Precision Medicine for Cancer and Health Equity in Latin America: Generating Understanding for Policy and Health System Shaping
Abstract
1. Introduction
2. Objectives and Methodologies
3. Background
3.1. Impact of Cancer in LatAm and the Caribbean
3.2. Challenges for Cancer Care in LatAm
3.3. Health Equity and Cancer
- The health sector needs to ensure that high-quality and effective services are available, accessible, and acceptable to everyone, everywhere, when they need them.
- Health and other sectors need to act on the wider structural determinants of health to tackle the inequitable distribution of power and resources, and to improve daily living conditions.
- The health sector needs to take the lead in monitoring health inequities through monitoring health outcomes and health service delivery, as well as working with other sectors to monitor people’s living conditions.
- Redesigning health systems for equity, e.g., pooling financial resources to enhance redistributive capacity.
- Prioritizing the primary health care approach, e.g., investment of 1% of GDP in PHC.
- Tackling structural determinants such as sexism, racism, ageism, classism, and ableism.
- Addressing harmful gender norms and gender inequalities in health policies/services/programs, and having more women in leadership positions and decision-making processes.
- Protecting and increasing investment in health and other social sectors (through universal health coverage [UHC], education, and broader social protection).
- Ensuring equitable services and infrastructure in both urban and rural areas to ensure everyone can lead healthy lives.
- Continuing to monitor health inequalities and the impact of action.
4. Literature Review
4.1. Literature Review of Precision Medicine, Its Application, Advancements, and Future Prospects
4.1.1. Precision Medicine
- Epigenomics: Chemical modifications of DNA, histones, non-histone chromatin proteins, and nuclear RNA;
- Transcriptomics: Gene expression pattern in a cell/tissue;
- Proteomics: Proteins expressed by a biological system;
- Metabolomics: Metabolites and their fluctuations related to internal (genetic) and external factors (environment);
- Phenomics: Measurable physical and chemical outcomes of the interactions between genes and the environment that are experienced by individuals and influence their phenotypes.
4.1.2. Precision Medicine and Oncology
- Checkpoint blockade: A type of drug that blocks proteins called checkpoints that are made by some types of immune system cells, such as T cells, and some cancer cells [181].
- CAR T-cells: Therapies made by collecting T cells from the patient and re-engineering them in the laboratory to produce proteins on their surface called chimeric antigen receptors, or CARs. The CARs recognize and bind to specific proteins, or antigens, on the surface of cancer cells to kill them. Currently available CAR T cell therapies are customized for each individual patient [182].
- Personalized vaccinomics: Vaccinomics combines the fields of immunogenetics, immunogenomics, immunoproteomics, and basic immunology to create vaccines that are tailor-made to an individual or groups of individuals. This broad range of omics applications to tumor immunology includes antigen discovery, diagnostic biomarkers, cancer vaccine development, predictors of immune response, and clinical response biomarkers [183].
- Liquid biopsy: Emerging technology that detects genomic information in bodily fluids and could alter traditional pathways of care for cancer. The technology is based on growing evidence that among certain cancers, tumor cells can release DNA into bodily fluids [184], known as circulating tumor DNA (ctDNA). ctDNA has been shown to harbor tumor-specific abnormalities, making it useful for diagnosis, treatment monitoring, and prognosis. It is increasingly used as a cancer biomarker, potentially contributing to improved clinical outcomes in certain cancer types, with ongoing clinical validation for different types of tumors, including non-small cell lung cancer and breast cancer [185].
4.1.3. The Value of Precision Medicine
- The human epidermal growth factor receptor (HER2) gene is overexpressed in 15% to 20% of breast cancers and in other cancer types such as gastric, colon, and head and neck. Drugs such as trastuzumab (ADC), pertuzumab (ADC), ado-trastuzumab emtansine (ADC), lapatinib (TKI), and trastuzumab deruxtecan (ADC) have been approved by the US FDA as targeted treatments.
- Epidermal growth factor receptor (EGFR) is expressed on the cell surface, with activating EGFR mutations commonly observed in patients with adenocarcinomas with no prior history of smoking, as well as in females and those of Asian descent, with targeted therapies including gefitinib, erlotinib, afatinib, dacomitinib, and osimertinib (all TKIs).
- Somatic rearrangements of the anaplastic lymphoma kinase (ALK) create common oncogenic fusions that lead to activation of different pathways in non-small cell lung cancer. Like HER2, ALK is a cell surface protein that regulates cell signaling pathways, and targeted therapy drugs include crizotinib, ceritinib, alectinib, and lorlatinib (all TKIs).
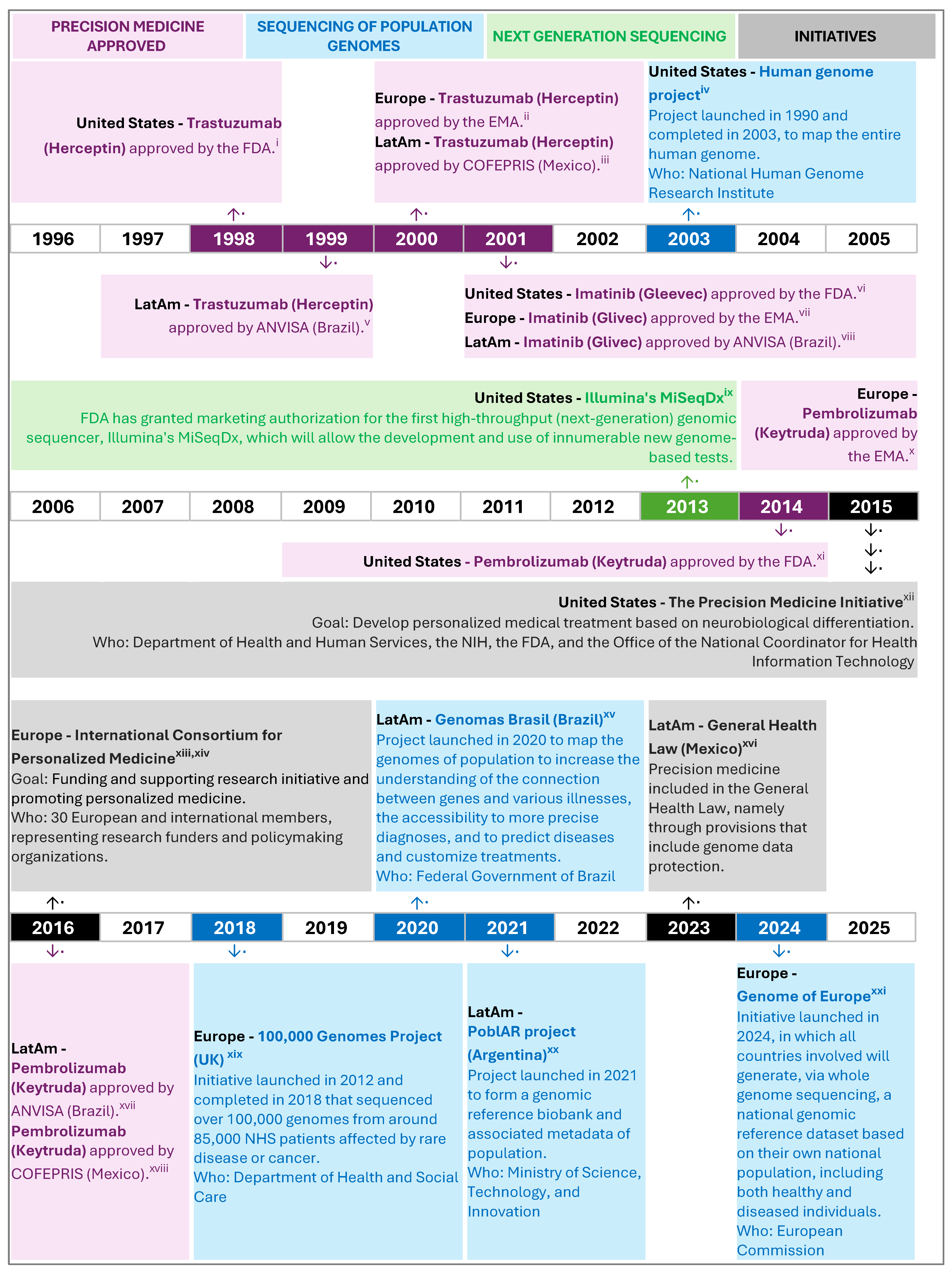
4.1.4. Policy Landscape of Precision Medicine and Oncology in LatAm
| Country | Precision Medicine Policies, Programs, Research Projects, Laws | Issuing Authority | Main Objectives |
|---|---|---|---|
| Argentina | Reference Program and genomic biobank of the Argentine population (2021) [237,238] | Ministry of Science, Technology, and Innovation | Design, launch, and consolidate a genomic reference biobank and associated metadata of the Argentine population [231]. |
| National Biobank of Biological Samples (2017–2019) [239] Clinical genomics of pediatric diseases (2018–2019) [240] Argentine tumor genomics action map (2018–2019) [233] Development of a biotechnology platform for the application of Precision Medicine in Cancer and Uncommon Diseases in Argentina (2018–2019) [234] | Ministry of Science, Technology, and Innovation | Strategic projects financed by the Argentine government to establish a national biobank, develop and locally implement genomics technologies and protocols, implement a second-generation massive sequencing platform to improve cancer treatments, facilitate the rapid translation of diagnostic/predictive products and services for clinical treatments in the field of cancer. | |
| Brazil | National Program for Genomics and Precision Health—Genomes Brazil (2020) [232] | Ministry of Health | Establish a reference genome for the Brazilian population, create a national database of genomic and clinical data, enhance scientific capacity and intellectual capital in genomic medicine, bolster the national industry for genomic products, and train the healthcare workforce in precision health. |
| Colombia | Law 2287—Regulates the operation of Biobanks and creates the National Biobank System (2023) [241] | Ministry of Health and Social Protection | Creates the National Biobank System and regulates the constitution, organization, and operation of biobanks in Colombia for the purposes of biomedical and technological research for the obtaining, use, processing, storage, transportation, and transfer of human biological samples, etc. |
| Mexico | General Health Law (LGS) (Last updated 2024) [235] | Ministry of Health | Provisions ensure individuals own their genomes, prevent genetic discrimination, mandate explicit consent for genome studies, uphold confidentiality of genetic data, respect individuals’ preferences on genetic test awareness, and prioritize health protection in related research and innovation. |
| Panama | No national precision medicine initiative was identified |
4.1.5. How Precision Medicine Can Enable Equitable Cancer Care
4.2. Literature Review of Policy Gaps and Challenges Implementing Precision Medicine in LatAm
4.2.1. Challenges Regarding Scientific Development of Precision Medicine
4.2.2. Challenges Implementing Precision Medicine in Healthcare Delivery Services
4.2.3. Challenges Regarding Economic Issues
4.2.4. Challenges Regarding Policies and Regulations for Implementation of Precision Medicine
5. Discussion
- (a)
- Explore the impact and implications of genetic heterogeneity in LatAm populations and address the low number of oncology-related clinical trials related to targeted therapies in the region.
- (b)
- Build adequate infrastructure and facilities for cancer care, train health professionals, and better distribute resources and/or deliver virtual consultations, when possible, to address geographic and cultural barriers whilst optimizing patient-centricity.
- (c)
- Sustainably finance public health systems, simplify reimbursement processes, and address issues of affordability and availability of targeted anti-cancer drugs in the public health system.
- (d)
- Ensure that regulations and policies keep pace with science to support the comprehensive implementation of precision medicine for cancer, including compliance with laws, regulatory pathways, and patient safety.
6. Recommendations (Suggested Solutions)
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mount Sinai. Targeted Therapies for Cancer. 2024. Available online: https://www.mountsinai.org/health-library/special-topic/targeted-therapies-for-cancer (accessed on 8 July 2024).
- National Library of Medicine. What Is Precision Medicine? 2022. Available online: https://medlineplus.gov/genetics/understanding/precisionmedicine/definition/ (accessed on 1 April 2024).
- National Cancer Institute. Biomarker Testing. 2022. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/biomarker-testing (accessed on 14 July 2025).
- National Cancer Institute. Targeted Therapy to Treat Cancer. 2022. Available online: https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies (accessed on 14 July 2025).
- World Health Organization. Health Technologies. 2023. Available online: https://www.who.int/europe/news-room/fact-sheets/item/health-technologies (accessed on 13 June 2024).
- Mathur, S.; Sutton, J. Personalized medicine could transform healthcare. Biomed. Rep. 2017, 7, 3–5. [Google Scholar] [CrossRef] [PubMed]
- CSDH. Closing the Gap in a Generation: Health Equity Through Action on the Social Determinants of Health. Final Report of the Commission on Social Determinants of Health. Geneva. 2008. Available online: https://iris.who.int/bitstream/handle/10665/43943/9789241563703_eng.pdf (accessed on 20 July 2024).
- World Health Organization. Health Equity. 2024. Available online: https://www.who.int/health-topics/health-equity (accessed on 16 April 2024).
- Nature Medicine. Precision medicine needs an equity agenda. Nat. Med. 2021, 27, 737. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.J.; Bowen, S.; Dotson, W.D.; Drzymalla, E.; Green, R.F.; Goldstein, R.; Kolor, K.; Liburd, L.C.; Spearling, L.S.; Bunnell, R. Health equity in the implementation of genomics and precision medicine: A public health imperative. Genet. Med. 2022, 24, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Global Cancer Observatory—United States of America. Cancer Today. [Fact Sheet]. 2024. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/840-united-states-of-america-fact-sheet.pdf (accessed on 15 August 2024).
- International Agency for Research on Cancer. Global Cancer Observatory—World. Cancer Today. [Fact Sheet]. 2024. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/900-world-fact-sheet.pdf (accessed on 29 February 2024).
- López-Cortés, A.; Guerrero, S.; Redal, M.; Alvarado, A.; Quiñones, L. State of Art of Cancer Pharmacogenomics in Latin American Populations. Int. J. Mol. Sci. 2017, 18, 639. [Google Scholar] [CrossRef]
- Ruiz, R.; Strasser-Weippl, K.; Touya, D.; Herrero Vincent, C.; Hernandez-Blanquisett, A.; St Louis, J.; Bukowski, A.; Goss, P.E. Improving access to high-cost cancer drugs in Latin America: Much to be done. Cancer 2017, 123, 1313–1323. [Google Scholar] [CrossRef]
- Organization for Economic Co-Operation and Development. Health at a Glance: Latin America and the Caribbean 2023; OECD: Paris, France, 2023. [Google Scholar]
- World Health Organization. Cancer Fact Sheet. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 10 April 2024).
- Piñeros, M.; Laversanne, M.; Barrios, E.; de Camargo Cancela, M.; de Vries, E.; Pardo, C.; Bray, F. An updated profile of the cancer burden, patterns and trends in Latin America and the Caribbean. Lancet Reg. Health Am. 2022, 13, 100294. [Google Scholar] [CrossRef]
- Sarfati, D. Why Social Inequalities Matter in the Cancer Continuum; IARC Scientific Publications: Lyon, France, 2019. [Google Scholar]
- Espina, C.; Feliu, A.; Maza, M.; Almonte, M.; Ferreccio, C.; Finck, C.; Herrero, F.; Dommarco, J.R.; Almeida, L.M.; Arrosi, S.; et al. Latin America and the Caribbean Code Against Cancer 1st Edition: 17 cancer prevention recommendations to the public and to policy-makers (World Code Against Cancer Framework). Cancer Epidemiol. 2023, 86, 102402. [Google Scholar] [CrossRef]
- Social Development Division of the Economic Commission for Latin America and the Caribbean. Social Panorama of Latin America and the Caribbean 2023: Labour Inclusion as a Key Axis of Inclusive Social Development. 2023. Available online: https://hdl.handle.net/11362/68703 (accessed on 15 April 2024).
- World Bank. Poverty Headcount Ratio at National Poverty Lines (% of Population)—Argentina, Brazil, Colombia, Mexico, Panama. 2024. Available online: https://data.worldbank.org/indicator/SI.POV.NAHC?locations=AR-BR-CO-MX-PA (accessed on 23 May 2024).
- Agência IBGE. Poverty Drops to 31.6% of the Population in 2022, After Reaching 36.7% in 2021. 2023. Available online: https://agenciadenoticias.ibge.gov.br/en/agencia-news/2184-news-agency/news/38574-poverty-drops-to-31-6-of-the-population-in-2022-after-reaching-36-7-in-2021 (accessed on 23 May 2024).
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results. [Database]. Institute for Health Metrics and Evaluation (IHME). 2020. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 29 February 2024).
- International Agency for Research on Cancer. Global Cancer Observatory—Latin America and the Caribbean. Cancer Today. [Fact Sheet]. 2024. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/904-latin-america-and-the-caribbean-fact-sheet.pdf (accessed on 29 February 2024).
- United Nations. Revision of World Population Prospects. Department of Economic and Social Affairs. Population Division. 2022. Available online: https://population.un.org/wpp/ (accessed on 19 June 2024).
- World Health Organization. Cancer Tomorrow: Estimated Number of New Cases from 2022 to 2050, Incidence, Both Sexes, Age [0–85+]. Available online: https://gco.iarc.who.int/tomorrow/en/dataviz/tables?mode=population&years=2050 (accessed on 31 July 2025).
- International Agency for Research on Cancer. Global Cancer Observatory—Argentina. Cancer Today. [Fact Sheet]. 2024. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/32-argentina-fact-sheet.pdf (accessed on 29 February 2024).
- International Agency for Research on Cancer. Global Cancer Observatory—Brazil. Cancer Today. [Fact Sheet]. 2024. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/76-brazil-fact-sheet.pdf (accessed on 29 February 2024).
- International Agency for Research on Cancer. Global Cancer Observatory—Colombia. Cancer Today. [Fact Sheet]. 2024. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/170-colombia-fact-sheet.pdf (accessed on 29 February 2024).
- International Agency for Research on Cancer. Global Cancer Observatory—Mexico. Cancer Today. [Fact Sheet]. 2024. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/484-mexico-fact-sheet.pdf (accessed on 29 February 2024).
- International Agency for Research on Cancer. Global Cancer Observatory—Panama. Cancer Today. [Fact Sheet]. 2024. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/591-panama-fact-sheet.pdf (accessed on 29 February 2024).
- Barrios, C.H.; Werutsky, G.; Mohar, A.; Ferrigno, A.S.; Müller, B.G.; Bychkovsky, B.L.; Castro, E.C.J.; Uribe, C.J.; Villarreal-Garza, C.; Soto-Perez-de-Celis, E.; et al. Cancer control in Latin America and the Caribbean: Recent advances and opportunities to move forward. Lancet Oncol. 2021, 22, e474–e487. [Google Scholar] [CrossRef]
- Prager, G.W.; Braga, S.; Bystricky, B.; Qvortrup, C.; Criscitiello, C.; Esin, E. Global cancer control: Responding to the growing burden, rising costs and inequalities in access. ESMO Open 2018, 3, e000285. [Google Scholar] [CrossRef]
- Worldometer. GDP per Capita. 2024. Available online: https://www.worldometers.info/gdp/gdp-per-capita/ (accessed on 29 February 2024).
- Ries, L.; Trama, A.; Nakata, K.; Gatta, G.; Botta, L.; Bleyer, A. Cancer Incidence, Survival, and Mortality Among Adolescents and Young Adults; Springer: Cham, Switzerland, 2017; pp. 7–42. [Google Scholar]
- Gupta, S.; Harper, A.; Ruan, Y.; Barr, R.; Frazier, A.L.; Ferlay, J.; Steliarova-Foucher, E.; Fidler-Benaoudia, M.M. International Trends in the Incidence of Cancer Among Adolescents and Young Adults. JNCI J. Natl. Cancer Inst. 2020, 112, 1105–1117. [Google Scholar] [CrossRef]
- Rice, D.P. Cost of illness studies: What is good about them? Inj. Prev. 2000, 6, 177–179. [Google Scholar] [CrossRef]
- Marmot, M.; Wilkinson, R. Social Determinants of Health; Marmot, M., Wilkinson, R., Eds.; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories From 2020 to 2050. JAMA Oncol. 2023, 9, 465. [Google Scholar] [CrossRef]
- de la Torre-Luque, A.; Gambara, H.; López, E.; Cruzado, J.A. Psychological treatments to improve quality of life in cancer contexts: A meta-analysis. Int. J. Clin. Health Psychol. 2016, 16, 211–219. [Google Scholar] [CrossRef]
- Finck, C.; Barradas, S.; Zenger, M.; Hinz, A. Quality of life in breast cancer patients: Associations with optimism and social support. Int. J. Clin. Health Psychol. 2018, 18, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Cardona, R.; Romero-Prada, M.; Ocampo, M.V.; Gallo, D.; Gómez, L.M.; Clavijo, N. Utility and health-related quality of life measures in adult Colombian patients with solid tumours. Ecancermedicalscience 2021, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Santos MN dos, Brito RG de. Qualidade de vida em pacientes com diagnóstico de câncer no Brasil: Uma revisão sistemática. Res. Soc. Dev. 2022, 19, e28511830635. [Google Scholar]
- Mejía-Rojas, M.E.; Contreras-Rengifo, A.; Hernández-Carrillo, M. Calidad de vida en mujeres con cáncer de mama sometidas a quimioterapia en Cali, Colombia. Biomédica 2020, 40, 349–361. [Google Scholar] [CrossRef]
- Gonzalez, L.; Bardach, A.; Palacios, A.; Peckaitis, C.; Ciapponi, A.; Pichón-Riviere, A.; Augustovski, F. Health-Related Quality of Life in Patients with Breast Cancer in Latin America and the Caribbean: A Systematic Review and Meta-Analysis. Oncologist 2021, 26, e794–806. [Google Scholar] [CrossRef]
- Sierra-Guerra, K.L.; Viveros-Contreras, C.; Martínez-Carrillo, G.; Hernández-León, O.; Caballero-Ambriz, G. Calidad de vida en pacientes con cáncer de próstata, operados de prostatectomía radical laparoscópica. Rev. Mex. Urol. 2014, 74, 133–140. [Google Scholar] [CrossRef][Green Version]
- Acevedo-Ibarra, J.N.; Juárez-García, D.M.; Espinoza-Velazco, A.; Buenaventura-Cisneros, S. Quality of life in Mexican colorectal cancer patients: Analysis with sociodemographic, medical, and psychological variables. Psychol Health Med. 2021, 26, 53–66. [Google Scholar] [CrossRef]
- Guerra-Martín, M.D.; Casado-Espinosa, M.D.R.; Gavira-López, Y.; Holgado-Castro, C.; López-Latorre, I.; Borrallo-Riego, Á. Quality of Life in Caregivers of Cancer Patients: A Literature Review. Int. J. Environ. Res. Public Health 2023, 20, 1570. [Google Scholar] [CrossRef]
- Molassiotis, A.; Wang, M. Understanding and Supporting Informal Cancer Caregivers. Curr. Treat. Options Oncol. 2022, 23, 494–513. [Google Scholar] [CrossRef]
- Espinola, N.; Pichon-Riviere, A.; Casarini, A.; Alcaraz, A.; Bardach, A.; Williams, C.; Cairoli, F.R.; Augustovski, F.; Palacios, A. Making visible the cost of informal caregivers’ time in Latin America: A case study for major cardiovascular, cancer and respiratory diseases in eight countries. BMC Public Health 2023, 23, 28. [Google Scholar] [CrossRef] [PubMed]
- Xiang, E.; Guzman, P.; Mims, M.; Badr, H. Balancing Work and Cancer Care: Challenges Faced by Employed Informal Caregivers. Cancers 2022, 14, 4146. [Google Scholar] [CrossRef] [PubMed]
- United Nations Economic Commission for Europe. Supporting Informal Carers—Six Policy Challenges and How to Meet Them. 2019. Available online: https://unece.org/population/news/supporting-informal-carers-six-policy-challenges-and-how-meet-them (accessed on 15 June 2024).
- World Health Organization. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All. 2020. Available online: https://www.who.int/publications/i/item/9789240001299 (accessed on 16 August 2024).
- Llera, A.S. A fresh perspective on Latin America cancer care: Uncovering hidden messages in unconventional data sources. Lancet Reg. Health Am. 2023, 24, 100559. [Google Scholar] [CrossRef] [PubMed]
- de Lemos, L.L.P.; Carvalho de Souza, M.; Pena Moreira, D.; Ribeiro Fernandes Almeida, P.H.; Godman, B.; Verguet, S.; Junior, A.A.G.; Cherchiglia, M.L. Stage at diagnosis and stage-specific survival of breast cancer in Latin America and the Caribbean: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0224012. [Google Scholar] [CrossRef]
- Carioli, G.; Bertuccio, P.; Malvezzi, M.; Rodriguez, T.; Levi, F.; Boffetta, P.; La Vecchia, C.; Negri, E. Cancer mortality predictions for 2019 in Latin America. Int. J. Cancer 2020, 147, 619–632. [Google Scholar] [CrossRef]
- Brand, N.R.; Qu, L.G.; Chao, A.; Ilbawi, A.M. Delays and Barriers to Cancer Care in Low- and Middle-Income Countries: A Systematic Review. Oncologist 2019, 24, e1371–e1380. [Google Scholar] [CrossRef]
- Unger-Saldaña, K. Challenges to the early diagnosis and treatment of breast cancer in developing countries. World J. Clin. Oncol. 2014, 5, 465. [Google Scholar] [CrossRef]
- Werutsky, G.; Barrios, C.H.; Cardona, A.F.; Albergaria, A.; Valencia, A.; Ferreira, C.G. Perspectives on emerging technologies, personalised medicine, and clinical research for cancer control in Latin America and the Caribbean. Lancet Oncol. 2021, 22, e488–e500. [Google Scholar] [CrossRef]
- Cazap, E. Breast Cancer in Latin America: A Map of the Disease in the Region. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Rosa, D.D.; Bines, J.; Werutsky, G.; Barrios, C.H.; Cronemberger, E.; Queiroz, G.S.; Lima, V.C.C.; Freitas-Júnior, R.; Couto, J.O.; Emerenciano, K.; et al. The impact of sociodemographic factors and health insurance coverage in the diagnosis and clinicopathological characteristics of breast cancer in Brazil: AMAZONA III study (GBECAM 0115). Breast Cancer Res. Treat. 2020, 183, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cannon, B.A.; Zertuche-Maldonado, T.; de la Rosa Pacheco, S.; Cardona-Huerta, S.; Canavati-Marcos, M.; Gomez-Macias, G.S.; Villarreal-Garza, C. Comparison of characteristics in Mexican women with breast cancer according to healthcare coverage. Women’s Health 2020, 16, 174550652094941. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro de Lima, V.C.; Gelatti, A.; Moura, J.F.P.; Fares, A.F.; de Castro, G.; Mathias, C.; Terra, R.M.; Werustky, G.; Corassa, M.; Araújo, L.H.L.; et al. Health Services Access Inequalities in Brazil Result in Poorer Outcomes for Stage III NSCLC—RELANCE/LACOG 0118. JTO Clin. Res. Rep. 2024, 5, 100646. [Google Scholar] [CrossRef]
- Nuche-Berenguer, B.; Sakellariou, D. Socioeconomic determinants of cancer screening utilisation in Latin America: A systematic review. PLoS ONE 2019, 14, e0225667. [Google Scholar] [CrossRef]
- Ruiz de Castilla, E.M.; Mayrides, M.; González, H.; Vidangossy, F.; Corbeaux, T.; Ortiz, N. Implementing precision oncology in Latin America to improve patient outcomes: The status quo and a call to action for key stakeholders and decision-makers. Ecancermedicalscience 2024, 8, 18. [Google Scholar] [CrossRef]
- Moye-Holz, D.; Soria Saucedo, R.; van Dijk, J.P.; Reijneveld, S.A.; Hogerzeil, H.V. Access to innovative cancer medicines in a middle-income country—The case of Mexico. J. Pharm. Policy Pract. 2018, 11, 25. [Google Scholar] [CrossRef]
- Vargas-Pelaez, C.M.; Rover, M.R.M.; Soares, L.; Blatt, C.R.; Mantel-Teeuwisse, A.K.; Rossi, F.A.; Restrepo, L.G.; Latorre, M.C.; López, J.J.; Bürgin, M.T.; et al. Judicialization of access to medicines in four Latin American countries: A comparative qualitative analysis. Int. J. Equity Health 2019, 18, 68. [Google Scholar] [CrossRef]
- Chagas, V.O.; Provin, M.P.; Mota, P.A.P.; Guimarães, R.A.; Amaral, R.G. Institutional strategies as a mechanism to rationalize the negative effects of the judicialization of access to medicine in Brazil. BMC Health Serv. Res. 2020, 20, 80. [Google Scholar] [CrossRef]
- Andia, T.S.; Lamprea, E. Is the judicialization of health care bad for equity? A scoping review. Int. J. Equity Health 2019, 18, 61. [Google Scholar] [CrossRef]
- Salha, L.A.; Reis, F.C.; Gonçalves, R.M.; Lima JHda, S.; Salha, N.A.; Pinto, R.P.; Menezes, J.E.; Oliveira, E.P.; Ferreira, P.L.; Barbosa, M.A. Judicialization of health: Profile of demands for oncological medicines in a state in the central region of Brazil. Int. J. Equity Health 2022, 21, 112. [Google Scholar] [CrossRef]
- Biehl, J.; Socal MP, Amon, J. J. The Judicialization of Health and the Quest for State Accountability: Evidence from 1262 Lawsuits for Access to Medicines in Southern Brazil. Health Hum. Rights 2016, 18, 209–220. [Google Scholar]
- Civil Society Engagement Mechanism. Why the 2019 UN High-Level Meeting on Universal Health Coverage Should Encourage All Countries to Achieve This Target. 2019. Available online: https://csemonline.net/wp-content/uploads/2019/07/WHY-5-of-GDP-1.pdf (accessed on 11 December 2024).
- Our World in Data. Government Health Expenditure as a Share of GDP, 1880 to 2021. 2024. Available online: https://ourworldindata.org/grapher/public-health-expenditure-share-gdp (accessed on 11 December 2024).
- Piñeros, M.; Abriata, M.G.; de Vries, E.; Barrios, E.; Bravo, L.E.; Cueva, P.; Cancela, M.C.; Fernández, L.; Gil, E.; Luciani, S.; et al. Progress, challenges and ways forward supporting cancer surveillance in Latin America. Int. J. Cancer 2021, 149, 12–20. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health Inequality Monitor- About WHO’s Work on Health Inequality Monitoring. 2024. Available online: https://www.who.int/data/inequality-monitor/about (accessed on 19 March 2024).
- Aday, L.A.; Fleming, G.V.; Andersen, R. Access to Medical Care in the U.S.: Who Has It, Who Doesn’t; Pluribus Press: Chicago, IL, USA, 1984; p. 240. [Google Scholar]
- Whitehead, M. The Concepts and Principles of Equity and Health. Int. J. Health Serv. 1992, 22, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, D.; Lee, S.; Fawcett, J. The concepts of health inequality, disparities and equity in the era of population health. Appl. Nurs. Res. 2020, 56, 151367. [Google Scholar] [CrossRef] [PubMed]
- Arcaya, M.C.; Arcaya, A.L.; Subramanian, S.V. Inequalities in health: Definitions, concepts, and theories. Glob. Health Action 2015, 8, 27106. [Google Scholar] [CrossRef]
- Pan American Health Organization. Methodological summaries: Measuring inequity in health. Epidemiol. Bull. 1999, 20, 11. [Google Scholar]
- Yao, Q.; Li, X.; Luo, F.; Yang, L.; Liu, C.; Sun, J. The historical roots and seminal research on health equity: A referenced publication year spectroscopy (RPYS) analysis. Int. J. Equity Health 2019, 18, 152. [Google Scholar] [CrossRef]
- World Health Organization. Constitution of the World Health Organization. 1948. Available online: https://www.who.int/about/governance/constitution (accessed on 23 May 2024).
- UN General Assembly. Universal Declaration of Human Rights|United Nations. General Assembly Resolution. 1948. Available online: https://documents.un.org/doc/resolution/gen/nr0/043/88/pdf/nr004388.pdf (accessed on 20 July 2024).
- World Health Organization. Report of the International Conference on Primary Health Care, Alma-Ata, USSR, 6–12 September 1978. 1978. Available online: https://iris.who.int/bitstream/handle/10665/39228/9241800011.pdf?sequence=1 (accessed on 17 May 2024).
- World Health Organization. Health in 2015: From MDGs, Millennium Development Goals to SDGs, Sustainable Development Goals. 2015. Available online: https://iris.who.int/handle/10665/200009 (accessed on 15 March 2024).
- Braveman, P.; Arkin, E.; Orleans, T.; Proctor, D.; Acker, J.; Plough, A. What is Health Equity? Behav. Sci. Policy 2018, 4, 1–14. [Google Scholar] [CrossRef]
- Aldrighetti, C.M.; Niemierko, A.; Van Allen, E.; Willers, H.; Kamran, S.C. Racial and Ethnic Disparities Among Participants in Precision Oncology Clinical Studies. JAMA Netw. Open 2021, 4, e2133205. [Google Scholar] [CrossRef]
- Edwards, T.L.; Breeyear, J.; Piekos, J.A.; Velez Edwards, D.R. Equity in Health: Consideration of Race and Ethnicity in Precision Medicine. Trends Genet. 2020, 36, 807–809. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, M.M.; Norato, L.F.; Friedman, E.A.; Armbrister, A.N. Planning for health equity in the Americas: An analysis of national health plans. Rev. Panam. Salud Pública 2021, 45, 1. [Google Scholar] [CrossRef]
- Ruano, A.L.; Rodríguez, D.; Rossi, P.G.; Maceira, D. Understanding inequities in health and health systems in Latin America and the Caribbean: A thematic series. Int. J. Equity Health 2021, 20, 94. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Equity in Cancer Prevention and Control. 2023. Available online: https://www.cdc.gov/cancer/health-equity/equity.htm (accessed on 16 April 2024).
- Kale, S.; Hirani, S.; Vardhan, S.; Mishra, A.; Ghode, D.B.; Prasad, R.; Wanjari, M. Addressing Cancer Disparities Through Community Engagement: Lessons and Best Practices. Cureus 2023, 14, e43445. [Google Scholar] [CrossRef]
- National Cancer Institute. Cancer Disparities. 2024. Available online: https://www.cancer.gov/about-cancer/understanding/disparities (accessed on 16 April 2024).
- World Health Organization. Improving Service Access and Quality. Available online: https://www.who.int/activities/improving-service-access-and-quality (accessed on 13 June 2024).
- World Health Organization. Universal Health Coverage. Report by the Director-General. 154th Session. 2023. Available online: https://apps.who.int/gb/ebwha/pdf_files/EB154/B154_6-en.pdf (accessed on 13 June 2024).
- World Health Organization. Retention of the Health Workforce in Rural and Remote Areas: A Systematic Review. 2020. Available online: https://iris.who.int/bitstream/handle/10665/337300/9789240013865-eng.pdf?sequence=1 (accessed on 16 August 2024).
- Thomas, S.; Sagan, A.; Larkin, J.; Cylus, J.; Figueiras, J.; Karanikolos, M. Strengthening Health Systems Resilience: Key Concepts and Strategies; European Observatory Policy Briefs: Copenhagen, Denmark, 2020. [Google Scholar]
- Kieny, M.P.; Bekedam, H.; Dovlo, D.; Fitzgerald, J.; Habicht, J.; Harrison, G.; Kluge, H.; Lin, V.; Menabde, N.; Mirza, Z.; et al. Strengthening health systems for universal health coverage and sustainable development. Bull. World Health Organ. 2017, 95, 537–539. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Patient Engagement: Technical Series on Safer Primary Care. 2016. Available online: https://iris.who.int/bitstream/handle/10665/252269/9789241511629-eng.pdf?sequence=1 (accessed on 12 June 2024).
- Handtke, O.; Schilgen, B.; Mösko, M. Culturally competent healthcare—A scoping review of strategies implemented in healthcare organizations and a model of culturally competent healthcare provision. PLoS ONE 2019, 14, e0219971. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Cabrero, I.; Doimi, F.; Ortega, V.; de Oliveira Lima, J.T.; Torres, R.; Torregrosa, L. Recommendations for streamlining precision medicine in breast cancer care in Latin America. Cancer Rep. 2021, 4, e1400. [Google Scholar]
- Gilardino, R.E.; Valanzasca, P.; Rifkin, S.B. Has Latin America achieved universal health coverage yet? Lessons from four countries. Arch. Public Health 2022, 80, 38. [Google Scholar] [CrossRef]
- Pan American Health Organization. Universal Health- On the Road to Universal Health for Everyone, Everywhere. 2024. Available online: https://www.paho.org/en/universal-health (accessed on 11 December 2024).
- Abramovich, V.; Pautassi, L. Judicial activism in the Argentine health system: Recent trends. Health Hum. Rights 2008, 10, 53–65. [Google Scholar] [CrossRef]
- Filho, L.B. The Right to Health as a Tool of Social Control: Compulsory Treatment Orders by Courts in Brazil. Health Hum. Rights 2022, 24, 159–169. [Google Scholar]
- Rosa, R.M.; Alberto, I.C. Universal health care for Colombians 10 years after Law 100: Challenges and opportunities. Health Policy 2004, 68, 129–142. [Google Scholar] [CrossRef]
- Block, M.A.G.; Morales, H.R.; Hurtado, L.C.; Balandrán, A.; Méndez, E. Mexico- Health System Review. 2020. Available online: https://iris.who.int/handle/10665/334334 (accessed on 20 July 2024).
- Pan American Health Organization. Health Systems Profile Panama: Monitoring and Analyzing Health Systems Change. 2007. Available online: https://www3.paho.org/hq/dmdocuments/2010/Health_System_Profile-Panama_2008.pdf (accessed on 12 March 2024).
- Arai, R.J.; Guindalini, R.S.C.; Llera, A.S.; O’Connor, J.M.; Muller, B.; Lema, M.; Freitas, H.C.; Soria, T.; Delgado, L.; Landaverde, D.; et al. Personalizing Precision Oncology Clinical Trials in Latin America: An Expert Panel on Challenges and Opportunities. Oncologist 2019, 24, e709–e719. [Google Scholar] [CrossRef]
- Sussman, L.; Garcia-Robledo, J.E.; Ordóñez-Reyes, C.; Forero, Y.; Mosquera, A.F. Ruíz-Patiño, A.; Chamorro, D.F.; Cardona, A.F. Integration of artificial intelligence and precision oncology in Latin America. Front. Med. Technol. 2022, 4, 1007822. [Google Scholar] [CrossRef] [PubMed]
- Penchansky, R.; Thomas, J.W. The Concept of Access. Med. Care 1981, 19, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Azeredo-da-Silva, A.F.; Zanotto, B.S.; Martins, F.; Navarro, N.; Alencar, R.; Medeiros, C. Health care accessibility and mobility in breast cancer: A Latin American perspective. BMC Health Serv. Res. 2024, 24, 764. [Google Scholar]
- Malek Pascha, V.A.; Sun, L.; Gilardino, R.; Legood, R. Telemammography for breast cancer screening: A cost-effective approach in Argentina. BMJ Health Care Inform. 2021, 28, e100351. [Google Scholar] [CrossRef]
- Bogdanova, A.; Andrawos, C.; Constantinou, C. Cervical cancer, geographical inequalities, prevention and barriers in resource depleted countries (Review). Oncol. Lett. 2022, 23, 113. [Google Scholar] [CrossRef]
- Patel, M.I.; Lopez, A.M.; Blackstock, W.; Reeder-Hayes, K.; Moushey, E.A.; Phillips, J.; Tap, W. Cancer Disparities and Health Equity: A Policy Statement From the American Society of Clinical Oncology. J. Clin. Oncol. 2020, 38, 3439–3448. [Google Scholar] [CrossRef]
- Marmot, M. Health equity, cancer, and social determinants of health. Lancet Glob. Health 2018, 6, S29. [Google Scholar] [CrossRef]
- National Human Genome Research Institute. Precision Medicine. 2024. Available online: https://www.genome.gov/genetics-glossary/Precision-Medicine (accessed on 13 March 2024).
- Nature Mental Health. The right treatment for each patient: Unlocking the potential of personalized psychiatry. Nat. Ment. Health 2023, 1, 607–608. [Google Scholar] [CrossRef]
- Jalilian, L.; Cannesson, M. Precision medicine in anesthesiology. Int. Anesth. Clin. 2020, 58, 17–22. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Williams, L.M.; Steiner, J.; Leboyer, M.; Carvalho, A.F.; Berk, M. The new field of ‘precision psychiatry’. BMC Med. 2017, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, M.K.; Karaglani, M.; Panagopoulou, M.; Fiska, A.; Chatzaki, E. Are the Origins of Precision Medicine Found in the Corpus Hippocraticum? Mol. Diagn. Ther. 2017, 21, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Committee on Quality of Health Care in America. Crossing the Quality Chasm; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Watanabe, J.H.; Tarn, D.M.; Hirsch, J.D. Evolution of Precision Medicine: Applying a Population-Based Evidence Assessment Repository to Achieve Patient-Centered Outcomes at the Point of Care. Value Outcomes Spotlight 2021, 7 (Suppl. S2). Available online: https://www.ispor.org/publications/journals/value-outcomes-spotlight/vos-archives/issue/patients-versus-process-the-data-and-the-drivers-behind-patient-centered-outcomes (accessed on 8 July 2024).
- National Human Genome Research Institute. The Human Genome Project. 2024. Available online: https://www.genome.gov/human-genome-project (accessed on 13 March 2024).
- Koboldt, D.C.; Steinberg, K.M.; Larson, D.E.; Wilson, R.K.; Mardis, E.R. The Next-Generation Sequencing Revolution and Its Impact on Genomics. Cell 2013, 155, 27–38. [Google Scholar] [CrossRef]
- Brittain, H.K.; Scott, R.; Thomas, E. The rise of the genome and personalised medicine. Clin. Med. 2017, 17, 545–551. [Google Scholar] [CrossRef]
- The White House. President Obama’s Precision Medicine Initiative. 2015. Available online: https://obamawhitehouse.archives.gov/the-press-office/2015/01/30/fact-sheet-president-obama-s-precision-medicine-initiative (accessed on 21 March 2024).
- US Food and Drug Administration. Precision Medicine. 2018. Available online: https://www.fda.gov/medical-devices/in-vitro-diagnostics/precision-medicine (accessed on 13 March 2024).
- Baird, A.; Westphalen, C.B.; Blum, S.; Nafria, B.; Knott, T.; Sargeant, I.; Harnik, H.; Brooke, N.; Wicki, N.; Wong-Rieger, D. How can we deliver on the promise of precision medicine in oncology and beyond? A practical roadmap for action. Health Sci. Rep. 2023, 6, e1349. [Google Scholar] [CrossRef]
- Khoury, M.J.; Holt, K.E. The impact of genomics on precision public health: Beyond the pandemic. Genome Med. 2021, 13, 67. [Google Scholar] [CrossRef]
- Tebani, A.; Afonso, C.; Marret, S.; Bekri, S. Omics-Based Strategies in Precision Medicine: Toward a Paradigm Shift in Inborn Errors of Metabolism Investigations. Int. J. Mol. Sci. 2016, 17, 1555. [Google Scholar] [CrossRef]
- Fox, B.I.; Felkey, B.G. Precision Medicine: Considerable Potential Combined with Important Challenges. Hosp. Pharm. 2016, 51, 950–951. [Google Scholar] [CrossRef]
- Carlsten, C.; Brauer, M.; Brinkman, F.; Brook, J.; Daley, D.; McNagny, K.; Pui, M.; Royce, D.; Takaro, T.; Denburg, J. Genes, the environment and personalized medicine. EMBO Rep. 2014, 15, 736–739. [Google Scholar] [CrossRef]
- McGrath, S.; Ghersi, D. Building towards precision medicine: Empowering medical professionals for the next revolution. BMC Med. Genom. 2016, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.B.; Wei, W.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision Medicine, AI, and the Future of Personalized Health Care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Brennan, M.; Mohamed, B.; Rashidi, P.; Modave, F.; Tighe, P. Accessing Artificial Intelligence for Clinical Decision-Making. Front. Digit. Health 2021, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Awan, F.M.; Naz, A.; deAndrés-Galiana, E.J.; Alvarez, O.; Cernea, A.; Fernández-Brillet, L.; Fernández-Martínez, J.L.; Kloczkowski, A. Innovations in Genomics and Big Data Analytics for Personalized Medicine and Health Care: A Review. Int. J. Mol. Sci. 2022, 23, 4645. [Google Scholar] [CrossRef]
- Kalra, D. The Importance of Real-World Data to Precision Medicine. Per. Med. 2019, 16, 79–82. [Google Scholar] [CrossRef]
- Lamichhane, P.; Agrawal, A. Precision medicine and implications in medical education. Ann. Med. Surg. 2023, 85, 1342–1345. [Google Scholar] [CrossRef]
- Panduro, A.; Roman, S. Personalized Medicine in Latin America. Per. Med. 2020, 17, 339–343. [Google Scholar] [CrossRef]
- Stenzinger, A.; Moltzen, E.K.; Winkler, E.; Molnar-Gabor, F.; Malek, N.; Costescu, A.; Jensen, B.N.; Nowak, F.; Pinto, C.; Ottersen, O.P.; et al. Implementation of precision medicine in healthcare—A European perspective. J. Intern. Med. 2023, 294, 437–454. [Google Scholar] [CrossRef]
- Argotti, U.; Leyens, L.; Lisbona, C.; López, P.; Alonso-Orgaz, S.; Nevado, A.; Cozzi, V. Comparison of the Latin America Regulation Landscape and International Reference Health Authorities to Hasten Drug Registration and Clinical Research Applications. Ther. Innov. Regul. Sci. 2023, 57, 1287–1297. [Google Scholar] [CrossRef]
- Slikker, W. Biomarkers and their impact on precision medicine. Exp. Biol. Med. 2018, 243, 211–212. [Google Scholar] [CrossRef]
- World Health Organization. International Programme on Chemical Safety Biomarkers and Risk Assessment: Concepts and Principles. 1993. Available online: www.inchem.org/documents/ehc/ehc/ehc155.htm (accessed on 13 March 2024).
- Food and Drug Administration. About Biomarkers and Qualification. 2021. Available online: https://www.fda.gov/drugs/biomarker-qualification-program/about-biomarkers-and-qualification (accessed on 15 August 2024).
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Hoque, M.O. Development of biomarkers for real precision medicine. Transl. Lung Cancer Res. 2018, 7, S228–S231. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A. Biomarkers and Precision Medicine in Oncology Practice and Clinical Trials. In Advancing the Science of Cancer in Latinos; Springer: Cham, Switzerland, 2020; pp. 113–123. [Google Scholar]
- Orsini, A.; Diquigiovanni, C.; Bonora, E. Omics Technologies Improving Breast Cancer Research and Diagnostics. Int. J. Mol. Sci. 2023, 24, 12690. [Google Scholar] [CrossRef]
- Liao, J.; Li, X.; Gan, Y.; Han, S.; Rong, P.; Wang, W.; Li, W.; Zhou, L. Artificial intelligence assists precision medicine in cancer treatment. Front. Oncol. 2023, 12, 998222. [Google Scholar]
- Bhinder, B.; Gilvary, C.; Madhukar, N.S.; Elemento, O. Artificial Intelligence in Cancer Research and Precision Medicine. Cancer Discov. 2021, 11, 900–915. [Google Scholar] [CrossRef]
- Stein, M.K.; Oluoha, O.; Patel, K.; VanderWalde, A. Precision Medicine in Oncology: A Review of Multi-Tumor Actionable Molecular Targets with an Emphasis on Non-Small Cell Lung Cancer. J. Pers. Med. 2021, 11, 518. [Google Scholar] [CrossRef]
- The Lancet. 20 years of precision medicine in oncology. Lancet 2021, 397, 1781. [Google Scholar] [CrossRef]
- Vargas, A.J.; Harris, C.C. Biomarker development in the precision medicine era: Lung cancer as a case study. Nat. Rev. Cancer 2016, 16, 525–537. [Google Scholar] [CrossRef]
- Personalized Medicine Coalition. Personalized Medicine at FDA: A Progress & Outlook Report. 2018. Available online: https://www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/PM_at_FDA_A_Progress_and_Outlook_Report.pdf (accessed on 13 March 2024).
- Murciano-Goroff, Y.R.; Taylor, B.S.; Hyman, D.M.; Schram, A.M. Toward a More Precise Future for Oncology. Cancer Cell. 2020, 37, 431–442. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.H.; Khaja, U.M.; Ahmed, M.; Khan, W.Y.; Ganie, S.A. Pharmacogenomics in cancer. In Pharmacogenomics; Elsevier: Amsterdam, The Netherlands, 2023; pp. 195–221. [Google Scholar]
- Drugan, T.; Leucuța, D. Evaluating Novel Biomarkers for Personalized Medicine. Diagnostics 2024, 14, 587. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.T.; Westergaard, N. Predictive biomarkers and personalized pharmacotherapy. Expert. Rev. Mol. Diagn. 2022, 22, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dey, M.K.; Devireddy, R.; Gartia, M.R. Biomarkers in Cancer Detection, Diagnosis, and Prognosis. Sensors 2023, 24, 37. [Google Scholar] [CrossRef]
- Sheng, K.L.; Kang, L.; Pridham, K.J.; Dunkenberger, L.E.; Sheng, Z.; Varghese, R.T. An integrated approach to biomarker discovery reveals gene signatures highly predictive of cancer progression. Sci. Rep. 2020, 10, 21246. [Google Scholar] [CrossRef]
- Al-Tashi, Q.; Saad, M.B.; Muneer, A.; Qureshi, R.; Mirjalili, S.; Sheshadri, A.; Le, X.; Vokes, N.I.; Zhang, J.; Wu, J. Machine Learning Models for the Identification of Prognostic and Predictive Cancer Biomarkers: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 7781. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, S.; Wang, H.; Cai, X.; Wang, Q. Review of Personalized Medicine and Pharmacogenomics of Anti-Cancer Compounds and Natural Products. Genes 2024, 15, 468. [Google Scholar] [CrossRef]
- Chen, J.; Sun, M.; Shen, B. Deciphering oncogenic drivers: From single genes to integrated pathways. Brief. Bioinform. 2015, 16, 413–428. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Table of Pharmacogenomic Biomarkers in Drug Labeling. 2024. Available online: https://www.fda.gov/drugs/science-and-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling (accessed on 15 June 2024).
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [CrossRef]
- Dienstmann, R. WS04.02 Access to Biomarker Testing in Latin America. J. Thorac. Oncol. 2021, 16, S842. [Google Scholar] [CrossRef]
- Calderon-Aparicio, A.; Orue, A. Precision oncology in Latin America: Current situation, challenges and perspectives. Ecancermedicalscience 2019, 13, 920. [Google Scholar] [CrossRef] [PubMed]
- Schienda, J.; Stopfer, J. Cancer Genetic Counseling—Current Practice and Future Challenges. Cold Spring Harb. Perspect. Med. 2020, 10, a036541. [Google Scholar] [CrossRef] [PubMed]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Gavan, S.P.; Thompson, A.J.; Payne, K. The economic case for precision medicine. Expert. Rev. Precis. Med. Drug Dev. 2018, 3, 1–9. [Google Scholar] [CrossRef]
- Quinn, R.; Patel, R.; Sison, C.; Singh, A.; Zhu, X.H. Impact of Precision Medicine on Clinical Outcomes: A Single-Institution Retrospective Study. Front. Oncol. 2021, 31, 11. [Google Scholar] [CrossRef]
- Anderson, E.C.; DiPalazzo, J.; Lucas, F.L.; Hall, M.J.; Antov, A.; Helbig, P.; Bourne, J.; Graham, L.; Gaitor, L.; Lu-Emerson, C.; et al. Genome-matched treatments and patient outcomes in the Maine Cancer Genomics Initiative (MCGI). NPJ Precis. Oncol. 2024, 8, 67. [Google Scholar] [CrossRef]
- Haslem, D.S.; Chakravarty, I.; Fulde, G.; Gilbert, H.; Tudor, B.P.; Lin, K.; Ford, J.M.; Nadauld, L.D. Precision oncology in advanced cancer patients improves overall survival with lower weekly healthcare costs. Oncotarget 2018, 9, 12316–12322. [Google Scholar] [CrossRef]
- Kato, S.; Kim, K.H.; Lim, H.J.; Boichard, A.; Nikanjam, M.; Weihe, E. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat. Commun. 2020, 11, 4965. [Google Scholar] [CrossRef]
- Song, I.W.; Vo, H.H.; Chen, Y.S.; Baysal, M.A.; Kahle, M.; Johnson, A.; Tsimberidou, A.M. Precision Oncology: Evolving Clinical Trials across Tumor Types. Cancers 2023, 15, 1967. [Google Scholar] [CrossRef]
- Rulten, S.L.; Grose, R.P.; Gatz, S.A.; Jones, J.L.; Cameron, A.J.M. The Future of Precision Oncology. Int. J. Mol. Sci. 2023, 24, 12613. [Google Scholar] [CrossRef]
- Senft, D.; Leiserson, M.D.M.; Ruppin, E.; Ronai, Z.A. Precision Oncology: The Road Ahead. Trends Mol. Med. 2017, 23, 874–898. [Google Scholar] [CrossRef]
- Pich, O.; Bailey, C.; Watkins, T.B.K.; Zaccaria, S.; Jamal-Hanjani, M.; Swanton, C. The translational challenges of precision oncology. Cancer Cell 2022, 40, 458–478. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Immune Checkpoint Inhibitor. 2022. Available online: https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/checkpoint-inhibitors (accessed on 20 March 2024).
- National Cancer Institute. CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers. 2022. Available online: https://www.cancer.gov/about-cancer/treatment/research/car-t-cells (accessed on 20 March 2024).
- O’Meara, M.M.; Disis, M.L. Therapeutic Cancer Vaccines and Translating Vaccinomics Science to the Global Health Clinic: Emerging Applications Toward Proof of Concept. OMICS 2011, 15, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Basharat, S.; Smith, A.; Darvesh, N.; Rader, T. Watch List: Top 10 Precision Medicine Technologies and Issues; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2023. [Google Scholar]
- Pessoa, L.S.; Heringer, M.; Ferrer, V.P. ctDNA as a cancer biomarker: A broad overview. Crit. Rev. Oncol. Hematol. 2020, 155, 103109. [Google Scholar] [CrossRef] [PubMed]
- Pereira Cabral, B.; da Graça Derengowski Fonseca, M.; Batista Mota, F. What is the future of cancer care? A technology foresight assessment of experts’ expectations. Econ. Innov. New Technol. 2019, 28, 635–652. [Google Scholar] [CrossRef]
- Baxevanis, C.N. Biomarkers in the Era of Precision Oncology. Cancers 2023, 15, 1782. [Google Scholar] [CrossRef]
- Seyhan, A.A.; Carini, C. Are innovation and new technologies in precision medicine paving a new era in patients centric care? J. Transl. Med. 2019, 17, 114. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Iskander, N.G.; Hong, D.S.; Wheler, J.J.; Falchook, G.S.; Fu, S.; Piha-Paul, S.; Naing, M.; Janku, F.; Luthra, R.; et al. Personalized Medicine in a Phase I Clinical Trials Program: The MD Anderson Cancer Center Initiative. Clin. Cancer Res. 2012, 18, 6373–6383. [Google Scholar] [CrossRef]
- Radovich, M.; Kiel, P.J.; Nance, S.M.; Niland, E.E.; Parsley, M.E.; Ferguson, M.E.; Jiang, G.; Ammakkanavar, N.R.; Einhorn, L.H.; Cheng, L.; et al. Clinical benefit of a precision medicine based approach for guiding treatment of refractory cancers. Oncotarget 2016, 7, 56491–56500. [Google Scholar] [CrossRef]
- Schwaederle, M.; Parker, B.A.; Schwab, R.B.; Daniels, G.A.; Piccioni, D.E.; Kesari, S.; Helsten, T.L.; Bazhenova, L.A.; Romero, J.; Fanta, P.T.; et al. Precision Oncology: The UC San Diego Moores Cancer Center PREDICT Experience. Mol. Cancer. Ther. 2016, 15, 743–752. [Google Scholar] [CrossRef]
- Kris, M.G.; Johnson, B.E.; Berry, L.D.; Kwiatkowski, D.J.; Iafrate, A.J.; Wistuba, I.I.; Varella-Garcia, M.; Franklin, W.A.; Aronson, S.L.; Su, P.; et al. Using Multiplexed Assays of Oncogenic Drivers in Lung Cancers to Select Targeted Drugs. JAMA 2014, 311, 1998. [Google Scholar] [CrossRef] [PubMed]
- Stockley, T.L.; Oza, A.M.; Berman, H.K.; Leighl, N.B.; Knox, J.J.; Shepherd, F.A.; Chen, E.X.; Krzyzanowska, M.K.; Dhani, N.; Joshua, A.M.; et al. Molecular profiling of advanced solid tumors and patient outcomes with genotype-matched clinical trials: The Princess Margaret IMPACT/COMPACT trial. Genome Med. 2016, 8, 109. [Google Scholar] [CrossRef]
- Kasztura, M.; Richard, A.; Bempong, N.E.; Loncar, D.; Flahault, A. Cost-effectiveness of precision medicine: A scoping review. Int. J. Public Health 2019, 64, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.; Keeling, P.; French, D.; Smart, D.; Sullivan, R.; Lawler, M. Cost-effectiveness of precision diagnostic testing for precision medicine approaches against non-small-cell lung cancer: A systematic review. Mol. Oncol. 2021, 15, 2672–2687. [Google Scholar] [CrossRef]
- Meehan, J.; Gray, M.; Martínez-Pérez, C.; Kay, C.; Pang, L.Y.; Fraser, J.; Poole, A.V.; Kunkler, L.H.; Langdon, S.P.; Argyle, D.; et al. Precision Medicine and the Role of Biomarkers of Radiotherapy Response in Breast Cancer. Front. Oncol. 2020, 10, 628. [Google Scholar]
- Mallarkey, G.; Mangoni, A.A. Targeting precision medicine toxicity: Recent developments. Ther. Adv. Drug Saf. 2015, 6, 4–14. [Google Scholar] [CrossRef]
- Sedhom, R.; Bates-Pappas, G.E.; Feldman, J.; Elk, R.; Gupta, A.; Fisch, M.J.; Soto-Perez-de-Celis, E. Tumor Is Not the Only Target: Ensuring Equitable Person-Centered Supportive Care in the Era of Precision Medicine. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e434026. [Google Scholar] [CrossRef]
- El-Alti, L.; Sandman, L.; Munthe, C. Person Centered Care and Personalized Medicine: Irreconcilable Opposites or Potential Companions? Health Care Anal. 2019, 27, 45–59. [Google Scholar] [CrossRef]
- Sarhadi, V.K.; Armengol, G. Molecular Biomarkers in Cancer. Biomolecules 2022, 12, 1021. [Google Scholar] [CrossRef]
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef]
- Schmidt, K.T.; Chau, C.H.; Price, D.K.; Figg, W.D. Precision Oncology Medicine: The Clinical Relevance of Patient-Specific Biomarkers Used to Optimize Cancer Treatment. J. Clin. Pharmacol. 2016, 56, 1484–1499. [Google Scholar] [CrossRef]
- National Cancer Institute. Tumor Marker Tests in Common Use. 2023. Available online: https://www.cancer.gov/about-cancer/diagnosis-staging/diagnosis/tumor-markers-list (accessed on 15 April 2024).
- Carmagnani Pestana, R.; Dias e Silva, D.; David, B.B.L.; Schmerling, R.A.; Filippi, R.Z.; De Camargo, V.P.; Mello, C.A.L.; Donna, L.M.G.; Munhoz, R.R.; Varas, J.C.C.H.; et al. Sarcoma drug approvals in Latin America compared to the FDA and EMA: An analysis by the LACOG Sarcoma Group. J. Clin. Oncol. 2024, 42 (Suppl. S16), e13731. [Google Scholar] [CrossRef]
- Ivama-Brummell, A.M.; Marciniuk, F.L.; Wagner, A.K.; Osorio-de-Castro, C.G.S.; Vogler, S.; Mossialos, E.; Tavares-de-Andrade, C.L.; Naci, H. Marketing authorisation and pricing of FDA-approved cancer drugs in Brazil: A retrospective analysis. Lancet Reg. Health Am. 2023, 22, 100506. [Google Scholar] [CrossRef]
- Barrios, C.H.; Reinert, T.; Werutsky, G. Access to high-cost drugs for advanced breast cancer in Latin America, particularly trastuzumab. Ecancermedicalscience 2019, 22, 13. [Google Scholar]
- Gomez, H.L.; Castañeda, C.; Valencia, F.; Muñoz-Bermeo, R.; Torrico Mdel, C.; Neciosup, S. ABC4 Consensus: First Latin American Meeting—Assessment, Comments, and Application of Its Recommendations. JCO Glob. Oncol. 2020, 6, 819–827. [Google Scholar] [CrossRef]
- World Health Organization. National Cancer Control Programmes: Policies and Managerial Guidelines. 2002. Available online: https://iris.who.int/bitstream/handle/10665/42494/9241545577.pdf?sequence=1 (accessed on 20 June 2024).
- Pan American Health Organization. Plan of Action for Cervical Cancer Prevention and Control 2018–2030. Washington, D.C. 2019. Available online: https://iris.paho.org/handle/10665.2/38574 (accessed on 1 March 2024).
- Pan American Health Organization. Breast Cancer: Knowledge Summaries for Health Professionals. Available online: https://www.paho.org/en/topics/cancer/breast-cancer-knowledge-summaries-health-professionals (accessed on 15 August 2024).
- Vásquez, L.; Fuentes-Alabi, S.; Benitez-Majano, S.; Ribeiro, K.B.; Abraham, M.; Agulnik, A.; Baker, J.N.; Blanco, D.B.; Caniza, N.A.; Cardenas-Aguirre, A.; et al. Collaboration for success: The Global Initiative for Childhood Cancer in Latin America. Rev. Panam. Salud Publica 2023, 47, e144. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health of Colombia. MinSalud and Cancerology Institute Will Integrate Public Health and Cancer Control Plans. [Minsalud y Cancerologico Integraran Planes de Salud Public y Control de Cancer]. 2021. Available online: https://www.minsalud.gov.co/Paginas/Minsalud-y-Cancerologico-integraran-planes-de-salud-publica-y-control-del-cancer.aspx (accessed on 18 February 2024).
- Loggetto, P.; Ritter, J.; Marx, K.; Metzger, M.L.; Lam, C.G. Equity in national cancer control plans in the region of the Americas. Lancet Oncol. 2022, 23, e209–e217. [Google Scholar] [CrossRef] [PubMed]
- Government Secretariat of Health/National Cancer Institute. Plan Nacional de Control de Cáncer 2018–2022 [National Cancer Control Plan 2018–2022]. 2018. Available online: https://www.iccp-portal.org/resources/plan-nacional-de-control-de-cancer-2018-2022 (accessed on 26 February 2024).
- Presidency of the Republic of Brazil. Lei No. 14.758 de 19 de Dezembro de 2023. [Law No. 14,758 of 19 December 2023]. 2023. Available online: https://www.in.gov.br/en/web/dou/-/lei-n-14.758-de-19-de-dezembro-de-2023-532172581 (accessed on 11 February 2024).
- Presidency of the Republic of Brazil. Lei No. 14.238, de 19 de Novembro de 2021. 2021. Available online: https://www.planalto.gov.br/ccivil_03/_Ato2019-2022/2021/Lei/L14238.htm (accessed on 15 June 2024).
- Presidency of the Republic of Brazil. Lei No. 12.732, de 22 de Novembro de 2012. 2012. Available online: https://www.planalto.gov.br/ccivil_03/_ato2011-2014/2012/lei/l12732.htm (accessed on 13 March 2024).
- Presidency of Republic of Brazil. Lei No. 12.715 de 17 de Setembro de 2012. [Law No. 12,715 of 17 September 2012]. 2012. Available online: https://www.planalto.gov.br/ccivil_03/_ato2011-2014/2012/Lei/L12715.htm (accessed on 15 May 2024).
- Ministry of Health of Brazil. Strategic Action Plan to Combat Chronic Diseases and Non-Communicable 2021–2030. [Plano de Ações Estratégicas para o Enfrentamento das Doenças Crônicas e Agravos não Transmissíveis no Brasil 2021–2030]. 2021. Available online: https://www.iccp-portal.org/system/files/plans/NCD%20plan%20Brazil%202021-2030.pdf (accessed on 18 February 2024).
- Ministry of Health of Colombia. 10-Year Cancer Control Plan 2012–2021. 2012. Available online: https://www.minsalud.gov.co/Documents/Plan-Decenal-Cancer/PlanDecenal_ControlCancer_2012-2021.pdf (accessed on 18 February 2024).
- Unified Regulatory Information System of Colombia. Ley 1388 de 2010. 2010. Available online: https://www.suin-juriscol.gov.co/viewDocument.asp?ruta=Leyes/1678530 (accessed on 18 March 2024).
- Ministry of Health of Colombia. Resolution No. 4496 of 2012. 2012. Available online: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/DIJ/Resolucion-4496-de-2012.PDF (accessed on 18 February 2024).
- Congress of Colombia. Law 1384 de 2010. 2010. Available online: https://www.funcionpublica.gov.co/eva/gestornormativo/norma_pdf.php?i=39368 (accessed on 18 April 2024).
- Ministry of Social Protection. Resolucion No. 0143. 2021. Available online: https://www.cancer.gov.co/recursos_user/Politicas/Resoluci%C3%B3n_0143_de_2021_(2).pdf (accessed on 18 February 2024).
- Ministry of Health of Mexico. Programa de Acción Específico de Prevencion y Control del Cáncer 2021–2024 [Specific Action Program of Prevention and Control of Cancer 2021–2024.]. 2021. Available online: https://www.gob.mx/cms/uploads/attachment/file/706943/PAE_CAN_cF.pdf (accessed on 28 February 2024).
- Ministry of Health of Mexico. Ley General Para la Detección Oportuna del Cáncer en la Infancia y la Adolescencia [General Law for the Timely Detection of Cancer in Childhood and Adolescence]. 2021. Available online: https://sidofqa.segob.gob.mx/notas/5609564 (accessed on 28 February 2024).
- Ministry of Health of Panama. Plan Estratégico Nacional para la Prevención y Control del Cáncer 2019–2029 [National Strategic Plan for Cancer Prevention and Control 2019–2029]. 2019. Available online: https://www.minsa.gob.pa/sites/default/files/publicaciones/plan_estrategico_nacional_para_la_prevencion_y_control_del_cancer_2019_-_2029.pdf (accessed on 8 March 2024).
- National Assembly of Panama. Ley 154, de 13 de mayo de 2020 [Law 154, of 13 May 2020]. 2020. Available online: https://w3.css.gob.pa/wp-content/wdocs/ASAMBLEA%20NACIONAL%20LEY%20154%20DEL%2013%20DE%20MAYO%20DE%202020.pdf (accessed on 12 March 2024).
- Ministry of Health of Panama. Decreto Ejecutivo No.382 de 4 de Septiembre de 2008. 2008. Available online: https://www.gacetaoficial.gob.pa/pdfTemp/26123/13250.pdf (accessed on 12 April 2024).
- Ministry of Health of Panama. Resolución No. 291 de 16 de Mayo de 2022. 2022. Available online: https://www.minsa.gob.pa/sites/default/files/normatividad/resolucion_no_291_de_16_de_mayo_de_2022_normativa.pdf (accessed on 15 March 2024).
- Secretariat of Planning and Policies in Science Technology and Innovation. Poblar. 2021. Available online: https://www.argentina.gob.ar/ciencia/seppcti/poblar (accessed on 10 March 2024).
- Ministry of Health of Brazil. Genomes Brasil. Available online: https://www.gov.br/saude/pt-br/composicao/sectics/decit/genomas-brasil (accessed on 12 February 2024).
- Argentinian Government. Mapa de Accionabilidad Genómica Tumoral Argentina (MAGenTa). 2018. Available online: https://www.argentina.gob.ar/ciencia/dnpe/proyectos/medicina-precision/MAGenTa (accessed on 23 May 2024).
- Argentinian Government. Medicina de Precisión en Cáncer y Enfermedades poco Frecuentes (Epof-PAMPA). 2018. Available online: https://www.argentina.gob.ar/ciencia/dnpe/proyectos/medicina-precision/epof-PAMPA (accessed on 23 May 2024).
- Diario Oficial de la Federación. Ley General de Salud [General Health Law] (1984, February 7 rev. 2024, January 3). 2024. Available online: https://www.diputados.gob.mx/LeyesBiblio/pdf/LGS.pdf (accessed on 12 March 2024).
- Legislative Information System. Iniciativa que Adiciona Diversas Disposiciones de la Ley General de Salud, en Materia de Medicina de Precisión, Suscrita por Diputados Integrantes del Grupo Parlamentario del Pan. 2023. Available online: http://sil.gobernacion.gob.mx/Archivos/Documentos/2023/04/asun_4562318_20230426_1680209383.pdf (accessed on 12 March 2024).
- Government of Argentina. Poblar: Un Proyecto federal Para Que la Población Argentina Cuente con un Biobanco Genómico propio. 2023. Available online: https://www.argentina.gob.ar/noticias/poblar-un-proyecto-federal-para-que-la-poblacion-argentina-cuente-con-un-biobanco-genomico (accessed on 22 March 2024).
- Economist Impact. The Journey Towards Health Improvement in Argentina: A Roadmap for Precision Medicine. 2022. Available online: https://impact.economist.com/perspectives/sites/default/files/economist_impact_precision_medicine_argentina_english_v2.pdf (accessed on 15 August 2024).
- Argentinian Government. Biobanco Nacional de Muestras Biológicas: Una Herramienta Esencial Para el Desarrollo de Proyectos de Investigación en el Campo de la Medicina de Precisión. 2017. Available online: https://www.argentina.gob.ar/ciencia/dnpe/proyectos/medicina-precision/biobanco-nacional (accessed on 23 May 2024).
- Argentinian Government. Genómica clínica de Enfermedades Pediátricas-GCEP. 2018. Available online: https://www.argentina.gob.ar/ciencia/dnpe/proyectos/medicina-precision/enfermedades-pediatricas (accessed on 23 May 2024).
- Centro de Estudios Regulatorios. Dapre, Ley 2287 de 2023. 2023. Available online: https://www.cerlatam.com/normatividad/dapre-ley-2287-de-2023/ (accessed on 11 December 2024).
- Yan, J.T.; Jin, Y.; Lo, E.; Chen, Y.; Hanlon Newell, A.E.; Kong, Y.; Inge, L.J. Real-World Biomarker Test Utilization and Subsequent Treatment in Patients with Early-Stage Non-small Cell Lung Cancer in the United States, 2011−2021. Oncol. Ther. 2023, 11, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Ge, W.; Quek, R.G.; Gleeson, M.; Pouliot, J.F.; Dietz, H.; Jalbert, J.J.; Harnett, J.; Antonia, S.J. Trends in Real-World Biomarker Testing and Overall Survival in US Patients with Advanced Non-Small-Cell Lung Cancer. Future Oncol. 2022, 18, 4385–4397. [Google Scholar] [CrossRef]
- Bruno, D.S.; Hess, L.M.; Li, X.; Su, E.W.; Patel, M. Disparities in Biomarker Testing and Clinical Trial Enrollment Among Patients With Lung, Breast, or Colorectal Cancers in the United States. JCO Precis. Oncol. 2022, 6, e2100427. [Google Scholar] [CrossRef]
- Dieguez, G.; Carioto, J. The Landscape of Biomarker Testing Coverage in the United States. 2022. Available online: https://www.fightcancer.org/sites/default/files/the_landscape_of_biomarker_testing_coverage_in_the_u_s_0.pdf (accessed on 15 August 2024).
- Norris, R.P.; Dew, R.; Sharp, L.; Greystoke, A.; Rice, S.; Johnell, K.; Todd, A. Are there socio-economic inequalities in utilization of predictive biomarker tests and biological and precision therapies for cancer? A systematic review and meta-analysis. BMC Med. 2020, 18, 282. [Google Scholar] [CrossRef]
- Normanno, N.; Apostolidis, K.; Wolf, A.; Al Dieri, R.; Deans, Z.; Fairley, J. Access and quality of biomarker testing for precision oncology in Europe. Eur. J. Cancer 2022, 176, 70–77. [Google Scholar] [CrossRef]
- EFPIA. IQNPath. European Cancer Patient Coallition. Unlocking the Potential of Precision Medicine in Europe—Improving Cancer Care Through Broader Access to Quality Biomarker Testing. 2021. Available online: https://www.efpia.eu/media/589673/biomarker-testing-summary-final-version.pdf (accessed on 15 August 2024).
- Memorial Sloan Kettering Cancer Center Library Precision Medicine: U.S. Gov’t Initiatives. 2024. Available online: https://libguides.mskcc.org/precisionmedicine/usgovt (accessed on 15 August 2024).
- National Cancer Institute. NCI-supported Precision Medicine Oncology Research Activities. 2023. Available online: https://dctd.cancer.gov/MajorInitiatives/NCI-supported_activities_in_precision_medicine.htm (accessed on 20 June 2024).
- National Cancer Institute. Center for Cancer Genomics. Supporting Genomic Science to Improve Cancer Diagnosis, Treatments, and Outcomes. 2024. Available online: https://www.cancer.gov/ccg/ (accessed on 20 June 2024).
- National Institutes of Health. All of Us Research Program. 2024. Available online: https://allofus.nih.gov/ (accessed on 20 June 2024).
- National Institutes of Health. Transdisciplinary Collaborative Centers for Health Disparities Research Program. 2024. Available online: https://www.nimhd.nih.gov/node/22006 (accessed on 15 June 2024).
- Beccia, F.; Hoxhaj, I.; Castagna, C.; Strohäker, T.; Cadeddu, C.; Ricciardi, W.; Boccia, S. An overview of Personalized Medicine landscape and policies in the European Union. Eur. J. Public Health 2022, 32, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Phanthunane, C.; Pongcharoen, S.; Pannarunothai, S.; Roboon, J.; Phanthunane, P.; Nontarak, J. Precision medicine in Asia enhanced by next-generation sequencing: Implications for Thailand through a scoping review and interview study. Clin. Transl. Sci. 2024, 17, e13868. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Bertin, N.; Hebrard, M.; Tirado-Magallanes, R.; Bellis, C.; Lim, W.K.; Chua, C.Y.; Tong, P.M.L.; Chua, R.; Mak, K.; et al. The Singapore National Precision Medicine Strategy. Nat. Genet. 2023, 55, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Susanti, S.; Subhandi Bakhtiar, H.; Prasetyo, H. Protection of Genetic Data in Health Services Based on Genomics Technology in Indonesia. Asian J. Healthy Sci. 2023, 2, 860–870. [Google Scholar] [CrossRef]
- Food and Drugs Administration. Herceptin. 1998. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf (accessed on 4 July 2024).
- European Medicines Agency. Herceptin. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/herceptin (accessed on 4 July 2024).
- Government of Mexico. Consulta de Registros Sanitarios. Available online: https://tramiteselectronicos02.cofepris.gob.mx/BuscadorPublicoRegistrosSanitarios/BusquedaRegistroSanitario.aspx (accessed on 4 July 2024).
- National Human Genome Research Institute. NHGRI History and Timeline of Events. 2024. Available online: https://www.genome.gov/about-nhgri/Brief-History-Timeline (accessed on 4 July 2024).
- Agencia Nacional de Vigilancia Sanitaria. Herceptine. Available online: https://consultas.anvisa.gov.br/#/medicamentos/8090?substancia=23119 (accessed on 4 July 2024).
- Food and Drugs Administration. Gleevec. 2001. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021588s024lbl.pdf (accessed on 4 July 2024).
- European Medicines Agency. Glivec. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/glivec (accessed on 4 July 2024).
- Agencia Nacional de Vigilancia Sanitaria. Glivec. Available online: https://consultas.anvisa.gov.br/#/medicamentos/5757?numeroRegistro=100680174 (accessed on 4 July 2024).
- Collins, F.S.; Hamburg, M.A. First FDA Authorization for Next-Generation Sequencer. N. Engl. J. Med. 2013, 369, 2369–2371. [Google Scholar] [CrossRef]
- European Medicines Agency. Keytruda. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/keytruda (accessed on 4 July 2024).
- Food and Drugs Administration. Food and Drugs Administration. 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s066lbl.pdf (accessed on 4 July 2024).
- International Consortium for Personalized Medicine. ICPerMed. 2023. Available online: https://www.icpermed.eu/ (accessed on 4 July 2024).
- European Commission. Personalised Medicine. Available online: https://research-and-innovation.ec.europa.eu/research-area/health/personalised-medicine_en (accessed on 4 July 2024).
- Ministry of Human Rights and Citizenship. Governo Federal Lança Programa Inédito de Medicina de Precisão. 2020. Available online: https://www.gov.br/mdh/pt-br/assuntos/noticias/2020-2/outubro/governo-federal-lanca-programa-inedito-de-medicina-de-precisao (accessed on 4 July 2024).
- Agencia Nacional de Vigilancia Sanitaria. Registrados Medicamentos Inovadores para Câncer de Pele. Available online: https://antigo.anvisa.gov.br/resultado-de-busca?p_p_id=101&p_p_lifecycle=0&p_p_state=maximized&p_p_mode=view&p_p_col_id=column-1&p_p_col_count=1&_101_struts_action=%2Fasset_publisher%2Fview_content&_101_assetEntryId=3037244&_101_type=content&_101_groupId=219201&_101_urlTitle=registrados-medicamentos-inovadores-para-cancer-de-pele&inheritRedirect=true (accessed on 4 July 2024).
- Genomics England. 100,000 Genomes Project. Available online: https://www.genomicsengland.co.uk/initiatives/100000-genomes-project (accessed on 4 July 2024).
- European Commission. European “1+ Million Genomes” Initiative. 2024. Available online: https://digital-strategy.ec.europa.eu/en/policies/1-million-genomes (accessed on 4 July 2024).
- Chong, H.Y.; Allotey, P.A.; Chaiyakunapruk, N. Current landscape of personalized medicine adoption and implementation in Southeast Asia. BMC Med. Genom. 2018, 11, 94. [Google Scholar] [CrossRef]
- Chumnumwat, S.; Lu, Z.H.; Sukasem, C.; Winther, M.D.; Capule, F.R.; Abdul Hamid, A.A.; Bhandari, B.; Chaikledkaew, U.; Chanhom, N.; Chantarangsu, S.; et al. Southeast Asian Pharmacogenomics Research Network (SEAPharm): Current Status and Perspectives. Public Health Genom. 2019, 22, 132–139. [Google Scholar] [CrossRef]
- de Vries, J.; Tindana, P.; Littler, K.; Ramsay, M.; Rotimi, C.; Abayomi, A.; Mulder, N.; Mayosi, B.M. The H3Africa policy framework: Negotiating fairness in genomics. Trends Genet. 2015, 31, 117–119. [Google Scholar] [CrossRef]
- Mulder, N.; Abimiku, A.; Adebamowo, S.N.; de Vries, J.; Matimba, A.; Olowoyo, P.; Ramsey, M.; Skelton, M.; Stein, D.J. H3Africa: Current perspectives. Pharmgenomics Pers. Med. 2018, 11, 59–66. [Google Scholar] [CrossRef]
- NHS England. NHS Genomic Medicine Service. Available online: https://www.england.nhs.uk/genomics/nhs-genomic-med-service/ (accessed on 8 July 2024).
- Neuhaus, C.P.; Pacia, D.M.; Crane, J.T.; Maschke, K.J.; Berlinger, N. All of Us and the Promise of Precision Medicine: Achieving Equitable Access for Federally Qualified Health Center Patients. J. Pers. Med. 2023, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.S.; Walker, R.J.; Egede, L.E. Achieving Equity in an Evolving Healthcare System: Opportunities and Challenges. Am. J. Med. Sci. 2016, 351, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Ory, M.G.; Adepoju, O.E.; Ramos, K.S.; Silva, P.S.; Vollmer Dahlke, D. Health equity innovation in precision medicine: Current challenges and future directions. Front. Public Health 2023, 15, 11. [Google Scholar] [CrossRef]
- Botham, J.; Shilling, V.; Jones, J. Patient and public understanding of the concept of ‘personalised medicine’ in relation to cancer treatment: A systematic review. Future Health J. 2021, 8, e703–e708. [Google Scholar] [CrossRef] [PubMed]
- Yelne, S.; Chaudhary, M.; Dod, K.; Sayyad, A.; Sharma, R. Harnessing the Power of AI: A Comprehensive Review of Its Impact and Challenges in Nursing Science and Healthcare. Cureus 2023, 22, e49252. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.; Cobanaj, M.; Dee, E.C.; Criscitiello, C.; Tolaney, S.M.; Celi, L.A.; Curigliano, G. Artificial intelligence in cancer research and precision medicine: Applications, limitations and priorities to drive transformation in the delivery of equitable and unbiased care. Cancer Treat. Rev. 2023, 112, 102498. [Google Scholar] [CrossRef]
- Seetharam, K.; Kagiyama, N.; Sengupta, P.P. Application of mobile health, telemedicine and artificial intelligence to echocardiography. Echo. Res. Pract. 2019, 6, R41–R52. [Google Scholar] [CrossRef]
- Goss, P.E.; Lee, B.L.; Badovinac-Crnjevic, T.; Strasser-Weippl, K.; Chavarri-Guerra, Y.; Louis, J.S.; Villarreal-Garza, C.; Unger-Saldaña, K.; Ferreyra, M.; Debiasi, M. Planning cancer control in Latin America and the Caribbean. Lancet Oncol. 2013, 14, 391–436. [Google Scholar] [CrossRef]
- Ferreira, C.G.; Achatz, M.I.; Ashton-Prolla, P.; Begnami, M.D.; Marchini, F.K.; Stefani, S.D. Brazilian health-care policy for targeted oncology therapies and companion diagnostic testing. Lancet Oncol. 2016, 17, e363–e370. [Google Scholar] [CrossRef]
- Fong, H.; Harris, E. Technology, innovation and health equity. Bull. World Health Organ. 2015, 93, 438–438A. [Google Scholar] [CrossRef]
- Gallifant, J.; Nakayama, L.F.; Gichoya, J.W.; Pierce, R.; Celi, L.A. Equity should be fundamental to the emergence of innovation. PLoS Digit. Health 2023, 2, e0000224. [Google Scholar] [CrossRef]
- Temporão, J.G.; Santini, L.A.; Santos ATCdos Fernandes, F.M.B.; Zoss, W.P. Current and future challenges of the use of precision medicine in cancer diagnosis and treatment in Brazil. Cad. Saude Publica. 2022, 38, e00006122. [Google Scholar] [CrossRef] [PubMed]
- Shirdarreh, M.; Aziza, O.; Pezo, R.C.; Jerzak, K.J.; Warner, E. Patients’ and Oncologists’ Knowledge and Expectations Regarding Tumor Multigene Next-Generation Sequencing: A Narrative Review. Oncologist 2021, 26, e1359–e1371. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, R. Healthcare Priority Setting in the Courts: A Reflection on Decision-Making When Healthcare Priority Setting Is Brought to Court. Available online: https://f1000research.com/documents/6-242 (accessed on 15 April 2024).
- Robles, M.Y. El derecho a la salud en la jurisprudencia de la corte Interamericana De Derechos Humanos (2004–2014). Cuest. Constitucl. 2016, 1, 199–246. [Google Scholar] [CrossRef][Green Version]
- Raez, L.E.; Santos, E.S.; Rolfo, C.; Lopes, G.; Barrios, C.; Cardona, A.; Mas, L.A.; Arrieta, O.; Richardet, E.; Vallejos, C.; et al. Challenges in Facing the Lung Cancer Epidemic and Treating Advanced Disease in Latin America. Clin. Lung Cancer 2017, 18, e71–e79. [Google Scholar] [CrossRef]
- Raez, L.E.; Cardona, A.F.; Santos, E.S.; Catoe, H.; Rolfo, C.; Lopes, G.; Barrios, C.; Mas, L.A.; Vallejos, C.; Zatarain-Barrón, Z.L.; et al. The burden of lung cancer in Latin-America and challenges in the access to genomic profiling, immunotherapy and targeted treatments. Lung Cancer 2018, 119, 7–13. [Google Scholar] [CrossRef]
- Balogun, O.D.; Olopade, O.I. Addressing health disparities in cancer with genomics. Nat. Rev. Genet. 2021, 22, 621–622. [Google Scholar] [CrossRef]
- Ginsburg, G.S.; Phillips, K.A. Precision Medicine: From Science To Value. Health Aff. 2018, 37, 694–701. [Google Scholar] [CrossRef]
- Bertier, G.; Carrot-Zhang, J.; Ragoussis, V.; Joly, Y. Integrating precision cancer medicine into healthcare—Policy, practice, and research challenges. Genome Med. 2016, 8, 108. [Google Scholar] [CrossRef]
- Lotsberg, M.L.; D’mello Peters, S.A. Publication Bias in Precision Oncology and Cancer Biomarker Research. In Challenges and Possible Implications; Cambridge University Press: Cambridge, UK, 2022; pp. 155–174. [Google Scholar]
- Zavala, V.A.; Serrano-Gomez, S.J.; Dutil, J.; Fejerman, L. Genetic Epidemiology of Breast Cancer in Latin America. Genes 2019, 10, 153. [Google Scholar] [CrossRef]
- Popejoy, A.B.; Fullerton, S.M. Genomics is failing on diversity. Nature 2016, 538, 161–164. [Google Scholar] [CrossRef]
- Vargas, R.J.; Cobar, O.M. The Urgent Need for Management of Biological Samples and Data Accessibility in Latin America. Front. Pharmacol. 2021, 12, 620043. [Google Scholar] [CrossRef]
- Cooke Bailey, J.N.; Bush, W.S.; Crawford, D.C. Editorial: The Importance of Diversity in Precision Medicine Research. Front. Genet. 2020, 26, 11. [Google Scholar] [CrossRef]
- Gössling, G.; Rebelatto, T.F.; Villarreal-Garza, C.; Ferrigno, A.S.; Bretel, D.; Sala, R.; Giacomazzi, J.; William, W.N.; Werutsky, G. Current Scenario of Clinical Cancer Research in Latin America and the Caribbean. Curr. Oncol. 2023, 30, 653–662. [Google Scholar] [CrossRef]
- Edsjö, A.; Holmquist, L.; Geoerger, B.; Nowak, F.; Gomon, G.; Alix-Panabières, C.; Ploeger, C.; Lassen, U.; Le Tourneau, C.; Lehtiö, J.; et al. Precision cancer medicine: Concepts, current practice, and future developments. J. Intern. Med. 2023, 294, 455–481. [Google Scholar] [CrossRef]
- Al Meslamani, A.Z. The future of precision medicine in oncology. Expert. Rev. Precis. Med. Drug Dev. 2023, 8, 43–47. [Google Scholar] [CrossRef]
- Bestari, M.B.; Joewono, I.R.; Syam, A.F. A Quest for Survival: A Review of the Early Biomarkers of Pancreatic Cancer and the Most Effective Approaches at Present. Biomolecules 2024, 14, 364. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gonzalez, L.; Sanchez Cendra, A.; Sanchez Cendra, C.; Roberts Cervantes, E.D.; Espinosa, J.C.; Pekarek, T.; Fraile-Martinez, O.; García-Montero, C.; Rodriguez-Sloker, A.M. Exploring Biomarkers in Breast Cancer: Hallmarks of Diagnosis, Treatment, and Follow-Up in Clinical Practice. Medicina 2024, 60, 168. [Google Scholar] [CrossRef] [PubMed]
- Amur, S.; LaVange, L.; Zineh, I.; Buckman-Garner, S.; Woodcock, J. Biomarker Qualification: Toward a Multiple Stakeholder Framework for Biomarker Development, Regulatory Acceptance, and Utilization. Clin. Pharmacol. Ther. 2015, 98, 34–46. [Google Scholar] [CrossRef]
- Hayes, D.F. Biomarker validation and testing. Mol. Oncol. 2015, 9, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Burke, W. Genetic Tests: Clinical Validity and Clinical Utility. Curr. Protoc. Hum. Genet. 2014, 24, 81. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Biomarker Testing for Cancer Treatment. 2021. Available online: https://www.cancer.gov/about-cancer/treatment/types/biomarker-testing-cancer-treatment (accessed on 15 June 2024).
- West, H.J.; Lovly, C.M. Ferrying Oncologists Across the Chasm of Interpreting Biomarker Testing Reports: Systematic Support Needed to Improve Care and Decrease Disparities. JCO Oncol. Pract. 2023, 19, 530–532. [Google Scholar] [CrossRef]
- Moore, D.C.; Guinigundo, A.S. The Advanced Practitioner’s Role in the Rapidly Evolving Landscape of Precision Medicine. J. Adv. Pract. Oncol. 2023, 14, 39–48. [Google Scholar]
- Al-Dewik, N.I.; Younes, S.N.; Essa, M.M.; Pathak, S.; Qoronfleh, M.W. Making Biomarkers Relevant to Healthcare Innovation and Precision Medicine. Processes 2022, 10, 1107. [Google Scholar] [CrossRef]
- Bakker, E.; Starokozhko, V.; Kraaijvanger, J.W.M.; Heerspink, H.J.L.; Mol, P.G.M. Precision medicine in regulatory decision making: Biomarkers used for patient selection in European Public Assessment Reports from 2018 to 2020. Clin. Transl. Sci. 2023, 16, 2394–2412. [Google Scholar] [CrossRef]
- Tseng, T.S. Impact of social behavioral and genomic sciences on translational cancer research. Transl. Cancer Res. 2016, 5, S936–S1397. [Google Scholar] [CrossRef]
- Alvarez-Gomez, R.M.; De la Fuente-Hernandez, M.A.; Herrera-Montalvo, L.; Hidalgo-Miranda, A. Challenges of diagnostic genomics in Latin America. Curr. Opin. Genet. Dev. 2021, 66, 101–109. [Google Scholar] [CrossRef]
- Moye-Holz, D.; Vogler, S. Comparison of Prices and Affordability of Cancer Medicines in 16 Countries in Europe and Latin America. Appl. Health Econ. Health Policy 2022, 20, 67–77. [Google Scholar] [CrossRef]
- Pichon-Riviere, A.; Garay, O.U.; Augustovski, F.; Vallejos, C.; Huayanay, L.; Bueno, M.P.N.; Rodriguez, A.; Andrade, C.J.C.; Buendía, J.A.; Drummond, M. Implications of global pricing policies on access to innovative drugs: The case of trastuzumab in seven Latin American countries. Int. J. Technol. Assess. Health Care 2015, 31, 2–11. [Google Scholar] [CrossRef]
- UNESCO. Fact Sheet No. 59-Global Investments in R&D. 2020. Available online: https://uis.unesco.org/sites/default/files/documents/fs59-global-investments-rd-2020-en.pdf (accessed on 11 December 2024).
- The Lancet Oncology. Violence, crime, corruption, and cuts in public spending: How to re-establish cancer control in Latin America? Lancet Oncol. 2018, 19, 1417. [Google Scholar] [CrossRef]
- FIFARMA & IQVIA. Patient WAIT Indicator 2023—Latin America: Final Report. 2024. Available online: https://fifarma.org/wp-content/uploads/2024/03/IQVIA-FIFARMA_WAIT-Indicator-2023_FinalPresentation_5Mar24_vS.pdf (accessed on 11 March 2024).
- Gilardino, R.E.; Mejía, A.; Guarín, D.; Rey-Ares, L.; Perez, A. Implementing Health Technology Assessments in Latin America: Looking at the Past, Mirroring the Future. A Perspective from the ISPOR Health Technology Assessment Roundtable in Latin America. Value Health Reg. Issues 2020, 23, 6–12. [Google Scholar] [CrossRef]
- Lessa, F.; Caccavo, F.; Curtis, S.; Ouimet-Rathé, S.; Lemgruber, A. Strengthening and implementing health technology assessment and the decision-making process in the Region of the Americas. Rev. Panam. Salud. Pública 2017, 41, e165. [Google Scholar]
- World Health Organization. Guidance for Human Genome Data Collection, Access, Use and Sharing; The World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
| Country/ Region | Cancer Incidence New Cases (2022) | Cancer Incidence Age-Standardized Rate Per 100,000 (2022) | Cancer Prevalence 5-Year Prevalence (2022) | Cancer Mortality Number of Deaths (2022) | Cancer Mortality Age-Standardized Rate Per 100,000 (2022) | Disability-Adjusted Life Years and % of Total DALYs (2019) |
|---|---|---|---|---|---|---|
| Global | 19,976,499 | 212.6 | 53,504,187 | 9,743,832 | 109.8 | 251,390,451.29 9.93% |
| Latin America and the Caribbean | 1,551,060 | 199.9 | 4,096,032 | 749,242 | 96.5 | 16,470,687.03 9.94% |
| Argentina | 133,420 | 231.8 | 395,958 | 70,251 | 123.6 | 1,977,228.47 15.76% |
| Brazil | 627,193 | 240.1 | 1,634,441 | 278,835 | 107.7 | 6,834,584.54 10.48% |
| Colombia | 117,620 | 183.3 | 303,656 | 56,719 | 87.6 | 1,235,853.45 10.68% |
| Mexico | 207,154 | 141.3 | 577,487 | 96,210 | 66.5 | 2,872,120.20 8.47% |
| Panama | 8353 | 158.3 | 24,610 | 3770 | 71.0 | 99,326.00 10.32% |
| Country | National Cancer Policy and Cancer-Related Legislation | Includes Precision/Personalized/Individualized Medicine | Includes Biomarkers | Includes Genetic Markers |
|---|---|---|---|---|
| Argentina | National Cancer Control Plan 2018–2022 [214] | Yes a | No | No |
| Brazil | National Policy for the Prevention and Control of Cancer 2023 [215] | No | No | No |
| Law No. 14.238 of 19 November 2021—Statute for Persons with Cancer [216] Encompasses objectives aimed at comprehensively addressing the needs of individuals with cancer | No | No | No | |
| Law No. 12,732 of 22 November 2012 [217] Provides for the treatment of cancer patients | Yes a | No | No | |
| National Program to Support Oncology Care (2012) [218] | No | No | No | |
| Strategic Action Plan to Combat Chronic and Non-communicable Diseases (2021–2030) [219] | No | No | No | |
| Colombia | 10-Year Cancer Control Plan 2012–2021 [220] | No | No | No |
| Law No. 1388 de 2010 [221] Ensures access to early detection and comprehensive treatment services | No | No | No | |
| Resolution No. 4496 of 2012 [222] Organizes the National Cancer Information System | No | No | No | |
| Resolution 247 of 2014 [221] Introduces a mandate for all health benefit plan administrators and healthcare institutions to report cancer patients | No | No | No | |
| Law No. 1384 of 2010 Sandra Ceballos [223] Establishes comprehensive measures for cancer control | No | No | No | |
| Institutional Public Health Policy for Cancer Control 2021–2023 of the National Institute of Cancerology [224] | No | No | No | |
| Mexico | Specific Action Program for the Prevention and Control of Cancer 2021–2024 [225] | No | No | No |
| General Law for the Timely Detection of Cancer in Childhood and Adolescence 2021 [226] | No | No | No | |
| Panama | National Strategic Plan for Cancer Prevention and Control 2019–2029 [227] | No | No | No |
| Law 154, 13 May 2020 [228] Creates the national program of support, prevention, and comprehensive care for people suffering from oncological diseases | No | No | No | |
| Executive Decree 382 2008 [229] Creates the National Commission for the Early Detection of Cancer | No | No | No | |
| Resolution 291, 16 May 2022 [230] Creates Regional Oncology Units | No | No | No |
| Country | Initiative |
|---|---|
| Sweden | The Genomic Medicine Sweden (GMS) was a bottom-up initiative set in 2017 that gathered a multiprofessional workforce of diagnosticians, clinicians, researchers, and informaticians to coordinate the implementation of genomic-based PM into Swedish healthcare. GMS has established regional Genomic Medicine Centers at all university hospitals across Sweden, stimulating the uptake of genomic-based diagnostics, developed by GMS together with the Swedish research infrastructure Science for Life Laboratory, and supporting equitable access to genomic-based PM across Sweden for patients with rare diseases, cancer, infectious diseases, and complex diseases [141]. |
| England | The NHS Genomic Medicine Service was launched in 2018 and aims to support equitable access to genomics across the NHS in England and provide standardized care across the population, through workforce development, the Genomics Clinical Reference Group, and working with communities and patient groups [279]. |
| US | The All of Us initiative of the NIH recruits participants from diverse backgrounds to improve the makeup of biobanks, considering that nearly all biospecimens used in research come from people of European ancestry. AoU has partnered with Federally Qualified Health Centers, which is a type of community health center whose patient base is comprised largely of people who are uninsured, underinsured, or on Medicaid [280]. In 2016, the US Congress allocated USD 1.5 billion for this program over ten years, subject to an annual appropriations process. |
| Challenges and Gaps Identified in the Literature | Suggested Solutions |
|---|---|
| Challenges regarding scientific development of precision medicine | |
| Genetic heterogeneity of LatAm’s population |
|
| Lack of representation among biomedical research participants | |
| The number of trials related to targeted therapies and molecular profiling in cancer in LatAm is drastically lower than in Europe and the US |
|
| Due to lack of government investment and other alternative support sources, most oncology trials in the region have been sponsored by pharmaceutical companies | |
| The time required for regulatory approval for clinical trial applications in LatAm can limit the interest of pharmaceutical companies in conducting clinical trials in the region | |
| Implementation of biomarkers in clinical practice requires that physicians make many decisions |
|
| Biomarker testing reports must be interpreted by an expert or a team of experts (including medical oncologists, surgical oncologists, pathologists, basic scientists, pharmacists, clinical nurses, physician assistants, and genetic counsellors) to select the optimal treatment to be prescribed | |
| Treatment decisions consider coverage and reimbursement aspects | |
| Challenges implementing precision medicine in healthcare delivery services | |
| Limited availability of adequate infrastructure and trained human resources and laboratories to perform genetic testing and molecular profiling, and to interpret results |
|
| Requires a multidisciplinary team comprised of clinicians, pathologists, molecular specialists, and others, but such teams are usually unavailable due to the short supply of both financial and human resources |
|
| Access to trained healthcare professionals, new therapies, and adequate facilities, due to inadequately distributed budget across locations and geographic barriers |
|
| A lack of stable logistical infrastructure to perform local sequencing and local genomic analysis, and a lack of cost-effective transportation of biological samples to molecular laboratories | |
| Laboratory infrastructure and specialized oncologists are mostly concentrated in urban centers | |
| Insufficient funding for the development of computing, AI, and machine learning to support clinical judgment and managing processes, both of which require improvements in health workforce capacity-building |
|
| Precision medicine is inherently patient-focused, but its application sometimes hyperfocuses on patient genetics and biomarker reports, rather than reflecting the patient’s values and preferences |
|
| Available epidemiological cancer data is not reliable, not all countries in the region have population-based cancer registries, and those that do exhibit varying levels of quality and population coverage |
|
| Challenges regarding economic issues | |
| Limited access to next-generation anti-cancer targeted drugs, mainly due to unaffordability |
|
| Inequities in access to biomarker testing | |
| Healthcare systems in LatAm are characterized by a lack of coverage for populations excluded from social security. Universal healthcare coverage available for only a minority of the LatAm population. In general, many therapeutic options are not reimbursed, leaving patients to face very high out-of-pocket costs | |
| Under-financing of public health systems The public investment in clinical research in LatAm is lower than the global average |
|
| Political instability, corruption, and weak governance lead to poor resource allocation, underfunded health systems, and limited prioritization of cancer and precision medicine in public policy agendas |
|
| Oncologists hesitate to prescribe targeted therapies because health insurance does not always cover them |
|
| Not all indicated targeted therapies are commercially available in LatAm, and not all patients can access therapies currently approved |
|
| Challenges regarding policies and regulations for implementation of precision medicine | |
| At the regional level, international agencies like PAHO lack comprehensive action plans or policies on cancer and precision medicine |
|
| Despite all national plans being issued after 2018, there was no mention of biomarkers or genetic markers in any of the national plans, nor in the supporting cancer legislation |
|
| Lack of national policies and laws regarding precision medicine |
|
| Delayed approval and registration processes for both broad access and clinical trials |
|
| Compliance with law, regulatory pathways, and safety planning is challenging for precision therapies and diagnostics in LatAm and contributes to regulatory lags |
|
| Regulatory processes are independent, but HTA evaluations may depend on the healthcare system’s capacity to adopt and reimburse new drugs |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, A.R.; Merchán, L.A.A.; Alexander, J.A.A.; Kaen, D.; Lopez-Correa, C.; Martin, C.; Attwill, A.; Marinetti, T.; Rocha, J.V.; Barrios, C. Precision Medicine for Cancer and Health Equity in Latin America: Generating Understanding for Policy and Health System Shaping. Int. J. Environ. Res. Public Health 2025, 22, 1220. https://doi.org/10.3390/ijerph22081220
González AR, Merchán LAA, Alexander JAA, Kaen D, Lopez-Correa C, Martin C, Attwill A, Marinetti T, Rocha JV, Barrios C. Precision Medicine for Cancer and Health Equity in Latin America: Generating Understanding for Policy and Health System Shaping. International Journal of Environmental Research and Public Health. 2025; 22(8):1220. https://doi.org/10.3390/ijerph22081220
Chicago/Turabian StyleGonzález, Ana Rita, Lizbeth Alexandra Acuña Merchán, Jorge A. Alatorre Alexander, Diego Kaen, Catalina Lopez-Correa, Claudio Martin, Allira Attwill, Teresa Marinetti, João Victor Rocha, and Carlos Barrios. 2025. "Precision Medicine for Cancer and Health Equity in Latin America: Generating Understanding for Policy and Health System Shaping" International Journal of Environmental Research and Public Health 22, no. 8: 1220. https://doi.org/10.3390/ijerph22081220
APA StyleGonzález, A. R., Merchán, L. A. A., Alexander, J. A. A., Kaen, D., Lopez-Correa, C., Martin, C., Attwill, A., Marinetti, T., Rocha, J. V., & Barrios, C. (2025). Precision Medicine for Cancer and Health Equity in Latin America: Generating Understanding for Policy and Health System Shaping. International Journal of Environmental Research and Public Health, 22(8), 1220. https://doi.org/10.3390/ijerph22081220






