Prevalence of Antibiotic Resistance Bacteria in Manure, Soil, and Vegetables in Urban Blantyre, Malawi, from a Farm-to-Fork Perspective
Abstract
1. Introduction
2. Materials and Methods
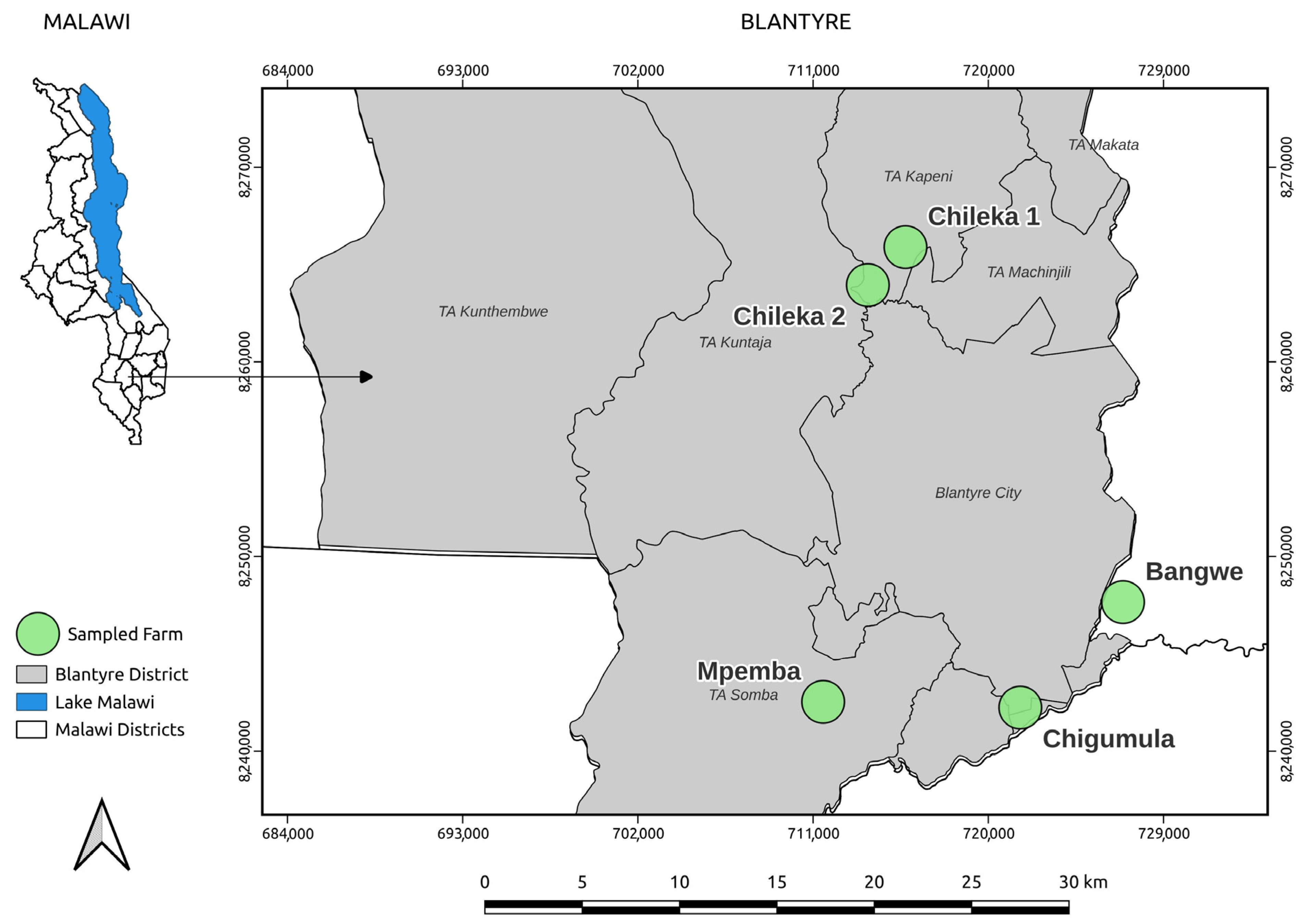
2.1. Study Area
2.1.1. Sample Size and Sampling Strategy
2.1.2. Farms Description
2.2. Sample Collection
2.2.1. Manure Samples Collection
2.2.2. Soil Samples Collection
2.2.3. Vegetable Samples Collection
2.3. Sample Preparation
2.3.1. Manure and Soil Sample Preparation
2.3.2. Vegetable Samples Preparation
2.4. Bacteria Culture Isolation
2.5. Antibiotic Susceptibility Tests
2.6. Data Analysis
3. Results and Discussion
3.1. Bacterial Identification in Samples
3.1.1. Bacterial Identification in Manure
3.1.2. Bacterial Identification in Soil Samples
3.1.3. Bacterial Identification in Vegetables
3.2. Antibiotic Resistance
3.2.1. Antibiotic Resistance in Chicken and Pig Manures
3.2.2. Antibiotic Resistance in Soils
3.2.3. Antibiotic Resistance in Vegetables
| Microorganism | Manure Type | Tests (n) | SXT | CIP | TGC | CTX | AMP | GM | MEM | ATM | CRO | VAN | Resistance Pattern |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acinetobacter spp. | Chicken | 6 | 100% (21–100) | 0% (0–79) | NT | NT | NT | 0% (0–79) | NT | 0% (0–79) | 0% (0–79) | 100% (21–100) | SXT-VAN |
| Pig | 6 | 100% (21–100) | 0% (0–79) | NT | NT | NT | 0% (0–79) | NT | NT | 100% (21–100) | 100% (21–100) | SXT–CRO–VAN | |
| E. coli | Chicken | 18 | 67% (21–94) | 0% (0–56) | NT | 50% (9–91) | 50% (9–91) | 33% (6–79) | NT | 100% (21–100) | NT | 100% (21–100) | ATM–VAN |
| Pig | 24 | 75% (30–95) | 0% (0–49) | 0% (0–66) | 0% (0–66) | 100% (34–100) | 25% (5–70) | NT | 0% (0–66) | 50% (9–91) | 100% (34–100) | SXT-AMP–VAN | |
| K. pneumoniae | Chicken | 12 | 100% (34–100) | 0% (0–66) | 50% (9–91) | NT | 100% (34–100) | 100% (34–100) | NT | NT | NT | NT | SXT–AMP–GM |
| Pig | 12 | 100% (34–100) | 0% (0–49) | 100% (34–100) | NT | 100% (34–100) | 100% (34–100) | NT | NT | NT | NT | SXT–TGC–AMP–GM |
| Microorganism | Soil Type | Tests (n) | SXT | CIP | TGC | CTX | AMP | GM | ATM | CRO | VAN | Critical Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | Farm | 30 | 60% (23–88) | 40% (12–77) | 0% (0–49) | 75% (30–95) | 75% (30–95) | 40% (12–77) | 0% (0–79) | 0% (0–79) | 0% (0–79) | AMP-CTX |
| K. pneumoniae | Farm | 24 | 100% (51–100) | 25% (5–70) | 50% (15–85) | 50% (15–85) | 100% (51–100) | 100% (51–100) | NT | NT | NT | AMP-GM-SXT |
| Home | 36 | 100% (61–100) | 33% (10–70) | 33% (10–70) | 0% (0–39) | 100% (61–100) | 100% (61–100) | NT | NT | NT | AMP-SXT-GM | |
| Salmonella cholerae | Home | 6 | 100% (21–100) | 0% (0–79) | NT | NT | NT | 100% (21–100) | 0% (0–79) | 0% (0–79) | 100% (21–100) | Pan-resistance |
| Stenotrophomonas spp. | Home | 12 | 0% (0–79) | 0% (0–79) | NT | NT | NT | 0% (0–79) | 0% (0–79) | 0% (0–79) | 100% (21–100) | Carbapenem resistance (VAN) |
| Microorganism | Vegetable | Test (n) | SXT | CIP | TGC | CTX | AMP | GM | ATM | CRO | VAN | Resistance Hotspot |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | Amaranthus spp. | 6 | 100% (21–100) | 0% (0–79) | 0% (0–79) | 0% (0–79) | 100% (21–100) | 100% (21–100) | NT | NT | NT | SXT-AMP-GM |
| Brassica rapa subsp. Chinensis | 6 | 100% (21–100) | 0% (0–79) | 0% (0–79) | 0% (0–79) | 100% (21–100) | 100% (21–100) | NT | NT | NT | SXT-AMP-GM | |
| K. pneumoniae | Amaranthus spp. | 18 | 67% (21–94) | 33% (6–79) | 33% (6–79) | 33% (6–79) | 100% (44–100) | 67% (21–94) | NT | NT | NT | AMP |
| Brassica rapa subsp. Chinensis | 18 | 67% (21–94) | 0% (0–56) | 33% (6–79) | 0% (0–56) | 100% (44–100) | 67% (21–94) | NT | NT | NT | AMP | |
| Brassica rapa | 36 | 83% (44–97) | 33% (10–70) | 20% (4–62) | 0% (0–43) | 100% (57–100) | 67% (30–90) | 100% (21–100) | 0% (0–79) | 100% (21–100) | AMP-SXT-GM-VAN | |
| Pseudomonascepacia | Brassica rapa | 26 | 100% (21–100) | 33% (6–79) | NT | 100% (34–100) | NT | 100% (44–100) | 100% (44–100) | 0% (0–56) | 100% (21–100) | SXT-CTX-GM-ATM-VAN |
3.3. Microbial Transmission Pathways
3.4. One Health Implications: The Silent AMR Crisis on Malawi’s Plate
3.4.1. Human Health Implications
3.4.2. Environmental Health Implications
3.4.3. Animal Health Implications
3.5. Study Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1
| Antibiotic | Microbe Diversity N = 18 n (%) | Number of Tests N = 370 n (%) | Mean Inhibition Zone | Sensitivity Distribution | ||
|---|---|---|---|---|---|---|
| I | R | S | ||||
| AMP | 4 (22) | 39 (10.54) | 7.153846 | 0 | 37 | 2 |
| ATM | 14 (78) | 26 (7.03) | 15.576923 | 0 | 11 | 15 |
| CIP | 18 (100) | 62 (16.76) | 25.419355 | 14 | 9 | 39 |
| CRO | 15 (83) | 27 (7.30) | 18.296296 | 6 | 5 | 16 |
| CTX | 9 (50) | 44 (11.89) | 20.613636 | 0 | 12 | 32 |
| GM | 18 (100) | 65 (17.57 | 12.307692 | 1 | 40 | 24 |
| MEM | 2 (11) | 2 (0.54) | 24.000000 | 0 | 0 | 2 |
| SXT | 12 (67) | 53 (14.32) | 8.566038 | 0 | 43 | 10 |
| TGC | 6 (33) | 40 (10.81) | 18.925000 | 1 | 12 | 27 |
| VAN | 7 (39) | 12 (3.24) | 2.500000 | 1 | 11 | 0 |
Appendix A.2
| Sample Type | Microbe | I | R | S |
|---|---|---|---|---|
| Chicken manure | Acinetobacter junni | 1 | 1 | 2 |
| Acinetobacter spp. | 1 | 2 | 3 | |
| E. coli | 0 | 7 | 11 | |
| K. pneumoniae | 1 | 7 | 4 | |
| Serratia odoniferia | 1 | 0 | 4 | |
| Farm soil | E. coli | 1 | 13 | 16 |
| K. pneumoniae | 2 | 17 | 5 | |
| Pseudomonas luteola | 1 | 0 | 4 | |
| Pseudomonas spp. | 1 | 2 | 2 | |
| Serratia odoniferia | 0 | 2 | 3 | |
| Xanthomonas maltophilia | 1 | 4 | 7 | |
| Y. pestis | 0 | 2 | 2 | |
| Home soil | K. pneumoniae | 4 | 22 | 10 |
| S. choleraesuis | 0 | 3 | 3 | |
| Stenotrophomonas | 0 | 1 | 5 | |
| S. maltophilia | 0 | 3 | 3 | |
| Pig manure | Acinetobacter pitii | 1 | 2 | 1 |
| Acinetobacter spp. | 0 | 3 | 3 | |
| E. coli | 1 | 9 | 14 | |
| K. pneumoniae | 0 | 8 | 4 | |
| Vegetables | E. coli | 1 | 6 | 5 |
| Erwinia spp. | 0 | 0 | 5 | |
| K. pneumoniae | 3 | 37 | 32 | |
| Pasteurella spp. | 2 | 15 | 7 | |
| Pseudomonas aeruginosa | 0 | 1 | 4 | |
| Pseudomonas cepacia | 1 | 11 | 4 | |
| Raoultella ornitholytica | 0 | 2 | 4 |
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef]
- Vezeau, N.; Kahn, L. Current understanding and knowledge gaps regarding wildlife as reservoirs of antimicrobial resistance. Am. J. Vet. Res. 2024, 85. [Google Scholar] [CrossRef]
- Dolejska, M.; Literak, I. Wildlife is Overlooked in the Epidemiology of Medically Important Antibiotic-Resistant Bacteria. Antimicrob. Agents Chemother. 2019, 63, e01167-19. [Google Scholar] [CrossRef]
- Ngunguni, S.M.; Moodley, A.; Msefula, C.; Mkakosya, R.; Muloi, D.M. Patterns and drivers of antibiotic use in small-scale broiler production systems in Lilongwe District, Malawi. Prev. Vet. Med. 2024, 230, 106263. [Google Scholar] [CrossRef]
- Chidziwisano, K.; Cocker, D.; Mwapasa Kumwenda, T.; Amos, S.; Feasey, N.; Morse, T. Risk Perception and Psychosocial Factors Influencing Exposure to Antimicrobial Resistance through Environmental Pathways in Malawi. Am. J. Trop. Med. Hyg. 2025, 112, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Musa, L.; Stefanetti, V.; Casagrande Proietti, P.; Grilli, G.; Gobbi, M.; Toppi, V.; Brustenga, L.; Magistrali, C.F.; Franciosini, M.P. Antimicrobial Susceptibility of Commensal E. coli Isolated from Wild Birds in Umbria (Central Italy). Animals 2023, 13, 1776. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, J.; Wang, J.; Zhu, L.; Zhang, W.; Zhao, X.; Ahmad, Z. Growth Inhibiting Effects of Four Antibiotics on Cucumber, Rape and Chinese Cabbage. Bull. Environ. Contam. Toxicol. 2019, 103, 187–192. [Google Scholar] [CrossRef]
- Zalewska, M.; Błażejewska, A.; Czapko, A.; Popowska, M. Antibiotics and Antibiotic Resistance Genes in Animal Manure—Consequences of Its Application in Agriculture. Front. Microbiol. 2021, 12, 610656. [Google Scholar] [CrossRef]
- Lee, E.; New, C.Y.; Thung, T.Y.; Tan, C.W.; Wendy, R.D.; Nuzul, N.J.; Radu, S.; Abdul-Mutalib, N. Detection and antibiotic resistance profile of extended-spectrum betalactamase—Producing Escherichia coli in raw vegetables. Food Res. 2023, 7, 45–54. [Google Scholar] [CrossRef]
- Usui, M.; Ozeki, K.; Komatsu, T.; Fukuda, A.; Tamura, Y. Prevalence of Extended-Spectrum β-Lactamase–Producing Bacteria on Fresh Vegetables in Japan. J. Food Prot. 2019, 82, 1663–1666. [Google Scholar] [CrossRef]
- Hölzel, C.S.; Tetens, J.L.; Schwaiger, K. Unraveling the Role of Vegetables in Spreading Antimicrobial-Resistant Bacteria: A Need for Quantitative Risk Assessment. Foodborne Pathog. Dis. 2018, 15, 671–688. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Fernández-Calviño, D.; Arias-Estévez, M. Tetracycline and Sulfonamide Antibiotics in Soils: Presence, Fate and Environmental Risks. Processes 2020, 8, 1479. [Google Scholar] [CrossRef]
- Ren, J.; Shi, H.; Liu, J.; Zheng, C.; Lu, G.; Hao, S.; Jin, Y.; He, C. Occurrence, source apportionment and ecological risk assessment of thirty antibiotics in farmland system. J. Environ. Manag. 2023, 335, 117546. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, A.; Olatunji, D.; Bayode, O. Occurrence of veterinary antibiotics in poultry manure from two farms in Ibadan, Nigeria: Ecotoxicological implications in manure-amended soil. Environ. Anal. Health Toxicol. 2022, 37, e2022038. [Google Scholar] [CrossRef]
- Patyra, E.; Nebot, C.; Gavilán, R.E.; Kwiatek, K.; Cepeda, A. Prevalence of veterinary antibiotics in natural and organic fertilizers from animal food production and assessment of their potential ecological risk. J. Sci. Food. Agric. 2023, 103, 3638–3644. [Google Scholar] [CrossRef]
- Rahman, M.; Alam, M.-U.; Luies, S.K.; Kamal, A.; Ferdous, S.; Lin, A.; Sharior, F.; Khan, R.; Rahman, Z.; Parvez, S.M.; et al. Contamination of Fresh Produce with Antibiotic-Resistant Bacteria and Associated Risks to Human Health: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 19, 360. [Google Scholar] [CrossRef] [PubMed]
- Materechera, S.A. Utilization and management practices of animal manure for replenishing soil fertility among smallscale crop farmers in semi-arid farming districts of the North West Province, South Africa. Nutr. Cycl. Agroecosyst. 2010, 87, 415–428. [Google Scholar] [CrossRef]
- Zuza, E.J.; Maseyk, K.; Bhagwat, S.A.; Chemura, A.; Brandenburg, R.L.; Emmott, A.; Rawes, W.; Hancock, W.; Mnthambala, F.; Araya, Y.N. Factors affecting soil quality among smallholder macadamia farms in Malawi. Agric. Food Secur. 2023, 12, 17. [Google Scholar] [CrossRef]
- Cai, T.; Steinfield, C.; Chiwasa, H.; Ganunga, T. Understanding Malawian farmers’ slow adoption of composting: Stories about composting using a participatory video approach. Land Degrad. Dev. 2019, 30, 1336–1344. [Google Scholar] [CrossRef]
- MacPherson, E.E.; Mankhomwa, J.; Dixon, J.; Pongolani, R.; Phiri, M.; Feasey, N.; O’bYrne, T.; Tolhurst, R.; MacPherson, P.; Hlongwa, M. Household antibiotic use in Malawi: A cross-sectional survey from urban and peri-urban Blantyre. PLOS Glob. Public Health 2023, 3, e0001946. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health. Malawi: Antimicrobial Resistance Strategy; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/publications/m/item/malawi-antimicrobial-resistance-strategy-2017-2022 (accessed on 22 February 2024).
- Banda, B.V.; Mapoma, H.W.T.; Thole, B. Investigating Antibiotic Susceptibility of Pathogenic Micro-Organisms in Groundwater from Boreholes and Shallow Wells in T/A Makhwira, Chikwawa. Microbiol. Res. 2025, 16, 137. [Google Scholar] [CrossRef]
- Malawi Department of Climate Change Meteorological Services. CLIMATE OF MALAWI. Malawi Meteorol Serv 2025. Available online: https://www.metmalawi.com/climate/climate.php (accessed on 9 July 2025).
- Xu, P.; Zhou, X.; Xu, D.; Xiang, Y.; Ling, W.; Chen, M. Contamination and Risk Assessment of Estrogens in Livestock Manure: A Case Study in Jiangsu Province, China. Int. J. Environ. Res. Public Health 2018, 15, 125. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Lu, C.; Liao, Q.; Gudda, F.O.; Ling, W. Antibiotics in animal manure and manure-based fertilizers: Occurrence and ecological risk assessment. Chemosphere 2020, 255, 127006. [Google Scholar] [CrossRef]
- Tadić, Đ.; Bleda Hernandez, M.J.; Cerqueira, F.; Matamoros, V.; Piña, B.; Bayona, J.M. Occurrence and human health risk assessment of antibiotics and their metabolites in vegetables grown in field-scale agricultural systems. J. Hazard. Mater. 2021, 401, 123424. [Google Scholar] [CrossRef] [PubMed]
- Marti, R.; Scott, A.; Tien, Y.-C.; Murray, R.; Sabourin, L.; Zhang, Y.; Topp, E. Impact of Manure Fertilization on the Abundance of Antibiotic-Resistant Bacteria and Frequency of Detection of Antibiotic Resistance Genes in Soil and on Vegetables at Harvest. Appl. Environ. Microbiol. 2013, 79, 5701–5709. [Google Scholar] [CrossRef]
- Jauregi, L.; Epelde, L.; Alkorta, I.; Garbisu, C. Antibiotic Resistance in Agricultural Soil and Crops Associated to the Application of Cow Manure-Derived Amendments From Conventional and Organic Livestock Farms. Front. Vet. Sci. 2021, 8, 633858. [Google Scholar] [CrossRef]
- Yévenes, K.; Pokrant, E.; Trincado, L.; Lapierre, L.; Galarce, N.; Martín, B.S.; Maddaleno, A.; Hidalgo, H.; Cornejo, J. Detection of Antimicrobial Residues in Poultry Litter: Monitoring a Risk through a Selective and Sensitive HPLC–MS/MS Method. Animals 2021, 11, 1399. [Google Scholar] [CrossRef]
- Cobbina, S.J.; Kotochi, M.C.; Korese, J.K.; Akrong, M.O. Microbial Contamination in Vegetables at the Farm Gate Due to Irrigation with Wastewater in the Tamale Metropolis of Northern Ghana. J. Environ. Prot. 2013, 4, 676–682. [Google Scholar] [CrossRef][Green Version]
- Degaga, B.; Sebsibe, I.; Belete, T.; Asmamaw, A. Microbial Quality and Safety of Raw Vegetables of Fiche Town, Oromia, Ethiopia. J. Environ. Public Health 2022, 2022, 2556858. [Google Scholar] [CrossRef]
- Tien, Y.-C.; Li, B.; Zhang, T.; Scott, A.; Murray, R.; Sabourin, L.; Marti, R.; Topp, E. Impact of dairy manure pre-application treatment on manure composition, soil dynamics of antibiotic resistance genes, and abundance of antibiotic-resistance genes on vegetables at harvest. Sci. Total Environ. 2017, 581–582, 32–39. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 24th ed.; American Water Works Association: Washington, DC, USA, 2023. [Google Scholar]
- Bonadonna, L.; Briancesco, R.; La Rosa, G. Innovative analytical methods for monitoring microbiological and virological water quality. Microchem. J. 2019, 150, 104160. [Google Scholar] [CrossRef]
- Alemu, G.; Mama, M.; Siraj, M. Bacterial contamination of vegetables sold in Arba Minch Town, Southern Ethiopia. BMC Res. Notes 2018, 11, 775. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. M100|Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
- Redman-White, C.J.; Moran, D.; Peters, A.R.; Muwonge, A. A review of the predictors of antimicrobial use and resistance in European food animal production. Front. Antibiot. 2023, 2, 1209552. [Google Scholar] [CrossRef] [PubMed]
- Khairullah, A.R.; Moses, I.B.; Yanestria, S.M.; Eka Puji Dameanti, F.N.A.; Effendi, M.H.; Huat Tang, J.Y.; Tyasningsih, W.; Budiastuti, B.; Kusala, M.; Kurniasih, D.; et al. Potential of the livestock industry environment as a reservoir for spreading antimicrobial resistance. Open Vet. J. 2025, 15, 504–518. [Google Scholar] [CrossRef]
- Mankhomwa, J.; Tolhurst, R.; M’biya, E.; Chikowe, I.; Banda, P.; Mussa, J.; Mwasikakata, H.; Simpson, V.; Feasey, N.; MacPherson, E.E. A Qualitative Study of Antibiotic Use Practices in Intensive Small-Scale Farming in Urban and Peri-Urban Blantyre, Malawi: Implications for Antimicrobial Resistance. Front. Vet. Sci. 2022, 9, 876543. [Google Scholar] [CrossRef]
- Gemeda, B.A.; Amenu, K.; Magnusson, U.; Dohoo, I.; Hallenberg, G.S.; Alemayehu, G.; Desta, H.; Wieland, B. Antimicrobial Use in Extensive Smallholder Livestock Farming Systems in Ethiopia: Knowledge, Attitudes, and Practices of Livestock Keepers. Front. Vet. Sci. 2020, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Morgan, J.; Doyle, M.P. Fate of Escherichia coli O157:H7 in Manure-Amended Soil. Appl. Environ. Microbiol. 2002, 68, 2605–2609. [Google Scholar] [CrossRef]
- Yao, Z.; Yang, L.; Wang, H.; Wu, J.; Xu, J. Fate of Escherichia coli O157: H7 in agricultural soils amended with different organic fertilizers. J. Hazard. Mater. 2015, 296, 30–36. [Google Scholar] [CrossRef]
- Ngogang, M.P.; Ernest, T.; Kariuki, J.; Mouliom Mouiche, M.M.; Ngogang, J.; Wade, A.; van der Sande, M.A.B. Microbial Contamination of Chicken Litter Manure and Antimicrobial Resistance Threat in an Urban Area Setting in Cameroon. Antibiotics 2020, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Igbinosa, E.O.; Beshiru, A.; Igbinosa, I.H.; Cho, G.-S.; Franz, C.M.A.P. Multidrug-resistant extended spectrum β-lactamase (ESBL)-producing Escherichia coli from farm produce and agricultural environments in Edo State, Nigeria. PLoS ONE 2023, 18, e0282835. [Google Scholar] [CrossRef] [PubMed]
- Takawira, F.T.; Pitout, J.D.D.; Thilliez, G.; Mashe, T.; Gutierrez, A.V.; Kingsley, R.A.; Peirano, G.; Matheu, J.; Midzi, S.M.; Mwamakamba, L.W.; et al. Faecal carriage of ESBL producing and colistin resistant Escherichia coli in avian species over a 2-year period (2017–2019) in Zimbabwe. Front. Cell. Infect. Microbiol. 2022, 12, 1035145. [Google Scholar] [CrossRef]
- Hou, J.; Wan, W.; Mao, D.; Wang, C.; Mu, Q.; Qin, S.; Luo, Y. Occurrence and distribution of sulfonamides, tetracyclines, quinolones, macrolides, and nitrofurans in livestock manure and amended soils of Northern China. Environ. Sci. Pollut. Res. 2015, 22, 4545–4554. [Google Scholar] [CrossRef]
- Agyare, C.; Etsiapa Boamah, V.; Ngofi Zumbi, C.; Boateng Osei, F. Antibiotic Use in Poultry Production and Its Effects on Bacterial Resistance; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Cruz-Córdova, A.; Esteban-Kenel, V.; Espinosa-Mazariego, K.; Ochoa, S.A.; Moreno Espinosa, S.; de la Garza Elhain, A.; Rendón, E.F.; Villegas, E.O.L.; Xicohtencatl-Cortes, J. Pathogenic athogenic determinants of clinical K. pneumoniae strains associated with their persistence in the hospital environment. Bol. Méd. Hosp. Infant México 2014, 71, 15–24. [Google Scholar]
- Junaid, K.; Ejaz, H.; Younas, S.; Alanazi, A.; Yasmeen, H.; Rehman, A. Detection of K. pneumoniae antibiotic-resistant genes: An impending source of multidrug resistance dissemination through raw food. Saudi J. Biol. Sci. 2022, 29, 3347–3353. [Google Scholar] [CrossRef]
- Pantel, A.; Richaud-Morel, B.; Cazaban, M.; Bouziges, N.; Sotto, A.; Lavigne, J.-P. Environmental persistence of OXA-48-producing K. pneumoniae in a French intensive care unit. Am. J. Infect. Control 2016, 44, 366–368. [Google Scholar] [CrossRef]
- Ndlovu, T.; Kgosietsile, L.; Motshwarakgole, P.; Ndlovu, S.I. Evaluation of Potential Factors Influencing the Dissemination of Multidrug-Resistant K. pneumoniae and Alternative Treatment Strategies. Trop. Med. Infect. Dis. 2023, 8, 381. [Google Scholar] [CrossRef]
- Kagambèga, A.B.; Dembélé, R.; Traoré, O.; Wane, A.A.; Mohamed, A.H.; Coulibaly, H.; Fall, C.; Bientz, L.; M’zali, F.; Mayonnove, L.; et al. Isolation and Characterization of Environmental Extended Spectrum β-Lactamase-Producing Escherichia coli and K. pneumoniae from Ouagadougou, Burkina Faso. Pharmacy 2024, 17, 305. [Google Scholar] [CrossRef] [PubMed]
- Diarra, F.B.J.; Bonkoungou, I.J.O.; Garba, Z.; Somda, N.S.; Soma, D.; Nikiema, M.E.M.; Bako, E.; Sore, S.; Sawadogo, N.; Barro, N.; et al. One Health Approach to Study the Occurrence and Antimicrobial Resistance of Extended-Spectrum β-Lactamase- and Carbapenemase-Producing Escherichia coli and Klebsiella spp. in Urban Agriculture in Burkina Faso. Microorganisms 2024, 12, 2170. [Google Scholar] [CrossRef] [PubMed]
- Kimera, Z.I.; Mgaya, F.X.; Mshana, S.E.; Karimuribo, E.D.; Matee, M.I.N. Occurrence of Extended Spectrum Beta Lactamase (ESBL) Producers, Quinolone and Carbapenem Resistant Enterobacteriaceae Isolated from Environmental Samples along Msimbazi River Basin Ecosystem in Tanzania. Int. J. Environ. Res. Public Health 2021, 18, 8264. [Google Scholar] [CrossRef]
- van Elsas, J.D.; Semenov, A.V.; Costa, R.; Trevors, J.T. Survival of Escherichia coli in the environment: Fundamental and public health aspects. ISME J. 2011, 5, 173–183. [Google Scholar] [CrossRef]
- Weber-Dąbrowska, B.; Żaczek, M.; Łobocka, M.; Łusiak-Szelachowska, M.; Owczarek, B.; Orwat, F.; Łodej, N.; Skaradzińska, A.; Łaczmański, Ł.; Martynowski, D.; et al. Characteristics of Environmental K. pneumoniae and Klebsiella oxytoca Bacteriophages and Their Therapeutic Applications. Pharmaceutics 2023, 15, 434. [Google Scholar] [CrossRef]
- Jacob, J.J.; Aravind, V.; Beresford-Jones, B.S.; Lal, Y.B.; Shankar, C.; Yesudoss, M.; Abdullah, F.; Priya, T.M.; Kulkarni, S.; Baker, S.; et al. Limited evidence of spill over of antimicrobial resistant Klebsiella pneumoniae from animal/environmental reservoirs to humans in India. medRxiv 2024. [Google Scholar] [CrossRef]
- Chomel, B.B.; Sykes, J.E. Y. pestis (Plague) and Other Yersinioses. In Greene’s Infectious Diseases of the Dog and Cat, 5th ed.; Sykes, J.E., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2021; pp. 905–915. [Google Scholar] [CrossRef]
- Loggenberg, S.R.; Twilley, D.; De Canha, M.N.; Lall, N. Chapter 4-Medicinal plants used in South Africa as antibacterial agents for wound healing. In Medicinal Plants as Anti-Infectives; Chassagne, F., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 139–182. [Google Scholar] [CrossRef]
- Richter, L.; Du Plessis, E.M.; Duvenage, S.; Korsten, L. Occurrence, Identification, and Antimicrobial Resistance Profiles of Extended-Spectrum and AmpC β-Lactamase-Producing Enterobacteriaceae from Fresh Vegetables Retailed in Gauteng Province, South Africa. Foodborne Pathog. Dis. 2019, 16, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Soré, S.; Sawadogo, Y.; Bonkoungou, J.I.; Kaboré, S.P.; Béogo, S.; Sawadogo, C.; Bationo, B.G.; Ky, H.; Madingar, P.D.-M.; Ouédraogo, A.S.; et al. Detection, identification and characterization of extended-spectrum beta-lactamases producing Enterobacteriaceae in wastewater and salads marketed in Ouagadougou, Burkina Faso. Int. J. Biol. Chem. Sci. 2020, 14, 2746–2757. [Google Scholar] [CrossRef]
- Kayombo, M.C.; Mayo, A.W. Assessment of Microbial Quality of Vegetables Irrigated with Polluted Waters in Dar es Salaam City, Tanzania. Environ. Ecol. Res. 2018, 6, 229–239. [Google Scholar] [CrossRef]
- Palma-Salgado, S.; Ku, K.-M.; Dong, M.; Nguyen, T.H.; Juvik, J.A.; Feng, H. Adhesion and removal of E. coli K12 as affected by leafy green produce epicuticular wax composition, surface roughness, produce and bacterial surface hydrophobicity, and sanitizers. Int. J. Food Microbiol. 2020, 334, 108834. [Google Scholar] [CrossRef]
- Doan, H.K.; Antequera-Gómez, M.L.; Parikh, A.N.; Leveau, J.H.J. Leaf Surface Topography Contributes to the Ability of Escherichia coli on Leafy Greens to Resist Removal by Washing, Escape Disinfection with Chlorine, and Disperse Through Splash. Front. Microbiol. 2020, 11, 1485. [Google Scholar] [CrossRef]
- Truschi, S.; Marini, L.; Cacciari, I.; Baldi, A.; Bruschi, P.; Lenzi, A.; Baales, J.; Zeisler-Diehl, V.V.; Schreiber, L.; Marvasi, M. Relationship between Salmonella enterica attachment and leaf hydrophobicity, roughness, and epicuticular waxes: A focus on 30 baby-leaf salads. J. Sci. Food Agric. 2024, 104, 9287–9296. [Google Scholar] [CrossRef]
- Song, H.J.; Kim, M.H.; Ku, K.-M. A Double-Edged Sword of Surfactant Effect on Hydrophobic Surface Broccoli Leaf as a Model Plant: Promotion of Pathogenic Microbial Contamination and Improvement to Disinfection Efficiency of Ozonated Water. Processes 2021, 9, 679. [Google Scholar] [CrossRef]
- Elshafiee, E.A.; Kadry, M.; Nader, S.M.; Ahmed, Z.S. Extended-spectrum-beta-lactamases and carbapenemase-producing K. pneumoniae isolated from fresh produce farms in different governorates of Egypt. Vet. World 2022, 15, 1191–1196. [Google Scholar] [CrossRef]
- Falomir, M.P.; Rico, H.; Gozalbo, D. Enterobacter and Klebsiella species isolated from fresh vegetables marketed in Valencia (Spain) and their clinically relevant resistances to chemotherapeutic agents. Foodborne Pathog. Dis. 2013, 10, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hua, Y.; Deng, L. Nutrient Status and Contamination Risks from Digested Pig Slurry Applied on a Vegetable Crops Field. Int. J. Environ. Res. Public Health 2016, 13, 406. [Google Scholar] [CrossRef] [PubMed]
- Atidégla, S.C.; Huat, J.; Agbossou, E.K.; Saint-Macary, H.; Glèlè Kakai, R. Vegetable Contamination by the Fecal Bacteria of Poultry Manure: Case Study of Gardening Sites in Southern Benin. Int. J. Food Sci. 2016, 2016, 4767453. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.C.; Maia, C.M.; Carvalho, I.D.; da Silva, N.F.; André, M.C.D.P.B.; Serafini, Á.B. Microbiological quality of organic vegetables produced in soil treated with different types of manure and mineral fertilizer. Braz. J. Microbiol. 2006, 37, 538–544. [Google Scholar] [CrossRef]
- Alifragki, A.; Kontogianni, A.; Protopapa, I.; Baliou, S.; Ioannou, P. Infective Endocarditis by Pasteurella Species: A Systematic Review. J. Clin. Med. 2022, 11, 5037. [Google Scholar] [CrossRef]
- Cooke, F.J.; Slack, M.P.E. 183-Gram-Negative Coccobacilli. In Infectious Diseases, 5th ed.; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1611–1627.e1. [Google Scholar] [CrossRef]
- Wilson, B.A.; Ho, M. Pasteurella multocida: From Zoonosis to Cellular Microbiology. Clin. Microbiol. Rev. 2013, 26, 631–655. [Google Scholar] [CrossRef]
- Brook, I. Microbiology and management of human and animal bite wound infections. Prim. Care Clin. Off. Pract. 2003, 30, 25–39. [Google Scholar] [CrossRef]
- Craig, M. Combination Therapy Successfully Treats Pasteurella Multocida-Related Invasive Infections. News-Med. 2023. Available online: https://www.news-medical.net/news/20230604/Combination-therapy-successfully-treats-Pasteurella-multocida-related-invasive-infections.aspx (accessed on 22 June 2025).
- Piorunek, M.; Brajer-Luftmann, B.; Walkowiak, J. Pasteurella Multocida Infection in Humans. Pathogens 2023, 12, 1210. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A. A spotlight on Raoultella ornithinolytica: A newly emerging life-threatening zoonotic pathogen. Int. J. One Health 2021, 7, 1–5. [Google Scholar] [CrossRef]
- Hajjar, R.; Ambaraghassi, G.; Sebajang, H.; Schwenter, F.; Su, S.-H. Raoultella ornithinolytica: Emergence and Resistance. Infect. Drug Resist. 2020, 13, 1091–1104. [Google Scholar] [CrossRef]
- Luo, J.; Yao, X.; Lv, L.; Doi, Y.; Huang, X.; Huang, S.; Liu, J.-H. Emergence of mcr-1 in Raoultella ornithinolytica and Escherichia coli Isolates from Retail Vegetables in China. Antimicrob. Agents Chemother. 2017, 61, e01139-17. [Google Scholar] [CrossRef]
- Al-Hulu, S.M.; Al-Charrakh, A.H. Isolation and characterization of Raoultella ornithinolytica from Clinical Specimens in Hilla City, Iraq. Hist. Relig. 2009, 5, 365–375. [Google Scholar]
- Opara, E.U.; Asuquo, A.A. An Overview of Characterization and Identification of Soft Rot Bacterium Erwinia in Some Vegetable Crops. Greener J. Biol. Sci. 2016, 6, 46–55. [Google Scholar] [CrossRef]
- Phokum, C.; Jitareerat, P.; Photchanachai, S.; Cheevadhanarak, S. Detection and Classification of Soft Rot Erwinia of Vegetables in Thailand by DNA Polymerase Chain Reaction. Acta Hortic. 2006, 712, 917–926. [Google Scholar] [CrossRef]
- Wakil Monilola, S.; Oyinlola Abiola, K. Diversity of pectinolytic bacteria causing soft rot disease of vegetables in Ibadan, Nigeria. J. Appl. Biosci. 2011, 38, 2540–2550. [Google Scholar]
- Ruiz-Roldán, L.; Rojo-Bezares, B.; Lozano, C.; López, M.; Chichón, G.; Torres, C.; Sáenz, Y. Occurrence of Pseudomonas spp. in Raw Vegetables: Molecular and Phenotypical Analysis of Their Antimicrobial Resistance and Virulence-Related Traits. Int. J. Mol. Sci. 2021, 22, 12626. [Google Scholar] [CrossRef]
- Tavares, M.; Kozak, M.; Balola, A.; Sá-Correia, I. Burkholderia cepacia Complex Bacteria: A Feared Contamination Risk in Water-Based Pharmaceutical Products. Clin. Microbiol. Rev. 2020, 33, e00139-19. [Google Scholar] [CrossRef]
- Holmes, A.; Govan, J.; Goldstein, R. Agricultural use of Burkholderia (Pseudomonas) cepacia: A threat to human health? Emerg. Infect. Dis. 1998, 4, 221–227. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, T.J.; Quinn, J.P.; Bettermann, A.; Bookland, R. Pseudomonas cepacia suppression of sunflower wilt fungus and role of antifungal compounds in controlling the disease. Appl. Environ. Microbiol. 1992, 58, 1760–1763. [Google Scholar] [CrossRef]
- Iglewski, B.H. Pseudomonas. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Allydice-Francis, K.; Brown, P.D. Diversity of Antimicrobial Resistance and Virulence Determinants in Pseudomonas aeruginosa Associated with Fresh Vegetables. Int. J. Microbiol. 2012, 2012, 426241. [Google Scholar] [CrossRef]
- Li, X.; Gu, N.; Huang, T.Y.; Zhong, F.; Peng, G. Pseudomonas aeruginosa: A typical biofilm forming pathogen and an emerging but underestimated pathogen in food processing. Front. Microbiol. 2023, 13, 1114199. [Google Scholar] [CrossRef] [PubMed]
- Ambreetha, S.; Marimuthu, P.; Mathee, K.; Balachandar, D. Rhizospheric and endophytic Pseudomonas aeruginosa in edible vegetable plants share molecular and metabolic traits with clinical isolates. J. Appl. Microbiol. 2022, 132, 3226–3248. [Google Scholar] [CrossRef] [PubMed]
- Kot, B.; Witeska, M. Review: Antimicrobial resistance of K. pneumoniae isolated from poultry, cattle and pigs. Animal 2024, 18, 101345. [Google Scholar] [CrossRef] [PubMed]
- Mourão, J.; Ribeiro-Almeida, M.; Novais, C.; Magalhães, M.; Rebelo, A.; Ribeiro, S.; Peixe, L.; Novais, Â.; Antunes, P.; Oladeinde, A. From Farm to Fork: Persistence of Clinically Relevant Multidrug-Resistant and Copper-Tolerant K. pneumoniae Long after Colistin Withdrawal in Poultry Production. Microbiol. Spectr. 2023, 11, e01386-23. [Google Scholar] [CrossRef]
- Chisembe, P.; Suzuki, M.; Dao, D.T.; Njunga, G.; Nkhoma, J.; Mthilakuwili, L.; Kinoshita-Daitoku, R.; Kuroda, E.; Kimura, K.; Shibayama, K. A nationwide survey of antimicrobial resistance of Escherichia coli isolated from broiler chickens in Malawi. JAC-Antimicrob. Resist. 2024, 6, dlae200. [Google Scholar] [CrossRef]
- Sinyawa, T.; Shawa, M.; Muuka, G.M.; Goma, F.; Fandamu, P.; Chizimu, J.Y.; Khumalo, C.S.; Mulavu, M.; Ngoma, M.; Chambaro, H.M.; et al. Antimicrobial Use Survey and Detection of ESBL-Escherichia coli in Commercial and Medium-/Small-Scale Poultry Farms in Selected Districts of Zambia. Antibiotics 2024, 13, 467. [Google Scholar] [CrossRef]
- Mudenda, S.; Malama, S.; Munyeme, M.; Matafwali, S.K.; Kapila, P.; Katemangwe, P.; Katemangwe, P.; Mainda, G.; Mukubesa, A.N.; Hadunka, M.A.; et al. Antimicrobial resistance profiles of Escherichia coli isolated from laying hens in Zambia: Implications and significance on one health. JAC-Antimicrob. Resist. 2023, 5, dlad060. [Google Scholar] [CrossRef]
- Hu, Z.; Huang, Y.; Cui, X.; Shi, T.; Ji, Q.; Wang, X.; Bao, G.; Liu, Y. Genomic epidemiology of antimicrobial resistance determinants in Chinese swine farm Escherichia coli isolates. Front. Microbiol. 2025, 16, 1575426. [Google Scholar] [CrossRef]
- Lv, C.; Shang, J.; Zhang, W.; Sun, B.; Li, M.; Guo, C.; Zhou, N.; Guo, X.; Huang, S.; Zhu, Y. Dynamic antimicrobial resistant patterns of Escherichia coli from healthy poultry and swine over 10 years in Chongming Island, Shanghai. Infect. Dis. Poverty 2022, 11, 98. [Google Scholar] [CrossRef]
- Fatoba, D.O.; Amoako, D.G.; Abia, A.L.K.; Essack, S.Y. Transmission of Antibiotic-Resistant Escherichia coli from Chicken Litter to Agricultural Soil. Front. Environ. Sci. 2022, 9, 751732. [Google Scholar] [CrossRef]
- Appau, A.A.A.; Ofori, L.A. Antibiotic Resistance Profile of E. coli Isolates from Lettuce, Poultry Manure, Irrigation Water, and Soil in Kumasi, Ghana. Int. J. Microbiol. 2024, 2024, 6681311. [Google Scholar] [CrossRef] [PubMed]
- Hailu, W.; Helmy, Y.A.; Carney-Knisely, G.; Kauffman, M.; Fraga, D.; Rajashekara, G. Prevalence and Antimicrobial Resistance Profiles of Foodborne Pathogens Isolated from Dairy Cattle and Poultry Manure Amended Farms in Northeastern Ohio, the United States. Antibiotics 2021, 10, 1450. [Google Scholar] [CrossRef]
- Adzitey, F. Antibiotic resistance of Escherichia coli and Salmonella enterica isolated from cabbage and lettuce samples in Tamale metropolis of Ghana. Int. J. Food Contam. 2018, 5, 7. [Google Scholar] [CrossRef]
- Liu, C.; Sun, S.; Sun, Y.; Li, X.; Gu, W.; Luo, Y.; Wang, N.; Wang, Q. Antibiotic resistance of Escherichia coli isolated from food and clinical environment in China from 2001 to 2020. Sci. Total Environ. 2024, 939, 173498. [Google Scholar] [CrossRef] [PubMed]
- Habib, I.; Al-Rifai, R.H.; Mohamed, M.-Y.I.; Ghazawi, A.; Abdalla, A.; Lakshmi, G.; Agamy, N.; Khan, M. Contamination Levels and Phenotypic and Genomic Characterization of Antimicrobial Resistance in Escherichia coli Isolated from Fresh Salad Vegetables in the United Arab Emirates. Trop. Med. Infect Dis. 2023, 8, 294. [Google Scholar] [CrossRef]
- Devi, L.; Broor, S.; Chakravarti, A.; Chattopadhya, D. Livestock Manure as Potential Reservoir of CTX-M Type Extended-spectrum β-lactamase Producing Escherichia coli and K. pneumoniae Associated with Carbapenemase Production. J. Pure Appl. Microbiol. 2020, 14, 171–181. [Google Scholar] [CrossRef]
- Guron, G.K.P.; Chen, C.; Du, P.; Pruden, A.; Ponder, M.A. Manure-Based Amendments Influence Surface-Associated Bacteria and Markers of Antibiotic Resistance on Radishes Grown in Soils with Different Textures. Appl. Environ. Microbiol. 2021, 87, e02753-20. [Google Scholar] [CrossRef]
- Sane, S.; Tene, S.D.; Diouara, A.A.M.; Coundoul, S.; Mbengue, M.; Dieye, Y. Bacterial community in fresh fruits and vegetables sold in streets and open-air markets of Dakar, Senegal. BMC Microbiol. 2024, 24, 471. [Google Scholar] [CrossRef]
- Mwanza, F.; Komba, E.V.G.; Kambarage, D.M. Occurrence and Determination of Antimicrobial Resistant Escherichia coli Isolates in Fish and Vegetables as Indicator Organism of Faecal Contamination in Dar es Salaam, Tanzania. Int. J. Microbiol. 2021, 2021, 6633488. [Google Scholar] [CrossRef]
- WHO. High Level of Resistant Bacteria Circulating in the Environment, Food Chain and Community: A Call to Strengthen One Health Surveillance on Antimicrobial Resistance in Zimbabwe; WHO Regional Office for Africa: Brazzaville, Congo, 2022; Available online: https://www.afro.who.int/countries/zimbabwe/news/high-level-resistant-bacteria-circulating-environment-food-chain-and-community-call-strengthen-one (accessed on 7 July 2025).
- Wang, Y.; Zou, Q. Deciphering Microbial Adaptation in the Rhizosphere: Insights into Niche Preference, Functional Profiles, and Cross-Kingdom Co-occurrences. Microb. Ecol. 2024, 87, 74. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Huo, L.; Guo, Y.; Gao, M.; Wang, G.; Hu, D.; Li, C.; Wang, Z.; Liu, G.; Wang, X. Metagenomic assembly reveals hosts and mobility of common antibiotic resistome in animal manure and commercial compost. Environ. Microbiome 2022, 17, 42. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, D.; Yan, Y.; Yang, C.; Fang, B.; Li, X.; Shao, Y.; Wang, H.; Yue, J.; Wang, Y.; et al. Short-term application of chicken manure under different nitrogen rates alters structure and co-occurrence pattern but not diversity of soil microbial community in wheat field. Front. Microbiol. 2022, 13, 975571. [Google Scholar] [CrossRef]
- Resendiz-Nava, C.N.; Alonso-Onofre, F.; Silva-Rojas, H.V.; Rebollar-Alviter, A.; Rivera-Pastrana, D.M.; Stasiewicz, M.J.; Nava, G.M.; Mercado-Silva, E.M. Tomato Plant Microbiota under Conventional and Organic Fertilization Regimes in a Soilless Culture System. Microorganisms 2023, 11, 1633. [Google Scholar] [CrossRef] [PubMed]
- Bottery, M.J.; Pitchford, J.W.; Friman, V.-P. Ecology and evolution of antimicrobial resistance in bacterial communities. ISME J. 2021, 15, 939–948. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Matoga, M.; Chen, J.S.; Krysiak, R.; Ndalama, B.; Massa, C.; Bonongwe, N.; Mathiya, E.N.; Kamtambe, B.N.; Jere, E.; Chikaonda, T.; et al. Gentamicin Susceptibility in Neisseria gonorrhoeae and Treatment Outcomes for Urogenital Gonorrhea After 25 Years of Sustained Gentamicin Use in Malawi. Sex. Transm. Dis. 2022, 49, 251–256. [Google Scholar] [CrossRef]
- Phiri, A.F.N.D.; Abia, A.L.K.; Amoako, D.G.; Mkakosya, R.; Sundsfjord, A.; Essack, S.Y.; Simonsen, G.S. Burden, Antibiotic Resistance, and Clonality of Shigella spp. Implicated in Community-Acquired Acute Diarrhoea in Lilongwe, Malawi. Trop. Med. Infect Dis. 2021, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.; Hoffman, I.; Hobbs, M.M.; Maida, M.; Zimba, D.; Davis, R.; Mughogho, G.; Cohen, M.S. Development of an Antimicrobial Susceptibility Surveillance System for Neisseria gonorrhoeae in Malawi: Comparison of Methods. Portal Periód CAPES. 1997. Available online: https://www.periodicos.capes.gov.br/index.php/acervo/buscador.html?task=detalhes&id=W2138732038 (accessed on 19 June 2025).
- Lester, R.; Maheswaran, H.; Jewell, C.P.; Lalloo, D.G.; Feasey, N.A. Estimating the burden of antimicrobial resistance in Malawi: Protocol for a prospective observational study of the morbidity, mortality and economic cost of third-generation cephalosporin resistant bloodstream infection. Wellcome Open Res. 2020, 5, 29. [Google Scholar] [CrossRef]
- Gray, K.J.; Wilson, L.K.; Phiri, A.; Corkill, J.E.; French, N.; Hart, C.A. Identification and characterization of ceftriaxone resistance and extended-spectrum β-lactamases in Malawian bacteraemic Enterobacteriaceae. J. Antimicrob. Chemother. 2006, 57, 661–665. [Google Scholar] [CrossRef][Green Version]
- Onduru, O.G.; Aboud, S.; Nyirenda, T.S.; Rumisha, S.F.; Mkakosya, R.S. Antimicrobial susceptibility testing profiles of ESBL-producing Enterobacterales isolated from hospital and community adult patients in Blantyre, Malawi. IJID Reg. 2021, 1, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Mwansa, M.; Mukuma, M.; Mulilo, E.; Kwenda, G.; Mainda, G.; Yamba, K.; Bumbangi, F.N.; Muligisa-Muonga, E.; Phiri, N.; Silwamba, I.; et al. Determination of antimicrobial resistance patterns of Escherichia coli isolates from farm workers in broiler poultry production and assessment of antibiotic resistance awareness levels among poultry farmers in Lusaka, Zambia. Front. Public Health 2023, 10, 998860. [Google Scholar] [CrossRef] [PubMed]
- Mwapasa, T.; Chidziwisano, K.; Mphasa, M.; Cocker, D.; Rimella, L.; Amos, S.; Feasey, N.; Morse, T. Key environmental exposure pathways to antimicrobial resistant bacteria in southern Malawi: A SaniPath approach. Sci. Total Environ. 2024, 945, 174142. [Google Scholar] [CrossRef]
- Hickman, R.A.; Leangapichart, T.; Lunha, K.; Jiwakanon, J.; Angkititrakul, S.; Magnusson, U.; Sunde, M.; Järhult, J.D. Exploring the Antibiotic Resistance Burden in Livestock, Livestock Handlers and Their Non-Livestock Handling Contacts: A One Health Perspective. Front. Microbiol. 2021, 12, 651461. [Google Scholar] [CrossRef]
- Ibrahim, E.; Mkwanda, C.; Masoambeta, E.; Scudeller, L.; Kostyanev, T.; Twabi, H.H.; Diness, Y.K.; Chinkhumba, J.; Musaya, J.; Mkakosya, R.S.; et al. Prevalence of Extended-Spectrum β-Lactamase-Producing Escherichia coli, K. pneumoniae and Enterobacter cloacae in Wastewater Effluent in Blantyre, Malawi. Antibiotics 2025, 14, 562. [Google Scholar] [CrossRef]
- Kainga, H.; Phonera, M.; Kallu, S.A.; Chatanga, E.; Mwenifumbo, M.; Nkhoma, J.; Njunga, G. Knowledge, attitudes and practices of veterinarians and para-veterinarians towards antimicrobial stewardship in Malawi: Underutilized strength in the fight against antimicrobial resistance. BMC Vet. Res. 2025, 21, 390. [Google Scholar] [CrossRef] [PubMed]
- Kalumbi, M.H.; Katuah, Z.J.; Nyirenda, A.E.; Katiniche, B.; Chinyama, R.; Moyo, D.; Thugo, S.; Mlozen, M.; Kamwendo, Z.; Bonya, E.; et al. Prevalence of Multidrug-Resistant Bacterial Isolates from Chickens, Goats, Cattle and Pigs in Bvumbwe, Malawi. Appl. Microbiol. Open Access 2022, 8. [Google Scholar] [CrossRef]

| Microbe | Chicken Manure (N = 45) | Pig Manure (N = 46) |
|---|---|---|
| Acinetobacter junii | 4 (8.9%) | 0 (0%) |
| Acinetobacter pitii | 0 (0%) | 4 (8.7%) |
| Acinetobacter spp. | 6 (13%) | 6 (13%) |
| E. coli | 18 (40%) | 24 (52%) |
| K. pneumoniae | 12 (27%) | 12 (26%) |
| Serratia odoniferia | 5 (11%) | 0 (0%) |
| Comparison | Difference | Lower CI | Upper CI | Adjusted p-Value 1 |
|---|---|---|---|---|
| Farm 4 and Farm 3 | 6.5 | 3.049183 | 9.950817 | 0.006 ** |
| Farm 5 and Farm 4 | −6.0 | −9.780181 | −2.219819 | 0.011 * |
| Microbe | Farm Soil (N = 85) | Home Soil (N = 54) |
|---|---|---|
| E. coli | 30 (35%) | 0 (0%) |
| K. pneumoniae | 24 (28%) | 36 (67%) |
| Pseudomonas luteola | 5 (5.9%) | 0 (0%) |
| Pseudomonas spp. | 5 (5.9%) | 0 (0%) |
| S. choleraesuis | 0 (0%) | 6 (11%) |
| Serratia odoniferia | 5 (5.9%) | 0 (0%) |
| S. maltophilia | 0 (0%) | 6 (11%) |
| Stenotrophomonas spp. | 0 (0%) | 6 (11%) |
| Xanthomonas maltophilia | 12 (14%) | 0 (0%) |
| Y. pestis | 4 (4.7%) | 0 (0%) |
| Comparison | Difference | Lower CI | Upper CI | Adjusted p-Value 1 |
|---|---|---|---|---|
| Farm 3 and Farm 1 | −12 | −12 | −12 | <0.001 *** |
| Farm 4 and Farm 1 | −12 | −12 | −12 | <0.001 *** |
| Farm 5 and Farm 1 | −12 | −12 | −12 | <0.001 *** |
| Microbe | Amaranthus spp. (N = 44) | Brassica rapa subsp. Chinensis (N = 30) | Brassica rapa (N = 66) |
|---|---|---|---|
| Erwinia spp. | 0 (0%) | 0 (0%) | 5 (7.6%) |
| E. coli | 6 (14%) | 6 (20%) | 0 (0%) |
| K. pneumoniae | 18 (41%) | 18 (60%) | 36 (55%) |
| Pasteurella spp. | 20 (45%) | 0 (0%) | 4 (6.1%) |
| P. aeruginosa | 0 (0%) | 0 (0%) | 5 (7.6%) |
| Pseudomonas cepacian | 0 (0%) | 0 (0%) | 16 (24%) |
| Raoultella ornitholytica | 0 (0%) | 6 (20%) | 0 (0%) |
| Comparison | Difference | Lower CI | Upper CI | Adjusted p-Value 1 |
|---|---|---|---|---|
| Farm 3 and Farm 1 | 6.5 | 2.912001 | 10.087999 | 0.004 ** |
| Farm 3 and Farm 2 | 6.0 | 2.412001 | 9.587999 | 0.006 ** |
| Farm 4 and Farm 3 | −6.0 | −9.587999 | −2.412001 | 0.006 ** |
| Farm 5 and Farm 3 | −6.0 | −9.587999 | −2.412001 | 0.006 ** |
| Comparison | Mean | Lower CI | Upper CI | T-Value | p-Value | Strength |
|---|---|---|---|---|---|---|
| Manure–soil | 0.247 | 0.0971 | 0.39 | 3.08 | 0.0185 | Weak |
| Manure–veg | 0.25 | 0.0833 | 0.383 | 3 | 0.02 | Weak |
| Soil–veg | 0.287 | 0.2 | 0.407 | 4.84 | 0.00421 | Weak |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abraham, A.; Mtewa, A.G.; Chiutula, C.; Mvula, R.L.S.; Maluwa, A.; Eregno, F.E.; Njalam’mano, J. Prevalence of Antibiotic Resistance Bacteria in Manure, Soil, and Vegetables in Urban Blantyre, Malawi, from a Farm-to-Fork Perspective. Int. J. Environ. Res. Public Health 2025, 22, 1273. https://doi.org/10.3390/ijerph22081273
Abraham A, Mtewa AG, Chiutula C, Mvula RLS, Maluwa A, Eregno FE, Njalam’mano J. Prevalence of Antibiotic Resistance Bacteria in Manure, Soil, and Vegetables in Urban Blantyre, Malawi, from a Farm-to-Fork Perspective. International Journal of Environmental Research and Public Health. 2025; 22(8):1273. https://doi.org/10.3390/ijerph22081273
Chicago/Turabian StyleAbraham, Amon, Andrew G. Mtewa, Chimwemwe Chiutula, Richard Lizwe Steven Mvula, Alfred Maluwa, Fasil Ejigu Eregno, and John Njalam’mano. 2025. "Prevalence of Antibiotic Resistance Bacteria in Manure, Soil, and Vegetables in Urban Blantyre, Malawi, from a Farm-to-Fork Perspective" International Journal of Environmental Research and Public Health 22, no. 8: 1273. https://doi.org/10.3390/ijerph22081273
APA StyleAbraham, A., Mtewa, A. G., Chiutula, C., Mvula, R. L. S., Maluwa, A., Eregno, F. E., & Njalam’mano, J. (2025). Prevalence of Antibiotic Resistance Bacteria in Manure, Soil, and Vegetables in Urban Blantyre, Malawi, from a Farm-to-Fork Perspective. International Journal of Environmental Research and Public Health, 22(8), 1273. https://doi.org/10.3390/ijerph22081273








