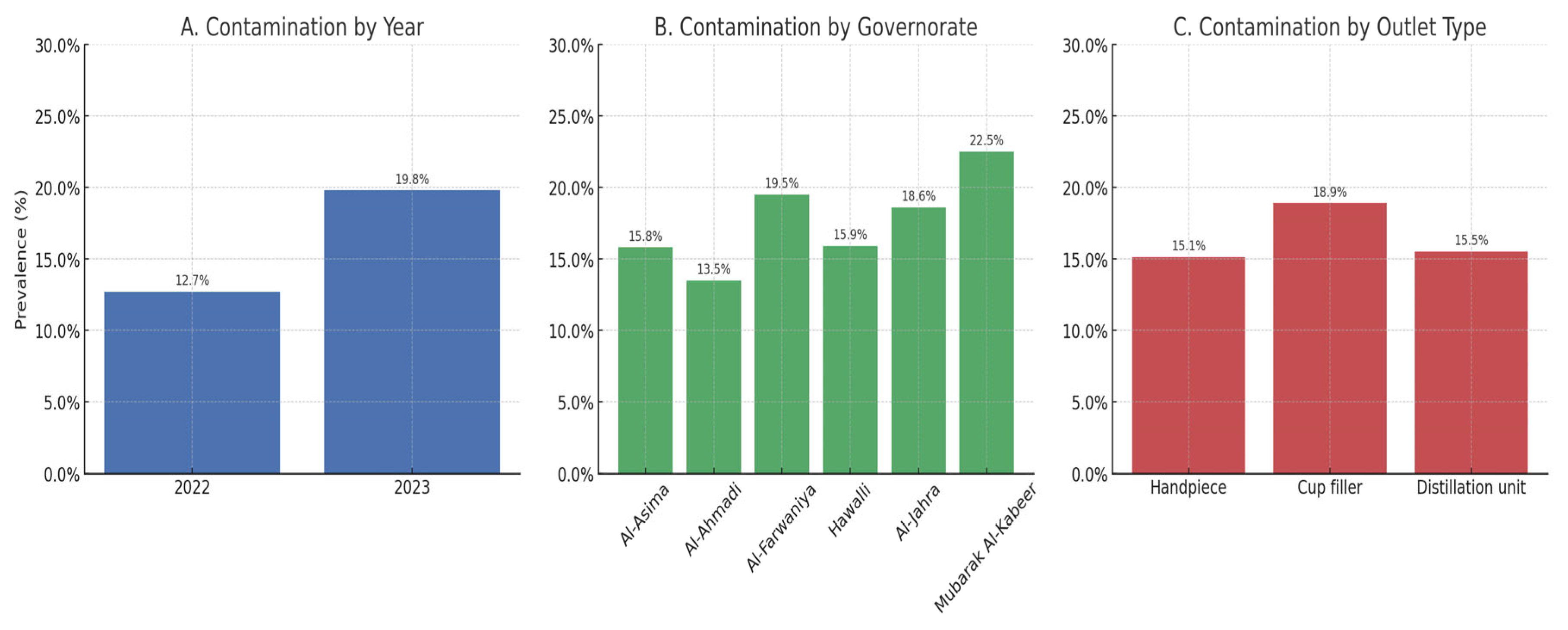
| Characteristic | Total | Contamination Status | p-Value | |
|---|---|---|---|---|
| Uncontaminated | Contaminated | |||
| n (%) | n (%) | n (%) | ||
| Total | 3290 (100%) | 2735 (83.1%) | 555 (16.9%) | |
| Year | <0.001 *** | |||
| 2022 | 1391 (42.3%) | 1213 (87.2%) | 178 (12.8%) | |
| 2023 | 1899 (57.7%) | 1522 (80.2%) | 377 (19.8%) | |
| Governorate | 0.001 ** | |||
| Asima | 919 (27.9%) | 774 (84.2%) | 145 (15.8%) | |
| Ahmadi | 734 (22.3%) | 635 (86.5%) | 99 (13.5%) | |
| Farwaniya | 394 (12.0%) | 317 (80.5%) | 77 (19.5%) | |
| Hawally | 603 (18.3%) | 507 (84.1%) | 96 (15.9%) | |
| Jahra | 156 (4.7%) | 127 (81.4%) | 29 (18.6%) | |
| Mubarak Alkabeer | 484 (14.7%) | 375 (77.5%) | 109 (22.5%) | |
| Water Sampling Outlet | 0.023 * | |||
| Handpiece | 1050 (31.9%) | 891 (84.9%) | 159 (15.1%) | |
| Cup filler | 1428 (43.4%) | 1158 (81.1%) | 270 (18.9%) | |
| Distillation unit | 812 (24.7%) | 686 (84.5%) | 126 (15.5%) | |
| Bacterial Finding | <0.01 ** | |||
| TPC 1 | 3290 (100%) | 2742 (83.4%) | 548 (16.6%) | |
| Coliform | 3290 (100%) | 0 (0.0%) | 0 (0.0%) | |
| E. coli | 3290 (100%) | 0 (0.0%) | 0 (0.0%) | |
| P. aeruginosa | 3290 (100%) | 3246 (98.7%) | 44 (1.3%) | |
| F. streptococci | 3290 (100%) | 0 (0.0%) | 0 (0.0%) | |
| Others | 3290 (100%) | 0 (0.0%) | 0 (0.0%) | |
| TPC descriptive statistics (CFU/mL) | ||||
| Mean (all samples) | 659.1 | – | – | |
| Median (all samples, IQR) | 0 (0–0) | – | – | |
| Mean (contaminated only) | 3957.0 | – | – | |
| Median (contaminated only, IQR) | 2700 (1200–4300) | – | – | |
| Characteristic | OR | (95% CI) | p-Value |
|---|---|---|---|
| Year | |||
| 2022 | Reference | ||
| 2023 | 1.6 | (1.3, 2.0) | <0.001 *** |
| Governorate | |||
| Asima | Reference | ||
| Ahmadi | 0.9 | (0.7, 1.2) | 0.332 |
| Farwaniya | 1.3 | (1.0, 1.8) | 0.104 |
| Hawally | 1.0 | (0.8, 1.4) | 0.877 |
| Jahra | 1.2 | (0.8, 1.9) | 0.369 |
| Mubarak Alkabeer | 1.4 | (1.1, 1.9) | 0.014 * |
| Water Sampling Outlet | |||
| Handpiece | Reference | ||
| Cup filler | 1.3 | (1.3, 1.6) | 0.017 * |
| Distillation unit | 1.1 | (0.8, 1.4) | 0.593 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamal, A.; Alawadhi, E. Bacterial Contamination in Dental Unit Water Lines at Primary Health Care Centers (2022–2023): A Nationwide Study. Int. J. Environ. Res. Public Health 2025, 22, 1406. https://doi.org/10.3390/ijerph22091406
Jamal A, Alawadhi E. Bacterial Contamination in Dental Unit Water Lines at Primary Health Care Centers (2022–2023): A Nationwide Study. International Journal of Environmental Research and Public Health. 2025; 22(9):1406. https://doi.org/10.3390/ijerph22091406
Chicago/Turabian StyleJamal, Abrar, and Eiman Alawadhi. 2025. "Bacterial Contamination in Dental Unit Water Lines at Primary Health Care Centers (2022–2023): A Nationwide Study" International Journal of Environmental Research and Public Health 22, no. 9: 1406. https://doi.org/10.3390/ijerph22091406
APA StyleJamal, A., & Alawadhi, E. (2025). Bacterial Contamination in Dental Unit Water Lines at Primary Health Care Centers (2022–2023): A Nationwide Study. International Journal of Environmental Research and Public Health, 22(9), 1406. https://doi.org/10.3390/ijerph22091406







