Abstract
Antimicrobial resistance (AMR) in environmental reservoirs is an emerging global health concern, particularly in urban settings with inadequate wastewater management. This study aimed to investigate the prevalence and resistance profiles of Salmonella spp. in canal water in Bangkok and assess the distribution of key antibiotic resistance genes (ARGs). Between 2016 and 2019, a total of 1381 water samples were collected from 29 canals. Salmonella spp. were isolated using standard microbiological methods and tested for susceptibility to 13 antibiotics. Polymerase chain reaction (PCR) was used to detect extended-spectrum β-lactamase (ESBL) genes and class 1 integron. Salmonella was found in 89.7% of samples. Among these, 62.1% showed resistance to at least one antimicrobial, and 54.8% were multidrug-resistant (MDR). The highest resistance was observed against streptomycin (41.4%). ESBL genes, predominantly blaCTX-M, were detected in 72.2% of tested isolates, while class 1 integrons were found in 67.8%, indicating a strong potential for gene dissemination. The results highlight urban canals as critical environment reservoirs of AMR Salmonella serovars, posing significant public health risks, particularly where canal water is used for agriculture, household, or recreational purposes. Strengthened environmental surveillance and effective wastewater regulation are urgently needed to mitigate AMR bacteria transmission at the human–environment–animal interface.
1. Introduction
Antimicrobial resistance (AMR) is an increasing major public health concern worldwide caused by excessive use of antibiotics in humans and agriculture for therapeutic and nontherapeutic purposes [1]. It claims more than 10 million deaths annually worldwide and may push 24 million people into severe poverty by 2050 [2].
A major concern is the release of AMR bacteria into the environment and food chain [3]. The One Health approach, recognizing the inter-connectedness of humans, animals, and the environment, is a vital mechanism in addressing AMR [4,5,6]. The Thai National Strategic Plan on Antimicrobial Resistance (2017–2021) was adopted and implemented with the aim of achieving a 20% reduction in antimicrobial consumption in the human sector and a 30% reduction in the animal sector through the One Health approach [7]. Under Strategy I, the plan seeks to develop a national integrated AMR surveillance system covering humans, animals, and the environment; however, progress in the environmental sector has been comparatively slow.
In the environment, soil and water serve as reservoirs for antibiotic residues and AMR bacteria [8]. One of the primary pathways for AMR bacteria contamination in the environment is through untreated waste and wastewater from households, hospitals, animal farms, and natural waterways [9]. Successful mitigation strategies to reduce AMR bacteria in environmental areas include (i) avoiding the creation of settings that select for, mobilize, and allow persistence of resistance genes in bacterial communities, (ii) reducing transmission routes for resistant bacteria from the diseased human’s (or animal’s) microbiome to environment, and (iii) limiting the selection pressure for resistant pathogens through prudent use of antibiotics for humans and animals.
Salmonella enterica subsp. enterica is responsible for the majority of Salmonella infections in humans and other warm-blooded animals, exhibits a ubiquitous distribution, and is frequently isolated from aquatic environments [10,11]. This pathogen is typically present in untreated sewage with large numbers of 103–104 colony-forming units per liter (CFU/L) and can be detected in effluent drains even after secondary advanced treatments [12,13]. Although most Salmonella infections are foodborne, cases of salmonellosis caused by contaminated recreational water and surface water have been reported [14]. While most Salmonella infections are self-limiting, antimicrobial treatment is necessary for invasive infections or severe diarrhea. The World Health Organization (WHO) recommends fluoroquinolones (FQs) and third-generation cephalosporins as choices for the treatment of salmonellosis, which are classified as critically important for human medicine [15]. However, these are extensively used in veterinary practices [16,17,18]. The use of antimicrobial agents in livestock production and in humans has resulted in the survival of AMR Salmonella isolates from different sources, which have been frequently reported worldwide [19].
Bangkok, the capital city of Thailand, has an extensive canal network surrounded by residential, farming, and small agricultural areas. Household and agricultural waste potentially releases AMR bacteria and antibiotic residues into the canal system. Despite growing concerns regarding AMR contamination in urban waterways, data on the presence and spread of AMR Salmonella in Bangkok’s canal network remains limited. Given that these canals are directly connected to communities and often serve for irrigation, aquaculture, and even informal recreational use, pathogen surveillance in canal water is essential for early detection of AMR threats and for guiding targeted public health interventions.
This study aims to investigate the occurrence of Salmonella spp., their AMR profiles, and antibiotic resistance genes (ARGs) in canal water in Bangkok. The findings are expected to provide a clearer understanding of the current situation and support data for the development of targeted mitigation policies and interventions to address AMR in the environment.
2. Materials and Methods
2.1. Sample Collection
A total of 1392 canal water samples were collected from 29 canals in Bangkok, Thailand, between 2016 and 2019 (Figure 1). Sampling was conducted by the Metropolitan Water Quality Control Division, the agency responsible for routine wastewater quality monitoring in Bangkok. The Bangkok Metropolitan Area has a population of approximately 10.5 million inhabitants, and more than 150 public and private hospitals are served by multiple wastewater treatment plants (WWTPs) of varying capacities. The selection of these 29 canals was based on their geographic distribution across different districts, their proximity to residential, agricultural, and industrial areas, and historical records of water quality concerns from the metropolitan monitoring program. Monthly sampling was systematically performed at all sites throughout the four-year study period (2016–2019), providing consistent temporal coverage across wet and dry seasons, a broad spatial representation of urban, agricultural, and industrial settings, and a sufficiently large sample size. This longitudinal and geographically stratified design yielded a robust dataset for reliable trend analysis, resistance profiling, and molecular characterization, ensuring strong validity for environmental surveillance.
Figure 1.
Sampling locations of canal water in Bangkok, Thailand (2016–2019). Blue markers represent the 29 canal sampling sites.
Bangkok’s canal network is closely linked to surrounding communities and is used for irrigation, aquaculture, and informal recreation, creating direct interfaces for human, animal, and environmental health risks. In densely populated urban areas, such canals can serve as critical reservoirs and transmission pathways for antimicrobial-resistant pathogens. Surveillance of pathogens in canal water is therefore essential for early detection of AMR threats and for informing targeted public health interventions, especially in rapidly urbanizing cities like Bangkok, where environmental AMR monitoring remains underdeveloped.
For each 200 mL water sample collection, the bottle was submerged facing upstream to a depth of approximately 20 cm below the water surface before the cap was removed underwater to allow natural filling into the bottle [20]. The sample was immediately placed in an ice box to maintain a cool temperature and transported to the Department of Microbiology, Faculty of Public Health, Mahidol University, for processing on the same day. Eleven of the samples were excluded from the study due to inadequate water quality for analysis after laboratory arrival.
2.2. Isolation and Identification of Salmonella
The bottles containing water samples were vigorously shaken, and then 50 mL of each water sample was mixed with an equal volume of double-strength buffered peptone water and incubated at 37 °C for 18 h. The enriched sample was streaked onto Modified Semisolid Rappaport–Vassiliadis (MSRV) medium and incubated at 42 °C for 18 h. Presumptive Salmonella colonies growing on the MSRV medium were sub-cultured by streaking onto Xylose Lysine Deoxycholate (XLD) agar and incubated at 37 °C for 18–24 h. Colonies suspected to be Salmonella (1–3 colonies) on XLD agar, appearing red with black centers, were further identified by using biochemical tests on Triple Sugar Iron (TSI) agar and Lysine Indole Motility (LIM) medium. The biochemical profile for Salmonella was as follows: TSI, alkaline (red), slant and acid (yellow), gas producing (+), hydrogen sulfide (H2S) production (+); LIM, lysine-positive, motility-positive, and indole-negative, in accordance with WHO guidelines [21].
Serogroups of identified Salmonella isolates were determined by slide agglutination according to the Kauffmann–White scheme [22] using the commercial polyvalent O antisera (S&A Reagents Lab, Bangkok, Thailand). To identify the Salmonella serogroups, the Salmonella culture was suspended in one drop of polyvalent O-antisera OMA, OMB, OMC, OMD, OME, OMF, and OMG antisera in an orderly manner until a positive-agglutinated reaction was observed. Then, the identified polyvalent group isolate was further examined with the corresponding monovalent O group antisera in the positive polyvalent O group. All identified Salmonella serogroups were kept at −80 °C in glycerol broth for further analysis.
2.3. Antimicrobial Susceptibility Determination
A total of 338 Salmonella isolates were randomly selected for the antimicrobial susceptibility test. We selected two to three isolates from each canal per year, ensuring representation across the serogroup and the month of sampling. Antimicrobial susceptibility was determined against 13 agents belonging to 8 antimicrobial classes using the agar disk diffusion method, in accordance with guidelines by the Clinical and Laboratory Standards Institute (CLSI) [23]. The antimicrobials tested were as follows: ampicillin (AMP), 10 g; ceftazidime (CAZ), 30 μg; chloramphenicol (CHL), 30 μg; ciprofloxacin (CIP), 5 μg; ceftriaxone (CRO), 30 μg; cefotaxime (CTX), 30 μg; gentamicin (GEN), 10 μg; imipenem (IMP), 10 μg; nalidixic acid (NAL), 30 μg; norfloxacin (NOR), 10 μg; streptomycin (STR), 10 μg; sulfamethoxazole-trimethoprim (SXT), 23.75/1.25 μg; and tetracycline (TET), 30 μg. Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922 were used as quality control strains. The inhibition zone diameter was measured, and the results were interpreted according to the CLSI guidelines. In the study area, commonly used antibiotics include ampicillin, third-generation cephalosporins, fluoroquinolones, aminoglycosides, tetracyclines, and sulfonamides. The 13 antimicrobials tested in this study cover most clinically relevant and commonly prescribed agents in Bangkok. Multi-drug resistance (MDR) was defined as resistance to one or more agents in at least three different antimicrobial classes [24].
2.4. Detection of ESBL Genes and Class 1 Integrons
Salmonella isolates that screened positive for ESBL, according to CLSI criteria, were examined for the presence of ESBL genes using multiplex polymerase chain reaction (PCR). Isolates were selected to minimize clonal duplication by excluding those with an identical serogroup, sampling site, and month of isolation. Three primer sets specific to blaCTX-M, blaTEM, and blaSHV were used, as previously designed [25]. It is acknowledged that certain blaCTX-M, blaTEM, and blaSHV variants represent non-ESBL β-lactamases; therefore, detection in this study reflects gene presence rather than functional confirmation of ESBL activity, as noted in the discussion. An initial annealing temperature gradient was performed to optimize the PCR conditions, after which all multiplex PCR assays were carried out in a final reaction volume of 25 μL containing 1 μL, alongside the DNA template, 12.5 μL of 2× Green Master Mix (New England Biolabs, MA 01938, USA), and 10 pmol of each gene-specific primer. The optimized cycling conditions were as follows: initial denaturation at 95 °C for 15 min; 30 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, elongation at 72 °C for 2 min; followed by final extension at 72 °C for 10 min [25].
The presence of class 1 integrons in MDR Salmonella isolates was determined by PCR targeting the intI1 gene. Each reaction mixture (25 μL) contained 1 μL of the DNA template, 12.5 μL of 2× Green Master Mix (New England Biolabs, MA 01938, USA), and 10 pmol of each primer. The cycling parameters were initial denaturation at 95 °C for 5 min; 30 cycles of denaturation at 94 °C for 60 s, annealing at 60 °C for 45 s, extension at 72 °C for 50 s; followed by a final extension at 72 °C for 5 min [26]. The PCR amplicons were separated by horizontal electrophoresis on a 1.2% agarose gel prepared in Tris-borate-EDTA (TBE) buffer and run at 120 V for 40 min. DNA bands were visualized under UV light and photographed using a UVITEC Fire Reader (Uvitec Ltd., Cambridge, CB4 0WS, UK).
3. Results
3.1. Salmonella Confirmation and Identification
Over the four-year period from 2016 to 2019, a total of 1381 canal water samples were collected from 29 canals in the Bangkok Metropolitan. Out of these samples, 1239 (89.7%) were culture positive for Salmonella. The highest percentage of Salmonella contamination was observed in 2017, with 95% (321/338) of samples, followed by 2018 with 90.4% (416/460), 2016 with 88.1% (288/327), and 2019 with 85.2% (218/256) indicating a persistently high environmental burden of Salmonella over the study period (Figure 2).
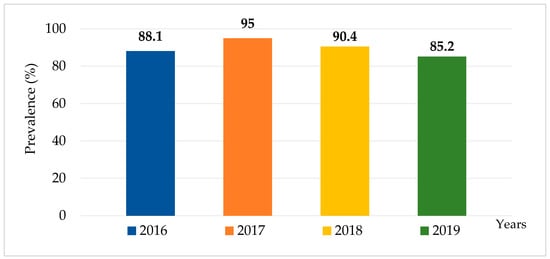
Figure 2.
Prevalence of Salmonella contamination in canal water in Bangkok, Thailand, 2016–2019.
From the 1239 positive samples, a total of 1748 Salmonella isolates were recovered from the 1239 culture-positive water samples for serogrouping. Twelve serogroups were identified, with serogroup B being the most prevalent (41%, namely 717/1748), followed by serogroup C (29.5%, 516/1748) and serogroup E (16.2%, 283/1748). The distribution of serogroups varied annually; detailed trends are presented in Supplementary Material Table S1.
3.2. Antimicrobial Resistance Profiles
Antimicrobial susceptibility testing was performed on 338 Salmonella isolates, selected through stratified random sampling (2–3 isolates per canal site per year) between 2016 and 2019 to represent geographic and temporal variation. Among these isolates, more than half (62.1%, 210/338) exhibited resistance to at least one antimicrobial, resulting in 65 resistant profiles; more details are reported in Supplementary Material Table S2. High resistance rates were observed for streptomycin (41.4%, 140/338), tetracycline (36.4%, 123/338), and ampicillin (35.8%, 121/338). Resistance to sulfamethoxazole–trimethoprim (24%, 81/338), ciprofloxacin (23.7%, 80/338), nalidixic acid (23.7%, 80/338), and chloramphenicol (14.2%, 48/338) was also noted. Notably, all isolates remained susceptible to imipenem. Of particular concern, over 23% of isolates were resistant to quinolones and fluoroquinolones, and 4.7% were resistant to 3rd generation cephalosporin, indicating the presence of ESBL strains in the aquatic environment (Table 1).

Table 1.
Number and percentage of Salmonella isolates from canal water in Bangkok, Thailand, which are resistant to antibiotics.
Overall, the Salmonella isolates exhibited various AMR profiles (altogether there are 63 patterns, see details in Annex 2), of which 54.8% (115/210) of the isolates were resistant to at least three classes of antibiotics, hence meeting the criteria for being MDR Salmonella. The prevalence of MDR Salmonella was highest in 2016 (71.2%, 37/52), followed by 2017 (51.7%, 31/60), 2018 (53.1%, 26/49), and 2019 (42.9%, 21/49), as seen in Supplementary Material Table S1. Furthermore, between 2016 and 2019, 51 isolates (24.3%, 51/210) were resistant to five or more antibiotic classes tested, indicating extensively drug-resistant (XDR) Salmonella, as shown in Table 2.

Table 2.
Number and percentages of Salmonella isolates resistant to 3–7 classes of antibiotics, 2016–2019.
3.3. Prevalence of ESBL Genes and Class 1 Integrons in Salmonella Isolates
Salmonella isolates that demonstrate positive screening as ESBL-producing strains by CLSI criteria were investigated further by using Multiplex PCR to detect the presence of ESBL genes. The PCR products of blaCTX-M, blaTEM, and blaSHV genes were applied. In total, 18 ESBL Salmonella isolates were investigated for the presence of ESBL genes. We found that the suspected ESBL Salmonella carried blaCTX-M (72.2%, 13/18), blaTEM (66.7%, 12/18), and blaSHV (5.6%, 1/18). In addition, the ESBL-producing isolates also exhibited the presence of multiple types of ESBL genes, of which 10/18 (55.6%) were blaCTX-M + blaTEM. Moreover, all 115 isolates of integron were screened for class 1 integrons by the specific class 1 integrase gene (intI1) using PCR. The integrase gene was detected in 67.8% (78/115) of these Salmonella isolates. Of these, Salmonella isolated in 2016 (62.2%; 23/37), 2017 (67.7%; 21/31), 2018 (69.2%; 18/26), and 2019 (76.2%; 16/21) were positive for the intI1 gene, with no significant difference across four years (Chi-squared test, p value = 0.74).
4. Discussion
4.1. AMR Challenges in Environment in Urban Setting
Bangkok, a densely populated city with over 10 million residents and 3503 inhabitants per square kilometer [27], has a massive network of canals that historically served as major routes for trade and transportation. However, rapid urbanization has led to environmental challenges, particularly water contamination due to inadequate wastewater management. Many residential and industrial areas release untreated sewage directly into canals, making these waterways reservoirs for AMR bacteria.
Salmonella is a major foodborne pathogen and a key contributor to AMR worldwide. Salmonellosis is globally recognized as one of the leading causes of acute human bacterial gastroenteritis [28]. It colonizes the gastrointestinal tract of humans and animals and is a primary indicator of fecal contamination in water, food, and the environment [29]. Moreover, as it has the ability to acquire and transfer resistance genes, particularly through horizontal gene transfer, Salmonella is considered a key marker for understanding the spread of AMR. Studying Salmonella provides insight into both its role in public health as a pathogen and its contribution to the overall AMR problem in environments.
Our findings indicate an alarmingly high prevalence of Salmonella in Bangkok’s urban canals, with the pathogen detected in 89.7% of water samples. This result indicates that these urban canals are significant reservoirs for bacterial pathogens. Similar findings have been reported in other rapidly urbanizing regions, such as Dhaka, Bangladesh, where high levels of bacterial contamination in urban rivers have been linked to untreated sewage disposal [30]. The widespread presence of Salmonella poses a serious public health risk, particularly in communities relying on canal water for agriculture, household activities, and recreation. The spread of Salmonella through urban environments highlights the urgent need for improving wastewater management to reduce bacterial contamination and address AMR.
This study also revealed high levels of AMR, with 62% of Salmonella isolates exhibiting resistance to at least one antibiotic. Resistance to streptomycin was most common (41.4%), consistent with the Suwannee River watershed in the Southeastern United States, where nearly all Salmonella isolates (98.9%) were resistant to streptomycin [31]. A systematic review and meta-analysis from Ethiopia reported the pooled proportion of streptomycin resistance of 47% (95% CI: 35–60%) [32]. Streptomycin is specifically used for the treatment of tuberculosis and is not commonly employed in other clinical practices [33]. Private pharmacies commonly dispense broad-spectrum antibiotics, including aminopenicillins and fluoroquinolones [34]. Streptomycin residues can exert selective pressure on bacterial populations, promoting the survival of resistant strains, which may transmit to humans via the food-chain system [32,35].
Of particular concern is the high prevalence of ESBL genes, particularly blaCTX-M, found in 72.2% of resistant isolates. This rate was slightly lower than 75% reported in northern Thailand [36], but it highlights the widespread occurrence of resistance mechanisms that undermine the effectiveness of beta-lactam antibiotics for treatment of serious infections. This observation is consistent with global reports identifying blaCTX-M as one of the most predominant ESBL genes in both clinical and environmental settings, reflecting its successful dissemination via mobile genetic elements. The presence of genes indicates that the bacteria have developed mechanisms to resist antibiotics that are commonly used to treat infections, thereby increasing the risk of treatment failure. The detection of blaCTX-M in the environment is a major concern as it can be horizontally transferred to other bacteria, a process that facilitates the emergence of multidrug resistance.
A study in Jordan reported a high prevalence of Salmonella resistant to tetracycline and multidrug-resistant Salmonella Typhimurium [37]. A systematic review and meta-analysis showed increasing prevalence of Salmonella to nalidixic acid and tetracycline in South Asia [38]. Further, Non-Typhoidal Salmonella (NTS) is a major cause of acute diarrhea with characteristic multidrug resistance (MDR), commonly reported in Asia [39].
4.2. MDR Challenges
Additionally, 54.8% of Salmonella isolates were MDR, which is lower than 80% reported from India [40] but higher than the 31.2% observed in the southeastern United States [41]. These variations in MDR prevalence may be attributed to differences in antibiotic regulations, wastewater treatment, and antibiotic use policies across countries. Antibiotic usage in agriculture is high in India, and the prevalence of MDR bacteria is also higher. In contrast, stricter regulations in some Western countries might contribute to lower rates of MDR Salmonella.
Furthermore, 67.8% of the MDR Salmonella isolates contained class 1 integrons, which are genetic elements that further facilitate the capture, integration, and expression of ARGs. Class 1 integrons are particularly significant because they are frequently associated with plasmids and transposons, enabling their mobility across diverse bacterial hosts and environments. They play a key critical role in the spread and persistence of AMR in the environment by promoting the acquisition and dissemination of resistance genes. The co-occurrence of blaCTX-M and class 1 integrons in a substantial proportion of isolates indicates a heightened potential for co-selection and maintenance of multiple resistance determinants in canal water ecosystems. These findings suggest that urban canals in Bangkok act as important environmental reservoirs for AMR genes, promoting their rapid spread among microbial communities. Similar studies in China have emphasized the role of integrons in the persistence of AMR in aquatic environments [42], underlining the urgent need for targeted interventions in environmental settings.
4.3. Weak Regulation in Environmental Sector
This highlights how policy and regulation play significant roles in determining the spread of AMR in the environment. The high prevalence of MDR Salmonella in Bangkok’s canals is closely linked to poor wastewater management. Many densely populated communities lack proper facilities for effective management of sewage, especially in congested communities in Bangkok. In 2023, out of 2.69 million cubic meters (0.95 million tonnes per year) of household sewage, only 16.7% were properly treated by well-designed 238 treatment facilities. Only 40% of municipalities have legally mandated facilities to manage sewage, and the management of sewage by sub-district local governments remains unclear [43]. Similar challenges have been reported in Nairobi, Kenya, where inadequate waste disposal practices have led to microbial contamination of water sources [44]. The continuous contamination of human and animal waste into the water systems contributes significantly to the persistence of bacteria, including resistant bacteria in the urban environment.
Furthermore, the presence of antibiotic residues in wastewater is a selective pressure for the emergence of AMR bacteria. Studies from India have shown that untreated wastewater containing antibiotic residues creates selective pressure, allowing resistant bacteria to thrive [36]. This situation allows AMR bacteria, including Salmonella, to survive and proliferate, leading to an increased risk of foodborne infections and treatment failures.
Addressing AMR in the environment requires urgent policy interventions. Despite Thailand’s National Strategic Plan on AMR (2017–2022), which has concluded, and the current second National Action Plan on AMR (2023–2027), environmental AMR surveillance remains underdeveloped [7]. AMR control efforts have largely focused on human and animal health, neglecting the environmental dimension. The result of this study highlights the urgent need for new policies to address AMR contamination in Bangkok’s canal water.
International examples such as Sweden have successfully lowered AMR levels by implementing strict antibiotic stewardship programs, thereby improving wastewater treatment infrastructure and facilitating effective regulation [45,46]. Implementing similar measures in Thailand could significantly reduce the spread of resistant bacteria. Multi-sectoral collaboration between public health and agriculture authorities, environmental agencies, and academic institutions is key to sustainable AMR mitigation in the environment.
4.4. Limitations of This Study
This study did not conduct phenotypic testing for ESBL production or PCR-based detection of Salmonella spp., which may limit the interpretation of functional resistance expression and species-level confirmation. Nevertheless, the findings provide valuable insights into the prevalence of antimicrobial resistance genes in canal water over four years. Future studies that include both phenotypic confirmation and molecular detection are recommended to strengthen the conclusions and advance an understanding of resistance dynamics in aquatic environments.
Despite the correlation between antibiotic residues and the abundance of antibiotic-resistant genes in environmental samples [47], this study did not quantify antibiotic residues in canal water, which could provide additional insights into the selective pressures driving AMR. Future work should integrate chemical analysis of residues with microbiological surveillance to better understand AMR emergence and persistence in aquatic environments.
This study was also limited by its geographic scope, its focus on a single bacterial species (Salmonella), the lack of integration of relevant environmental parameters, and the absence of intervention assessment. Future studies should address these limitations by expanding the geographic coverage, including multiple bacterial species, incorporating environmental factors, and evaluating the impact of interventions to provide a more comprehensive understanding of AMR in urban water environments. In addition, this study focused exclusively on Salmonella in canal water in Bangkok, which limits the generalizability of the findings to other regions, to other bacterial species, or to other water sources that may also contribute to AMR. To capture a broader picture, future research could apply advanced techniques, such as metagenomics, to detect a wider range of AMR genes across diverse environmental settings. Furthermore, assessing the influence of additional environmental drivers—such as heavy metal pollution—on the emergence and spread of AMR would enhance our understanding of the underlying dynamics. A nationwide study that assesses AMR in various water systems across Thailand would be invaluable for guiding policymakers in developing more effective AMR control measures. Finally, establishing long-term monitoring of AMR trends in urban waterways would also provide insights into the effectiveness of wastewater treatment improvements in reducing AMR bacteria.
5. Conclusions
The high prevalence of AMR Salmonella in Bangkok’s urban canals underscores the urgent need for improved wastewater management and stronger environmental AMR monitoring. Without effective interventions in managing effluent from households, hospitals, and animal farms before releasing this to the environment, these waterways will continue to serve as reservoirs and transmission pathways for AMR bacteria, threatening public health.
We recommend three synergistic policies. First, strengthening regular AMR monitoring in water sources will help to identify contamination hotspots. Second, implementing effective and affordable treatment of household sewage and enforced by municipality regulations, effluent from public and private hospitals, and animal farms regulated by the Ministry of Public Health and the Ministry of Agriculture and Cooperatives. Third, regular monitoring of the quality of effluents from the human and animal sectors for improvement of sewage management and other relevant policy actions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijerph22091333/s1, Table S1: Prevalence of AMR Salmonella spp.; Table S2: AMR profiles.
Author Contributions
S.K.: Conceptualization, Methodology, Investigation, Formal analysis, Data curation, Writing—Original Draft, Writing—Review and Editing; F.U.: Methodology, Investigation, Formal analysis, Data curation, Writing—Original Draft, Writing—Review and Editing; A.L.: Conceptualization, Methodology, Investigation, Formal analysis, Data curation, Writing—Original Draft; W.K.: Methodology, Formal analysis, Data curation, Writing—Original Draft; V.T.: Conceptualization, Methodology, Investigation, Formal analysis, Data curation, Writing—Original Draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy reasons.
Acknowledgments
We would like to thank all the staff from the Department of Microbiology, Faculty of Public Health, Mahidol University, who provided support during the course and kind assistance with laboratory work. Without their contributions, this research would not have been possible. We are also thankful to the admin staff of the International Health Policy Program Foundation for their assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AMP | Ampicillin |
| AMR | Antimicrobial resistance |
| ARGs | Antibiotic resistance genes |
| CAZ | Ceftazidime |
| CFU/L | Colony forming unit per liter |
| CHL | Chloramphenicol |
| CIP | Ciprofloxacin |
| CLSI | Clinical and Laboratory Standards Institute |
| CRO | Ceftriaxone |
| CTX | Cefotaxime |
| °C | Degree Celsius |
| DNA | Deoxyribonucleic acid |
| ESBL | Extended-spectrum β-lactamase |
| FQs | Fluoroquinolones |
| GEN | Gentamicin |
| H2S | Hydrogen sulfide |
| intI1 | Class 1 integrase gene |
| IMP | Imipenem |
| LIM | Lysine Indole Motility |
| μg | microgram |
| μL | microliter |
| MDR | Multidrug resistance |
| MSRV | Modified semisolid Rappaport Vasiliadis |
| NAL | Nalidixic acid |
| NOR | Norfloxacin |
| pmol | Picomole |
| PCR | Polymerase chain reaction |
| STR | Streptomycin |
| SXT | Sulfamethoxazole-trimethoprim |
| TET | Tetracycline |
| TSI | Triple Sugar Iron |
| WHO | World Health Organization |
| XDR | Extensively drug-resistant |
| XLD | Xylose Lysine Deoxycholate |
References
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- World Health Organization. GLASS Whole-Genome Sequencing for Surveillance of Antimicrobial Resistance. 2020. Available online: https://www.who.int/publications/i/item/9789240011007 (accessed on 1 August 2024).
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Jeggo, M. The One Health Approach-Why Is It So Important? Trop. Med. Infect. Dis. 2019, 4, 88. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Leaders Join Forces to Fight the Accelerating Crisis of Antimicrobial Resistance. 2020. Available online: https://www.who.int/news/item/20-11-2020-world-leaders-join-forces-to-fight-the-accelerating-crisis-of-antimicrobial-resistance (accessed on 25 December 2024).
- Nadimpalli, M.; Astagneau, E.D.; Love, D.C.; Price, L.B.; Huynh, B.-T.; Collard, J.-M.; Lay, K.S.; Borand, L.; Ndir, A.; Walsh, T.R.; et al. Bacterial Infections and antibiotic-Resistant Diseases among Young Children in low-income countries (BIRDY) Study Group. Combating Global Antibiotic Resistance: Emerging One Health Concerns in Lower- and Middle-Income Countries. Clin. Infect. Dis. 2018, 66, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Thailand National Strategic Plan on Antimicrobial Resistance 2017–2021. Available online: https://amrthailand.net/ (accessed on 25 December 2024).
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; et al. Role played by the environment in the emergence and spread of antimicrobial resistance (AMR) through the food chain. EFSA J. 2021, 19, e06651. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef]
- Mezal, E.H.; Sabol, A.; Khan, M.A.; Ali, N.; Stefanova, R.; Khan, A.A. Isolation and molecular characterization of Salmonella enterica serovar Enteritidis from poultry house and clinical samples during 2010. Food Microbiol. 2014, 38, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Lianou, A.; Panagou, E.Z.; Nychas, G.J.E. Chapter 17—Meat Safety—I Foodborne Pathogens and Other Biological Issues. In Lawrie’s Meat Science; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2017; pp. 521–552. [Google Scholar] [CrossRef]
- Chahal, C.; van den Akker, B.; Young, F.; Franco, C.; Blackbeard, J.; Monis, P. Pathogen and particle associations in wastewater significance and implications for treatment and disinfection processes. Adv. Appl. Microbiol. 2016, 97, 63–119. [Google Scholar] [CrossRef]
- Liu, H.; Whitehouse, C.A.; Li, B. Presence and Persistence of Salmonella in Water: The Impact on Microbial Quality of Water and Food Safety. Front. Public Health 2018, 6, 159. [Google Scholar] [CrossRef]
- Collier, S.A.; Deng, L.; Adam, E.A.; Benedict, K.M.; Beshearse, E.M.; Blackstock, A.J.; Bruce, B.B.; Derado, G.; Edens, C.; Fullerton, K.E.; et al. Estimate of Burden and Direct Healthcare Cost of Infectious Waterborne Disease in the United States. Emerg. Infect. Dis. 2021, 1, 140–149. [Google Scholar] [CrossRef]
- World Health Organization. Drug-Resistant Salmonella. 2022. Available online: http://www.who.int/mediacentre/factsheets/fs139/en/ (accessed on 20 December 2024).
- Lekagul, A.; Kirivan, S.; Tansakul, N.N.; Krisanaphan, C.; Srinha, J.; Laoprasert, T.; Kaewkhankhaeng, W.; Tangcharoensathien, V.; Odetokun, I.A. Antimicrobial consumption in food-producing animals in Thailand between 2017 and 2019: The analysis of national importation and production data. PLoS ONE 2023, 18, e0283819. [Google Scholar] [CrossRef]
- Lekagul, A.; Tangcharoensathien, V.; Mills, A.; Rushton, J.; Yeung, S. How antibiotics are used in pig farming: A mixed- methods study of pig farmers, feed mills and veterinarians in Thailand. BMJ Glob. Health 2020, 5, e001918. [Google Scholar] [CrossRef]
- Legakul, A.; Yeung, S.; Tangcharoensathien, V. Patterns of antibiotic use in global pig production: A systematic review. Vet. Anim. Sci. 2019, 7, 100058. [Google Scholar] [CrossRef]
- Manyi-Loh, C.M.S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; incorporating the 1st addendum; World Health Organization: Geneva, Switzerland, 2017. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 30 May 2024).
- WHO GFN Laboratory Protocol: “Biochemical Identification of Salmonella and Shigella, Using an Abbreviated Panel of Tests”—Version 002. 2015. Available online: https://www.researchgate.net/publication/242575055_Biochemical_Identification_of_Salmonella_and_Shigella_Using_an_Abbreviated_Panel_of_Tests (accessed on 1 June 2024).
- WHO Collaborating Centre for Reference and Research on Salmonella. Antigenic Formulae of the Salmonella Serovars, 9th ed.; Pasteur Institute: Paris, France, 2007; Available online: https://www.pasteur.fr/sites/default/files/veng_0.pdf (accessed on 1 June 2024).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020; Available online: https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf (accessed on 1 June 2024).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Monstein, H.J.; Balkhed, A.O.; Nilsson, M.V.; Dornbusch, K.; Nilsson, L.E. PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. APMIS 2007, 115, 1400–1408. [Google Scholar] [CrossRef]
- Ebrahim-Saraie, H.S.; Nezhad, N.Z.; Heidari, H.; Motamedifar, A.; Motamedifar, M. Detection of Antimicrobial Susceptibility and Integrons Among Extended-spectrum β-lactamase Producing Uropathogenic Escherichia coli Isolates in Southwestern Iran. Oman Med. J. 2018, 33, 218–223. [Google Scholar] [CrossRef]
- Population Density in Bangkok, Thailand from 2014 to 2023. Available online: https://www.statista.com/statistics/1422857/thailand-population-density-in-bangkok/ (accessed on 20 January 2025).
- Galán-Relaño, Á.; Díaz, A.V.; Lorenzo, B.H.; Gómez-Gascón, L.; Rodríguez, M.Á.M.; Jiménez, E.C.; Rodríguez, F.P.; Márquez, R.J.A. Salmonella and Salmonellosis: An Update on Public Health Implications and Control Strategies. Animals 2023, 13, 3666. [Google Scholar] [CrossRef]
- Mohammad, M.B.; Saydur Rahman, M. Salmonella in the environment: A review on ecology, antimicrobial resistance, seafood contaminations, and human health implications. J. Hazard. Mater. Adv. 2024, 13, 100407. [Google Scholar] [CrossRef]
- Amin, N.; Rahman, M.; Raj, S.; Ali, S.; Green, J.; Das, S.; Doza, S.; Mondol, M.H.; Wang, Y.; Islam, M.A.; et al. Quantitative assessment of fecal contamination in multiple environmental sample types in urban communities in Dhaka, Bangladesh using SaniPath microbial approach. PLoS ONE 2019, 14, e0221193. [Google Scholar] [CrossRef]
- Luo, Z.; Gu, G.; Ginn, A.; Giurcanu, M.C.; Adams, P.; Vellidis, G.; van Bruggen, A.H.C.; Danyluk, M.D.; Wright, A.C.; Griffiths, M.W. Distribution and Characterization of Salmonella enterica Isolates from Irrigation Ponds in the Southeastern United States. Appl. Environ. Microbiol. 2015, 8, 13. [Google Scholar] [CrossRef]
- Mengistu, G.; Dejenu, G.; Tesema, C.; Arega, B.; Awoke, T.; Alemu, K.; Moges, F.; Clegg, S. Epidemiology of streptomycin resistant Salmonella from humans and animals in Ethiopia: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0244057. [Google Scholar] [CrossRef]
- Clinical Practice Guideline (CPG) of Tuberculosis Treatment in Thailand. Available online: https://www2.si.mahidol.ac.th/km/download/20796/?tmstv=1739241009 (accessed on 20 January 2025).
- Siltrakool, B.; Berrou, I.; Griffiths, D. Alghamdi. Antibiotics’ Use in Thailand: Community Pharmacists’ Knowledge, Attitudes and Practices. Antibiotics 2021, 10, 137. [Google Scholar] [CrossRef]
- Miller, S.A.; Ferreira, J.P.; LeJeune, J.T. Antimicrobial Use and Resistance in Plant Agriculture: A One Health Perspective. Agriculture 2022, 12, 289. [Google Scholar] [CrossRef]
- Assawatheptawee, K.; Tansawai, U.; Kiddee, A.; Thongngen, P.; Punyadi, P.; Romgaew, T.; Kongthai, P.; Sumpradit, T.; Niumsup, P.R. Occurrence of Extended-Spectrum and AmpC-Type β-Lactamase Genes in Escherichia coli Isolated from Water Environments in Northern Thailand. Microbes Environ. 2017, 32, 293–296. [Google Scholar] [CrossRef]
- Burjaq, S.Z.; Abu-Romman, S.M. Prevalence and Antimicrobial Resistance of Salmonella spp. From Irrigation Water in Two Major Sources in Jordan. Curr. Microbiol. 2020, 77, 3760–3766. [Google Scholar] [CrossRef]
- Talukder, H.; Roky, S.A.; Debnath, K.; Sharma, B.; Ahmed, J.; Roy, S. Prevalence and Antimicrobial Resistance Profile of Salmonella Isolated from Human, Animal and Environment Samples in South Asia: A 10-Year Meta-analysis. J. Epidemiol. Glob. Health 2023, 13, 637–652. [Google Scholar] [CrossRef]
- Sharma, N.C.; Kumar, D.; Sarkar, A.; Chowdhury, G.; Mukhopadhyay, A.K.; Ramamurthy, T. Prevalence of Multidrug Resistant Salmonellae with Increasing Frequency of Salmonella enterica Serovars Kentucky and Virchow among Hospitalized Diarrheal Cases in and around Delhi, India. Jpn. J. Infect. Dis. 2020, 73, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Kapley, A.; Sheeraz, M.S.; Kukade, S.; Ansari, A.; Qureshi, A.; Bajaj, A.; Khan, N.A.; Tandon, S.; Jain, R.; Dudhwadkar, S.; et al. Antibiotic resistance in wastewater: Indian scenario. Environ. Pollut. 2023, 337, 122586. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, C.G.; Macklin, K.S.; Kumar, S.; Bailey, M.; Ebner, P.; Oliver, H.; Martin-Gonzalez, F.; Singh, M. Prevalence and antimicrobial resistance of Salmonella isolated from poultry in the southeastern United States. Poult. Sci. 2021, 100, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Sheng, D.; Xu, H. Role of Escherichia coli strain subgroups, integrons, and integron-associated gene cassettes in dissemination of antimicrobial resistance in aquatic environments of Jinan, China. Water Sci. Technol. 2012, 66, 2385–2392. [Google Scholar] [CrossRef]
- Project to Develop and Promote Toilet and Waste Management for Safety in Sanitation and Health (In Thai). Available online: https://env.anamai.moph.go.th/th/kpi68/download?id=123434&mid=39402&mkey=m_document&lang=th&did=35932 (accessed on 20 January 2025).
- Mohamed, S.A.; Nyerere; Sang, W.K.; Ngayo, M. Bottled water brands are contaminated with multidrug resistant bacteria in Nairobi, Kenya. F1000Research 2021, 9, 1337. [Google Scholar] [CrossRef] [PubMed]
- Mölstad, S.; Löfmark, S.; Carlin, K.; Erntell, M.; Aspevall, O.; Blad, L.; Hanberger, H.; Hedin, K.; Hellman, J.; Norman, C.; et al. Lessons learnt during 20 years of the Swedish strategic programme against antibiotic resistance. Bull. World Health Organ. 2017, 95, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Swedish Strategy to Combat Antibiotic Resistance 2024–2025. Available online: https://www.government.se/contentassets/1fedc516373d421f919814f1963e2fe1/amr_strategi_eng_web_ny2.pdf (accessed on 20 November 2024).
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).