Thallium Exposure Secondary to Commercial Kale Chip Consumption: California Case Highlights Opportunities for Improved Surveillance and Toxicological Understanding
Abstract
1. Introduction
2. Objectives
3. Household Thallium Investigation
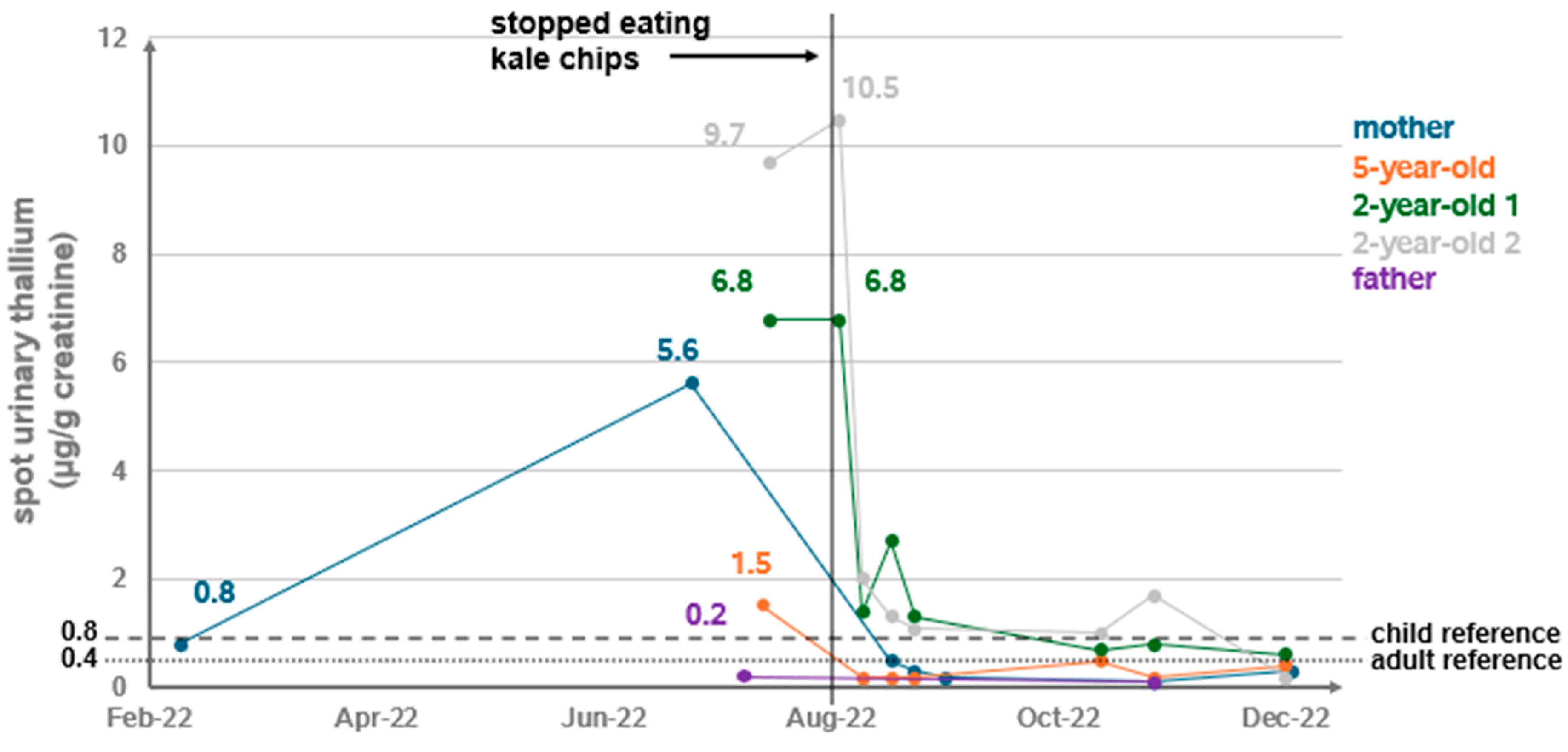
3.1. A Household Cluster of Elevated Random Urine Thallium Levels
3.2. Public Health Investigation and Findings
3.2.1. Household Exposure Survey and Environmental Assessment
3.2.2. Clinical Intervention and Ongoing Monitoring
4. Discussion
4.1. Opportunities for Improved Toxicological Understanding
4.1.1. Lack of Human Studies Representing Chronic Thallium Exposure
4.1.2. Role for Further Animal Studies
4.2. Opportunities for Improved Food Safety Surveillance and Regulatory Actions
4.2.1. Thallium Accumulation in Brassicas
4.2.2. Role for Further Monitoring of Thallium in Food Supply
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDPH | California Department of Public Health |
| NHANES | National Health and Nutrition Examination Survey |
| EPA | United Stated Environmental Protection Agency |
| FDA | United States Food and Drug Agency |
| IPCS | International Programme on Chemical Safety |
| RfD | Reference dose |
| NTP | National Institutes of Health National Toxicology Program |
Appendix A
| Organization | Reference Levels (mg/kg-day) or Urine Tl (µg/L) | Supporting Studies | Limitations |
|---|---|---|---|
| U.S. Environmental Protection Agency (EPA) | RfD range: 8.0 × 10−5 to 9.0 × 10−5 (withdrawn in 2009) | [18] U.S. EPA. IRIS Toxicological Review of Thallium And Compounds. U.S. Environmental Protection Agency. September 2009.EPA/635/R-08/001F. [31] Stoltz ML, Stedham MA, Brown LK, et al. 1986, Subchronic (90 day) toxicity of thallium (I) sulfate in Sprague-Dawley rats. Report to U.S. EPA, Office of Solid Waste, Washington, DC, by Midwest Research Institute, Kansas City, MO. | Lack of confidence in the key toxicological study in rats (Stoltz 1986; as cited by EPA 2009). The data gaps of the 90-day oral gavage study discussed by EPA in their review included: “High background incidence of alopecia, lack of histopathological examination of skin tissue in low- and mid-dose groups, and inadequate examination of objective measures of neurotoxicity”. |
| U.S. EPA Superfund Risk Assessment | PPRTV: subchronic and chronic provisional RfDs ranging from 1 × 10−5 to 4 × 10−5 | [54] U.S. EPA. Provisional Peer-Reviewed Toxicity Values for Thallium (Soluble Salts). U.S. EPA, Washington, DC, EPA/690/R-12/026F, 2012. | Used data from 1986 study by Midwest Research Institute. |
| International Programme on Chemical Safety (IPCS) proposed in 1996 Environmental Health Criteria (EHC) 182 | EHC 182 aligned with the conclusion of the 2009 U.S. EPA review that no health-based guidance value could be determined. IPCS proposed to base a safe intake of thallium on the 5 µg/L urine value, which was reported to be roughly equivalent to a daily intake of 10 µg/day. | [15] International Programme on Chemical Safety. Environmental Health Criteria 182 Thallium. World Health Organization. 1996. | Based on two epidemiological studies involving thallium-exposed workers and persons living near an industrial source. No developmental neurotoxicity studies that could serve to guide a health protective risk assessment threshold for thallium in pregnant women, adults of reproductive age, or young children. |
References
- Lennartson, A. Toxic thallium. Nat. Chem. 2015, 7, 610. [Google Scholar] [CrossRef]
- Thallium: Systemic Agent. Emergency Response Safety and Health Database. National Institute for Occupational Safety and Health. Updated 12 May 2011. Available online: https://www.cdc.gov/niosh/ershdb/ (accessed on 12 February 2024).
- Karbowska, B. Presence of thallium in the environment: Sources of contaminations, distribution and monitoring methods. Environ. Monit. Assess. 2016, 188, 640. [Google Scholar] [CrossRef]
- Peter, A.L.; Viraraghavan, T. Thallium: A review of public health and environmental concerns. Environ. Int. 2005, 31, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhou, W.; Guo, Z.; Peng, C.; Xu, R.; Zhang, Y.; Yang, Y. Thallium content in vegetables and derivation of threshold for safe food production in soil: A meta-analysis. Sci. Total Environ. 2024, 912, 168845. [Google Scholar] [CrossRef]
- Lin, G.; Yuan, L.; Peng, X.; Long, J.; Wang, C.; Bai, L.; Lu, X.; Dong, J.; Liu, Y.; Wang, Y.; et al. Clinical characteristics and treatment of thallium poisoning in patients with delayed admission in China. Medicine 2019, 98, e16471. [Google Scholar] [CrossRef]
- Gummin, D.D.; Mowry, J.B.; Spyker, D.A.; Brooks, D.E.; Beuhler, M.C.; Rivers, L.J.; Hashem, H.A.; Ryan, M.L. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin. Toxicol. 2019, 57, 1220–1413. [Google Scholar] [CrossRef]
- Gummin, D.D.; Mowry, J.B.; Beuhler, M.C.; Spyker, D.A.; Brooks, D.E.; Dibert, K.W.; Rivers, L.J.; Pham, N.P.T.; Ryan, M.L. 2019 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 37th Annual Report. Clin. Toxicol. 2020, 58, 1360–1541. [Google Scholar] [CrossRef]
- Gummin, D.D.; Mowry, J.B.; Beuhler, M.C.; Spyker, D.A.; Bronstein, A.C.; Rivers, L.J.; Pham, N.P.T.; Weber, J. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin. Toxicol. 2021, 59, 1282–1501. [Google Scholar] [CrossRef] [PubMed]
- Gummin, D.D.; Mowry, J.B.; Beuhler, M.C.; Spyker, D.A.; Rivers, L.J.; Feldman, R.; Brown, K.; Nathaniel, P.T.P.; Bronstein, A.C.; Weber, J.A. 2021 Annual Report of the National Poison Data System (NPDS) from America’s Poison Centers: 39th Annual Report. Clin. Toxicol. 2022, 60, 1381–1643. [Google Scholar] [CrossRef] [PubMed]
- Gummin, D.D.; Mowry, J.B.; Beuhler, M.C.; Spyker, D.A.; Rivers, L.J.; Feldman, R.; Brown, K.; Pham, N.P.; Bronstein, A.C.; DesLauriers, C. 2022 Annual Report of the National Poison Data System (NPDS) from America’s Poison Centers: 40th Annual Report. Clin. Toxicol. 2023, 61, 717–939. [Google Scholar] [CrossRef] [PubMed]
- National Center for Environmental Health. National Report on Human Exposure to Environmental Chemicals; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2022; Updated September 2023. [CrossRef]
- Smith, D.; Cannon, W.F.; Woodruff, L.G.; Solano Federico Kilburn, J.E.; Fey, D.L. Geochemical and Mineralogical Data for Soils of the Conterminous United States. 2013, 19. Data Series 801. Available online: https://pubs.usgs.gov/ds/801/ (accessed on 12 February 2024).
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef]
- International Programme on Chemical Safety. Environmental Health Criteria 182 Thallium; World Health Organization: Geneva, Switzerland, 1996; Available online: https://www.inchem.org/documents/ehc/ehc/ehc182.htm (accessed on 12 February 2024).
- Brockhaus, A.; Dolgner, R.; Ewers, U.; Krämer, U.; Soddemann, H.; Wiegand, H. Intake and health effects of thallium among a population living in the vicinity of a cement plant emitting thallium containing dust. Int. Arch. Occup. Environ. Health 1981, 48, 375–389. [Google Scholar] [CrossRef]
- Ludolph, A.; Elger, C.E.; Sennhenn, R.; Bertram, H.P. Chronic thallium exposure in cement plant workers: Clinical and electrophysiological data. Trace Elem. Med. 1986, 3, 121–125. [Google Scholar]
- US EPA. IRIS Toxicological Review of Thallium and Compounds. U.S. Environmental Protection Agency. September 2009. EPA/635/R-08/001F. Available online: https://iris.epa.gov/static/pdfs/1012tr.pdf (accessed on 12 February 2024).
- Agency for Toxic Substances Disease Registry Toxicological Profile for Thallium July 1992, U.S. Department of Health and Human Services. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp54.pdf (accessed on 12 February 2024).
- Chang, Y.; Chiang, C.K. The Impact of Thallium Exposure in Public Health and Molecular Toxicology: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 4750. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Wang, Y.; Li, Z.; Fu, G.; Mao, L.; Song, Y.; Qu, Y.; Ye, L.; Zhou, Q.; Yang, F.; et al. Thallium exposure at low concentration leads to early damage on multiple organs in children: A case study followed-up for four years. Environ. Pollut. 2020, 258, 113319. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xia, W.; Zhang, B.; Pan, X.; Liu, W.; Jin, S.; Huo, W.; Liu, H.; Peng, Y.; Sun, X.; et al. Predictors of thallium exposure and its relation with preterm birth. Environ. Pollut. 2018, 233, 971–976. [Google Scholar] [CrossRef]
- Govarts, E.; Remy, S.; Bruckers, L.; Hond, E.D.; Sioen, I.; Nelen, V.; Baeyens, W.; Nawrot, T.S.; Loots, I.; Van Larebeke, N.; et al. Combined Effects of Prenatal Exposures to Environmental Chemicals on Birth Weight. Int. J. Environ. Res. Public Health 2016, 13, 495. [Google Scholar] [CrossRef]
- Xia, W.; Du, X.; Zheng, T.; Zhang, B.; Li, Y.; Bassig, B.A.; Zhou, A.; Wang, Y.; Xiong, C.; Li, Z.; et al. A Case-Control Study of Prenatal Thallium Exposure and Low Birth Weight in China. Environ. Health Perspect. 2016, 124, 164–169. [Google Scholar] [CrossRef]
- Wu, M.; Shu, Y.; Song, L.; Liu, B.; Zhang, L.; Wang, L.; Liu, Y.; Bi, J.; Xiong, C.; Cao, Z.; et al. Prenatal exposure to thallium is associated with decreased mitochondrial DNA copy number in newborns: Evidence from a birth cohort study. Environ. Int. 2019, 129, 470–477. [Google Scholar] [CrossRef]
- Vriens, A.; Nawrot, T.S.; Baeyens, W.; Hond, E.D.; Bruckers, L.; Covaci, A.; Croes, K.; De Craemer, S.; Govarts, E.; Lambrechts, N.; et al. Neonatal exposure to environmental pollutants and placental mitochondrial DNA content: A multi-pollutant approach. Environ. Int. 2017, 106, 60–68. [Google Scholar] [CrossRef]
- Dai, J.; Wu, X.; Bai, Y.; Feng, W.; Wang, S.; Chen, Z.; Fu, W.; Li, G.; Chen, W.; Wang, G.; et al. Effect of thallium exposure and its interaction with smoking on lung function decline: A prospective cohort study. Environ. Int. 2019, 127, 181–189. [Google Scholar] [CrossRef]
- Yorita Christensen, K.L. Metals in blood and urine, and thyroid function among adults in the United States 2007–2008. Int. J. Hyg. Environ. Health 2013, 216, 624–632. [Google Scholar] [CrossRef]
- Campanella, B.; Colombaioni, L.; Benedetti, E.; Di Ciaula, A.; Ghezzi, L.; Onor, M.; D’Orazio, M.; Giannecchini, R.; Petrini, R.; Bramanti, E. Toxicity of Thallium at Low Doses: A Review. Int. J. Environ. Res. Public Health 2019, 16, 4732. [Google Scholar] [CrossRef]
- US EPA. Basic Information about the Integrated Risk Information System. U.S. Environmental Protection Agency. Updated November 9, 2023. Available online: https://www.epa.gov/iris/basic-information-about-integrated-risk-information-system#guidance (accessed on 12 February 2024).
- Stoltz, M.L.; Stedhan, M.A.; Brown, L.K.; Laber, L.; El-hawari, A.M. Subchronic (90 Day) Toxicity of Thallium (I) Sulfate in Sprague-Dawley Rats; Report to U.S. Environmental Protection Agency, Office of Solid Waste, Washington, DC, by Midwest Research Institute, Kansas City, MO; Midwest Research Institute: Kansas City, MO, USA, 1986. [Google Scholar]
- US EPA. IRIS Summary. U.S. Environmental Protection Agency. September 2009. EPA/635/R-08/001F. Available online: https://iris.epa.gov/static/pdfs/1012_summary.pdf (accessed on 12 February 2024).
- Sánchez-Chapul, L.; Santamaría, A.; Aschner, M.; Ke, T.; Tinkov, A.A.; Túnez, I.; Osorio-Rico, L.; Galván-Arzate, S.; Rangel-López, E. Thallium-induced DNA damage, genetic, and epigenetic alterations. Front. Genet. 2023, 14, 1168713. [Google Scholar] [CrossRef] [PubMed]
- Summary Minutes 15–16 June 2016 NTP Board of Scientific Counselors. Available online: https://ntp.niehs.nih.gov/sites/default/files/ntp/about_ntp/bsc/2016/june/minutes201606_508.pdf (accessed on 12 February 2024).
- Thallium (I) Sulfate (7446-18-6). National Toxicology Program. Available online: https://cebs.niehs.nih.gov/cebs/test_article/7446-18-6 (accessed on 12 February 2024).
- Shipkowski, K.A.; Hubbard, T.D.; Ryan, K.; Waidyanatha, S.; Cunny, H.; Shockley, K.R.; Allen, J.L.; Toy, H.; Levine, K.; Harrington, J.; et al. Short-term toxicity studies of thallium (I) sulfate administered in drinking water to Sprague Dawley rats and B6C3F1/N mice. Toxicol. Rep. 2023, 10, 621–632. [Google Scholar] [CrossRef]
- Pearson, A.J.; Ashmore, E. Risk assessment of antimony, barium, beryllium, boron, bromine, lithium, nickel, strontium, thallium and uranium concentrations in the New Zealand diet. Food Addit. Contam. Part A 2020, 37, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.B.N. Total Diet Study of Metals and Other Elements in Food. 2015; FS102081. Available online: https://www.food.gov.uk/sites/default/files/media/document/total-diet-study-of-metals-and-other-elements-in-food_0.pdf (accessed on 12 February 2024).
- Filippini, T.; Tancredi, S.; Malagoli, C.; Malavolti, M.; Bargellini, A.; Vescovi, L.; Nicolini, F.; Vinceti, M. Dietary Estimated Intake of Trace Elements: Risk Assessment in an Italian Population. Expo. Health 2020, 12, 641–655. [Google Scholar] [CrossRef]
- Pavlíčková, J.; Zbíral, J.; Smatanová, M.; Habarta, P.; Houserová, P.; Kubáň, V. Uptake of thallium from artificially contaminated soils by kale (Brassica oleracea L., var. acephala). Plant Soil Environ. 2006, 52, 544–549. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Schwarzbauer, J.; Robert, D. Environmental Chemistry: Green Chemistry and Pollutants in Ecosystems; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Wang, C.; Chen, Y.; Liu, J.; Wang, J.; Li, X.; Zhang, Y.; Liu, Y. Health risks of thallium in contaminated arable soils and food crops irrigated with wastewater from a sulfuric acid plant in western Guangdong province, China. Ecotoxicol. Environ. Saf. 2013, 90, 76–81. [Google Scholar] [CrossRef]
- Xiao, T.; Guha, J.; Boyle, D.; Liu, C.-Q.; Chen, J. Environmental concerns related to high thallium levels in soils and thallium uptake by plants in southwest Guizhou, China. Sci. Total Environ. 2004, 318, 223–244. [Google Scholar] [CrossRef]
- Mourato, M.P.; Moreira, I.N.; Leitão, I.; Pinto, F.R.; Sales, J.R.; Martins, L.L. Effect of Heavy Metals in Plants of the Genus Brassica. Int. J. Mol. Sci. 2015, 16, 17975–17998. [Google Scholar] [CrossRef] [PubMed]
- Environment and Climate Change Canada. Draft Screening Assessment—Thallium and Its Compounds. September 2020. Available online: https://www.canada.ca/en/environment-climate-change/services/evaluating-existing-substances/draft-screening-assessment-thallium-compounds.html (accessed on 12 February 2024).
- Baxter, M.; Brereton, N. Measurement of the Concentrations of Metals and Other Elements from the 2014 UK Total Diet Study. March 2015. Report for the UK Food Standards Agency (FS102081), by The Food and Environment Agency. Fera Report Number 15/06. Available online: https://www.food.gov.uk/sites/default/files/media/document/measurement-of-the-concentrations-of-metals-and-other-elements-from-the-2014-uk-total-diet-study.pdf (accessed on 12 February 2024).
- Codex Committee on Contaminants in Foods 16th Session Agenda Comments Submitted by United States of America. April 2023. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-735-16%252FCRDs%252Fcf16_CRD19%2528Rev%2529x.pdf (accessed on 12 February 2024).
- Report of the United States Delegate on the 16th Session of the Codex Committee on Contaminants in Foods. April 2023. Available online: https://www.usda.gov/sites/default/files/documents/cccf16-us-delegate-report.pdf (accessed on 12 February 2024).
- Codex Committee on Contaminants in Foods 16th Session Report of the Virtual Working Group on Priority List of Contaminants and Naturally Occurring Toxicants Proposed for Evaluation by JECFA. April 2023. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-735-16%252FCRDs%252Fcf16_CRD05x.pdf (accessed on 1 February 2024).
- Fong Sam, J.; Barber, C.; Gray, P. Determination of Thallium in Foods Containing Brassica. American Chemical Society Fall 2023 National Meeting. 16 August 2023. Available online: https://acs.digitellinc.com/p/s/determination-of-thallium-in-foods-containing-brassica-580534 (accessed on 2 July 2024).
- New Zealand Food Safety. 2024 NZTDS (Infants and Toddlers) Second Quarter Results. Updated 16 December 2024. Available online: https://www.mpi.govt.nz/food-business/food-monitoring-surveillance/new-zealand-total-diet-study/2024-nztds-infants-and-toddlers-second-quarter-results/ (accessed on 8 May 2025).
- Food and Drug Administration. List of Select Chemicals in the Food Supply Under FDA Review. Updated 4 March 2024. Available online: https://www.fda.gov/food/food-chemical-safety/list-select-chemicals-food-supply-under-fda-review (accessed on 12 August 2024).
- Food and Drug Administration. Environmental Contaminants in Food. Updated 24 July 2024. Available online: https://www.fda.gov/food/chemical-contaminants-pesticides/environmental-contaminants-food (accessed on 15 September 2024).
- U.S. EPA. Provisional Peer-Reviewed Toxicity Values for Thallium (Soluble Salts). U.S. Environmental Protection Agency, Washington, DC, EPA/690/R-12/026F. 2012. Available online: https://cfpub.epa.gov/ncea/pprtv/documents/ThalliumSolubleSalts.pdf (accessed on 12 February 2024).
| Sample Media | Number of Samples Collected | Range of Tl Found | Summary of Sample Locations |
|---|---|---|---|
| Surface soil * | 6 | ND (<0.11)–0.16 mg/kg | Children’s play area and various backyard locations, and family’s vacuum cleaner. |
| Drinking water | 4 | ND (<0.001) mg/L | Family’s private well and purchased water the family uses for drinking. |
| Indoor air | 7 | ND (<0.009) µg/m3 | Crawl space, attic, children’s bedrooms, living room, and kitchen. |
| Surface dust (wipe) ** | 8 | ND (<0.013) mg/sample | HVAC filter, used mobile air filter, the children’s slide in the backyard play area, and floor vents in all bedrooms and living room. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhury, A.; Fowles, J.; Bartlett, R.; Miller, M.D.; Durrani, T.; Harrison, R.; Barreau, T. Thallium Exposure Secondary to Commercial Kale Chip Consumption: California Case Highlights Opportunities for Improved Surveillance and Toxicological Understanding. Int. J. Environ. Res. Public Health 2025, 22, 1235. https://doi.org/10.3390/ijerph22081235
Choudhury A, Fowles J, Bartlett R, Miller MD, Durrani T, Harrison R, Barreau T. Thallium Exposure Secondary to Commercial Kale Chip Consumption: California Case Highlights Opportunities for Improved Surveillance and Toxicological Understanding. International Journal of Environmental Research and Public Health. 2025; 22(8):1235. https://doi.org/10.3390/ijerph22081235
Chicago/Turabian StyleChoudhury, Asha, Jefferson Fowles, Russell Bartlett, Mark D. Miller, Timur Durrani, Robert Harrison, and Tracy Barreau. 2025. "Thallium Exposure Secondary to Commercial Kale Chip Consumption: California Case Highlights Opportunities for Improved Surveillance and Toxicological Understanding" International Journal of Environmental Research and Public Health 22, no. 8: 1235. https://doi.org/10.3390/ijerph22081235
APA StyleChoudhury, A., Fowles, J., Bartlett, R., Miller, M. D., Durrani, T., Harrison, R., & Barreau, T. (2025). Thallium Exposure Secondary to Commercial Kale Chip Consumption: California Case Highlights Opportunities for Improved Surveillance and Toxicological Understanding. International Journal of Environmental Research and Public Health, 22(8), 1235. https://doi.org/10.3390/ijerph22081235






