The Effect of Long-Term Exposure to O3 and PM2.5 on Allergies and Asthma in Adolescents and Young Adults
Abstract
1. Introduction
2. Methods
2.1. Pollution Exposure Variables
2.2. Health Outcomes
2.3. Other Variables
3. Empirical Model and Estimation Strategy
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daniels, M.J.; Dominici, F.; Samet, J.M.; Zeger, S.L. Estimating particulate matter-mortality dose-response curves and threshold levels: An analysis of daily time-series for the 20 largest US cities. Am. J. Epidemiol. 2000, 152, 397–406. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Air Quality Criteria For Ozone and Related Photochemical Oxidants; U.S. Environmental Protection Agency: Washington, DC, USA, 2006.
- World Health Organization. Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- World Health Organization. Review of Evidence on Health Aspects of Air Pollution; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Beatty, T.K.; Shimshack, J.P. Air pollution and children’s respiratory health: A cohort analysis. J. Environ. Econ. Manag. 2014, 67, 39–57. [Google Scholar] [CrossRef]
- Bravo, M.A.; Ebisu, K.; Dominici, F.; Wang, Y.; Peng, R.D.; Bell, M.L. Airborne fine particles and risk of hospital admissions for understudied populations: Effects by urbanicity and short-term cumulative exposures in 708 US counties. Environ. Health Perspect. 2017, 125, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Chay, K.Y.; Greenstone, M. The impact of air pollution on infant mortality: Evidence from geographic variation in pollution shocks induced by a recession. Q. J. Econ. 2003, 118, 1121–1167. [Google Scholar] [CrossRef]
- Neidell, M.J. Air pollution, health, and socio-economic status: The effect of outdoor air quality on childhood asthma. J. Health Econ. 2004, 23, 1209–1236. [Google Scholar] [CrossRef]
- Koop, G.; Tole, L. Measuring the health effects of air pollution: To what extent can we really say that people are dying from bad air? J. Environ. Econ. Manag. 2004, 47, 30–54. [Google Scholar] [CrossRef]
- Neidell, M. Information, avoidance behavior, and health: The effect of ozone on asthma hospitalizations. J. Hum. Resour. 2009, 44, 450–478. [Google Scholar] [CrossRef]
- Lleras-Muney, A. The needs of the Army: Using compulsory relocation in the military to estimate the effect of air pollutants on children’s health. J. Hum. Resour. 2010, 45, 549–590. [Google Scholar] [CrossRef]
- Krall, J.R.; Anderson, G.B.; Dominici, F.; Bell, M.L.; Peng, R.D. Short-term exposure to particulate matter constituents and mortality in a national study of US urban communities. Environ. Health Perspect. 2013, 121, 1148–1153. [Google Scholar] [CrossRef]
- Crouse, D.L.; Peters, P.A.; Hystad, P.; Brook, J.R.; van Donkelaar, A.; Martin, R.V.; Burnett, R.T. Ambient PM2.5, O3, and NO2 exposures and associations with mortality over 16 years of follow-up in the Canadian Census Health and Environment Cohort (CanCHEC). Environ. Health Perspect. 2015, 123, 1180–1186. [Google Scholar] [CrossRef]
- Kaufman, J.D.; Elkind, M.S.; Bhatnagar, A.; Koehler, K.; Balmes, J.R.; Sidney, S.; American Heart Association Advocacy Coordinating Committee. Guidance to reduce the cardiovascular burden of ambient air pollutants: A policy statement from the American Heart Association. Circulation 2020, 142, e432–e447. [Google Scholar] [CrossRef]
- Kirwa, K.; Eckert, C.M.; Vedal, S.; Hajat, A.; Kaufman, J.D. Ambient air pollution and risk of respiratory infection among adults: Evidence from the multiethnic study of atherosclerosis (MESA). BMJ Open Respir. Res. 2021, 8, e000866. [Google Scholar] [CrossRef] [PubMed]
- Makar, M.; Antonelli, J.; Di, Q.; Cutler, D.; Schwartz, J.; Dominici, F. Estimating the causal effect of low levels of fine particulate matter on hospitalization. Epidemiology 2017, 28, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, C.; Huang, X. Air pollution, student health, and school absences: Evidence from China. J. Environ. Econ. Manag. 2018, 92, 465–497. [Google Scholar] [CrossRef]
- Moretti, E.; Neidell, M. Pollution, health, and avoidance behavior: Evidence from the ports of Los Angeles. J. Hum. Resour. 2011, 46, 154–175. [Google Scholar] [CrossRef]
- Jerrett, M.; Burnett, R.T.; Pope, C.A., III; Ito, K.; Thurston, G.; Krewski, D.; Thun, M. Long-term ozone exposure and mortality. N. Engl. J. Med. 2009, 360, 1085–1095. [Google Scholar] [CrossRef]
- Seaton, A.; Godden, D.; MacNee, W.; Donaldson, K. Particulate air pollution and acute health effects. Lancet 1995, 345, 176–178. [Google Scholar] [CrossRef]
- Delfino, R.J. Epidemiologic evidence for asthma and exposure to air toxics: Linkages between occupational, indoor, and community air pollution research. Environ. Health Perspect. 2002, 110 (Suppl. S4), 573–589. [Google Scholar] [CrossRef]
- Ling, S.H.; van Eeden, S.F. Particulate matter air pollution exposure: Role in the development and exacerbation of chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2009, 4, 233–243. [Google Scholar] [CrossRef]
- Pope, C.A., III. Epidemiology of fine particulate air pollution and human health: Biologic mechanisms and who’s at risk? Environ. Health Perspect. 2000, 108 (Suppl. S4), 713–723. [Google Scholar] [CrossRef]
- Committee on Environmental Health. Ambient air pollution: Health hazards to children. Pediatrics 2004, 114, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Bravo, M.A.; Fang, F.; Hancock, D.B.; Johnson, E.O.; Harris, K.M. Long-term air pollution exposure and markers of cardiometabolic health in the National Longitudinal Study of Adolescent to Adult Health (Add Health). Environ. Int. 2023, 177, 107987. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Jarolim, E.; Untersmayr, E. Gender-medicine aspects in allergology. Allergy 2008, 63, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Almqvist, C.; Worm, M.; Leynaert, B.; Working Group of GA2LEN WP 2. 5 ‘Gender’. Impact of gender on asthma in childhood and adolescence: A GA2LEN review. Allergy 2008, 63, 47–57. [Google Scholar] [CrossRef]
- Shah, R.; Newcomb, D.C. Sex bias in asthma prevalence and pathogenesis. Front. Immunol. 2018, 9, 2997. [Google Scholar] [CrossRef]
- Chowdhury, N.U.; Guntur, V.P.; Newcomb, D.C.; Wechsler, M.E. Sex and gender in asthma. Eur. Respir. Rev. 2021, 30, 210067. [Google Scholar] [CrossRef]
- Harris, K.M.; Halpern, C.T.; Biemer, P.; Liao, D.; Dean, S.C. Add Health Wave V Documentation: Sampling and Mixed-Mode Survey Design. Add Health. 2019. Available online: http://www.cpc.unc.edu/projects/addhealth/documentation/guides/ (accessed on 8 May 2025).
- Chen, P. Appropriate Analysis in Add Health: Correcting for Design Effects & Selecting Weights; University of North Carolina at Chapel Hill: Chapel Hill, NC, USA, 2014. [Google Scholar]
- Bravo, M.A.; Fang, F.; Hancock, D.B.; Johnson, E.O.; Harris, K.M. Waves III-V Multi-Year Air Pollution Exposure Estimates; University of North Carolina at Chapel Hill: Chapel Hill, NC, USA, 2023. [Google Scholar]
- Premji, S.; Bertrand, F.; Smargiassi, A.; Daniel, M. Socio-economic correlates of municipal-level pollution emissions on Montreal Island. Can. J. Public Health 2007, 98, 138–142. [Google Scholar] [CrossRef]
- Grineski, S.; Bolin, B.; Boone, C. Criteria air pollution and marginalized populations: Environmental inequity in metropolitan Phoenix, Arizona. Soc. Sci. Q. 2007, 88, 535–554. [Google Scholar] [CrossRef]
- Pearce, J.R.; Richardson, E.A.; Mitchell, R.J.; Shortt, N.K. Environmental justice and health: The implications of the socio-spatial distribution of multiple environmental deprivation for health inequalities in the United Kingdom. Trans. Inst. Br. Geogr. 2010, 35, 522–539. [Google Scholar] [CrossRef]
- Viel, J.F.; Hägi, M.; Upegui, E.; Laurian, L. Environmental justice in a French industrial region: Are polluting industrial facilities equally distributed? Health Place 2011, 17, 257–262. [Google Scholar] [CrossRef]
- Gunasekara, F.I.; Carter, K.; Blakely, T. Change in income and change in self-rated health: Systematic review of studies using repeated measures to control for confounding bias. Soc. Sci. Med. 2011, 72, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Cambois, E.; Jusot, F. Contribution of lifelong adverse experiences to social health inequalities: Findings from a population survey in France. Eur. J. Public Health 2011, 21, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Von Hertzen, L.C.; Haahtela, T. Asthma and atopy–the price of affluence? Allergy 2004, 59, 124–137. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Integrated Science Assessment (ISA) for Particulate Matter; U.S. Environmental Protection Agency: Washington, DC, USA, 2019.
- Kelly, F.J.; Fussell, J.C. Air pollution and airway disease. Clin. Exp. Allergy 2011, 41, 1059–1071. [Google Scholar] [CrossRef]
- Murrison, L.B.; Brandt, E.B.; Myers, J.B.; Hershey, G.K.K. Environmental exposures and mechanisms in allergy and asthma development. J. Clin. Investig. 2019, 129, 1504–1515. [Google Scholar] [CrossRef]
- Atkinson, C.E.; Kesic, M.J.; Hernandez, M.L. Ozone in the development of pediatric asthma and atopic disease. Immunol. Allergy Clin. North Am. 2022, 42, 701–713. [Google Scholar] [CrossRef]
- Bronte-Moreno, O.; González-Barcala, F.J.; Muñoz-Gall, X.; Pueyo-Bastida, A.; Ramos-González, J.; Urrutia-Landa, I. Impact of air pollution on asthma: A scoping review. Open Respir. Arch. 2023, 5, 100229. [Google Scholar] [CrossRef]
- Mukharesh, L.; Phipatanakul, W.; Gaffin, J.M. Air pollution and childhood asthma. Curr. Opin. Allergy Clin. Immunol. 2023, 23, 100–110. [Google Scholar] [CrossRef]
- Ronen, O.; Malhotra, A.; Pillar, G. Influence of gender and age on upper-airway length during development. Pediatrics 2007, 120, e1028–e1034. [Google Scholar] [CrossRef]
- Ripoll, J.G.; Guo, W.; Andersen, K.J.; Baker, S.E.; Wiggins, C.C.; Shepherd, J.R.; Dominelli, P.B. Sex differences in paediatric airway anatomy. Exp. Physiol. 2020, 105, 721–731. [Google Scholar] [CrossRef]
- Carey, M.A.; Card, J.W.; Voltz, J.W.; Arbes Jr, S.J.; Germolec, D.R.; Korach, K.S.; Zeldin, D.C. It’s all about sex: Male-female differences in lung development and disease. Trends Endocrinol. Metab. TEM 2007, 18, 308. [Google Scholar] [CrossRef]
- Kelly, A.; Lavender, P. Epigenetic approaches to identifying asthma endotypes. Allergy Asthma Immunol. Res. 2024, 16, 130. [Google Scholar] [CrossRef]
- Jung, C.R.; Chen, W.T.; Tang, Y.H.; Hwang, B.F. Fine particulate matter exposure during pregnancy and infancy and incident asthma. J. Allergy Clin. Immunol. 2019, 143, 2254–2262. [Google Scholar] [CrossRef]
- Shin, H.H.; Maquiling, A.; Thomson, E.M.; Park, I.W.; Stieb, D.M.; Dehghani, P. Sex-difference in air pollution-related acute circulatory and respiratory mortality and hospitalization. Sci. Total Environ. 2022, 806, 150515. [Google Scholar] [CrossRef]

| Wave IV (2008) | Mean | SD | Min | Max |
|---|---|---|---|---|
| Allergy | 0.094 | 0.292 | 0 | 1 |
| Asthma | 0.102 | 0.303 | 0 | 1 |
| O3, ppb | 39.06 | 3.608 | 24.83 | 53.72 |
| PM2.5, µg/m3 | 12.17 | 2.269 | 4.850 | 18.73 |
| Time-varying covariates: | ||||
| Household size | 3.421 | 1.439 | 1 | 14 |
| Parent has college degree | 0.183 | 0.386 | 0 | 1 |
| Parent works for pay | 0.613 | 0.487 | 0 | 1 |
| Parent work hours | 0.349 | 1.550 | 0 | 14 |
| Household income bracket | 7.441 | 2.874 | 1 | 12 |
| Parental employment HI | 0.683 | 0.466 | 0 | 1 |
| Parent smoking days | 8.412 | 12.92 | 0 | 30 |
| Parent marijuana use days | 0.441 | 1.381 | 0 | 6 |
| Grouping characteristics: | ||||
| Age | 5.778 | 3.339 | 1 | 16 |
| Male | 0.505 | 0.500 | 0 | 1 |
| Wave V (2016–2018) | ||||
| Allergy | 0.157 | 0.363 | 0 | 1 |
| Asthma | 0.136 | 0.343 | 0 | 1 |
| O3, ppb | 38.79 | 3.100 | 25.87 | 53.85 |
| PM2.5, µg/m3 | 9.467 | 1.407 | 4.717 | 14.61 |
| Time-varying covariates: | ||||
| Household size | 4.255 | 3.831 | 1 | 22.60 |
| Parent has college degree | 0.245 | 0.430 | 0 | 1 |
| Parent works for pay | 0.801 | 0.399 | 0 | 1 |
| Parent work hours | 4.259 | 2.542 | 0 | 15 |
| Household income bracket | 7.668 | 3.537 | 1 | 12 |
| Parental employment HI | 0.617 | 0.486 | 0 | 1 |
| Parent smoking days | 7.489 | 12.57 | 0 | 30 |
| Parent marijuana use days | 0.704 | 1.738 | 0 | 6 |
| Grouping characteristics: | ||||
| Age | 14.72 | 3.398 | 10 | 25 |
| Male | 0.505 | 0.500 | 0 | 1 |
| Allergy, All | Allergy, Males | Allergy, Females | Asthma, All | Asthma, Males | Asthma, Females | |
|---|---|---|---|---|---|---|
| O3, ppb | 0.005 * | 0.011 ** | −0.001 | 0.002 | 0.003 | 0.001 |
| (0.002) | (0.003) | (0.003) | (0.002) | (0.003) | (0.003) | |
| PM2.5, µg/m3 | 0.009 * | 0.021 ** | −0.002 | 0.009 ** | 0.004 | 0.013 ** |
| (0.004) | (0.006) | (0.005) | (0.003) | (0.005) | (0.005) | |
| Household size | −0.000 | −0.002 | 0.001 | 0.000 | 0.002 | −0.001 |
| (0.001) | (0.002) | (0.002) | (0.001) | (0.002) | (0.001) | |
| Parent has college degree | 0.053 ** | 0.054 * | 0.052 * | 0.006 | 0.018 | −0.008 |
| (0.017) | (0.025) | (0.023) | (0.014) | (0.020) | (0.020) | |
| Parent works for pay | 0.003 | −0.018 | 0.027 * | −0.014 | 0.015 | −0.047 ** |
| (0.010) | (0.014) | (0.013) | (0.008) | (0.011) | (0.011) | |
| Parent work hours | −0.006 ** | −0.004 | −0.008 ** | −0.003 | −0.002 | −0.003 |
| (0.002) | (0.003) | (0.002) | (0.002) | (0.002) | (0.002) | |
| Household income bracket | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| (0.002) | (0.002) | (0.002) | (0.001) | (0.002) | (0.002) | |
| Parental employment HI | −0.007 | −0.006 | −0.006 | 0.014 | 0.004 | 0.025 * |
| (0.009) | (0.013) | (0.012) | (0.008) | (0.011) | (0.011) | |
| Parent smoking days | −0.000 | 0.001 | −0.001 | 0.000 | 0.001 | −0.001 |
| (0.000) | (0.001) | (0.001) | (0.000) | (0.001) | (0.001) | |
| Parent marijuana use days | −0.010 ** | −0.012 ** | −0.009 * | −0.002 | −0.004 | −0.001 |
| (0.003) | (0.004) | (0.004) | (0.002) | (0.003) | (0.004) | |
| Wave 5 dummy | 0.108 ** | 0.143 ** | 0.076 ** | 0.065 ** | 0.037 * | 0.094 ** |
| (0.014) | (0.021) | (0.018) | (0.012) | (0.017) | (0.016) | |
| N. of children | 6860 | 3462 | 3398 | 6860 | 3462 | 3398 |
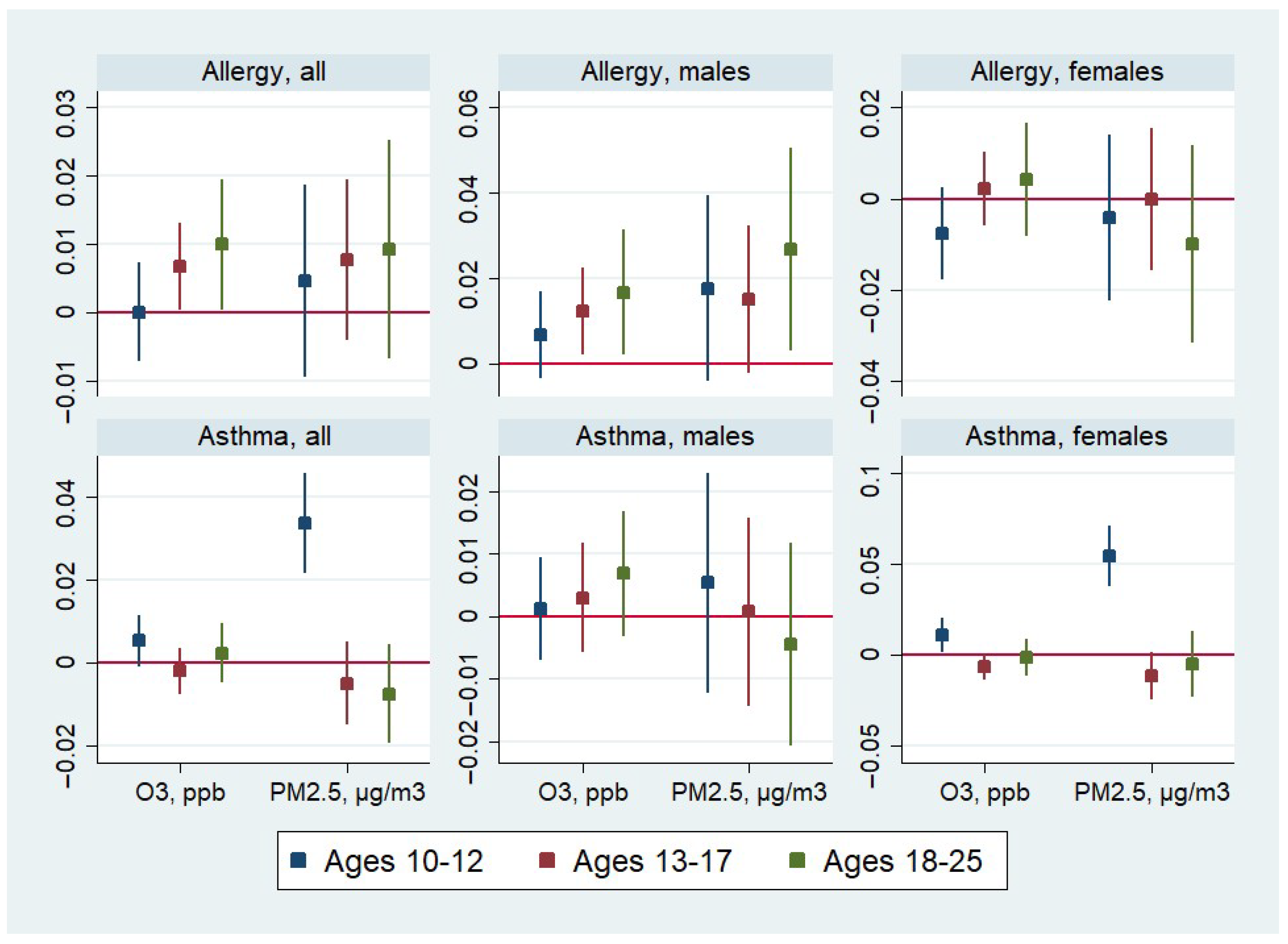
| Allergy, All | Allergy, Males | Allergy, Females | Asthma, All | Asthma, Males | Asthma, Females | |
|---|---|---|---|---|---|---|
| Age in Wave V: 10–12 | ||||||
| O3, ppb | 0.000 | 0.007 | −0.008 | 0.005 | 0.001 | 0.011 * |
| (0.00) | (0.01) | (0.01) | (0.00) | (0.00) | (0.00) | |
| PM2.5, µg/m3 | 0.005 | 0.018 | −0.004 | 0.034 ** | 0.005 | 0.055 ** |
| (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | |
| N. of children | 2216 | 1110 | 1106 | 2216 | 1110 | 1106 |
| Age in Wave V: 13–17 | ||||||
| O3, ppb | 0.007 * | 0.012 * | 0.002 | −0.002 | 0.003 | −0.006 |
| (0.00) | (0.01) | (0.00) | (0.00) | (0.00) | (0.00) | |
| PM2.5, µg/m3 | 0.008 | 0.015 | −0.000 | −0.005 | 0.001 | −0.011 |
| (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | |
| N. of children | 3082 | 1554 | 1528 | 3082 | 1554 | 1528 |
| Age in Wave V: 18–25 | ||||||
| O3, ppb | 0.010 * | 0.017 * | 0.004 | 0.002 | 0.007 | −0.001 |
| (0.00) | (0.01) | (0.01) | (0.00) | (0.01) | (0.01) | |
| PM2.5, µg/m3 | 0.009 | 0.027 * | −0.010 | −0.007 | −0.005 | −0.005 |
| (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | |
| N. of children | 1562 | 798 | 764 | 1562 | 798 | 764 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amialchuk, A.; Sapci, O. The Effect of Long-Term Exposure to O3 and PM2.5 on Allergies and Asthma in Adolescents and Young Adults. Int. J. Environ. Res. Public Health 2025, 22, 1262. https://doi.org/10.3390/ijerph22081262
Amialchuk A, Sapci O. The Effect of Long-Term Exposure to O3 and PM2.5 on Allergies and Asthma in Adolescents and Young Adults. International Journal of Environmental Research and Public Health. 2025; 22(8):1262. https://doi.org/10.3390/ijerph22081262
Chicago/Turabian StyleAmialchuk, Aliaksandr, and Onur Sapci. 2025. "The Effect of Long-Term Exposure to O3 and PM2.5 on Allergies and Asthma in Adolescents and Young Adults" International Journal of Environmental Research and Public Health 22, no. 8: 1262. https://doi.org/10.3390/ijerph22081262
APA StyleAmialchuk, A., & Sapci, O. (2025). The Effect of Long-Term Exposure to O3 and PM2.5 on Allergies and Asthma in Adolescents and Young Adults. International Journal of Environmental Research and Public Health, 22(8), 1262. https://doi.org/10.3390/ijerph22081262






