Investigating the Factors Influencing Traditional Male Circumcision and Its Contribution to HIV Transmission Amongst Men in Lesotho: A Multilevel Binary Logistic Regression Approach
Abstract
1. Introduction
2. Methods and Materials
2.1. Source of Data, Study Area, and Study Design
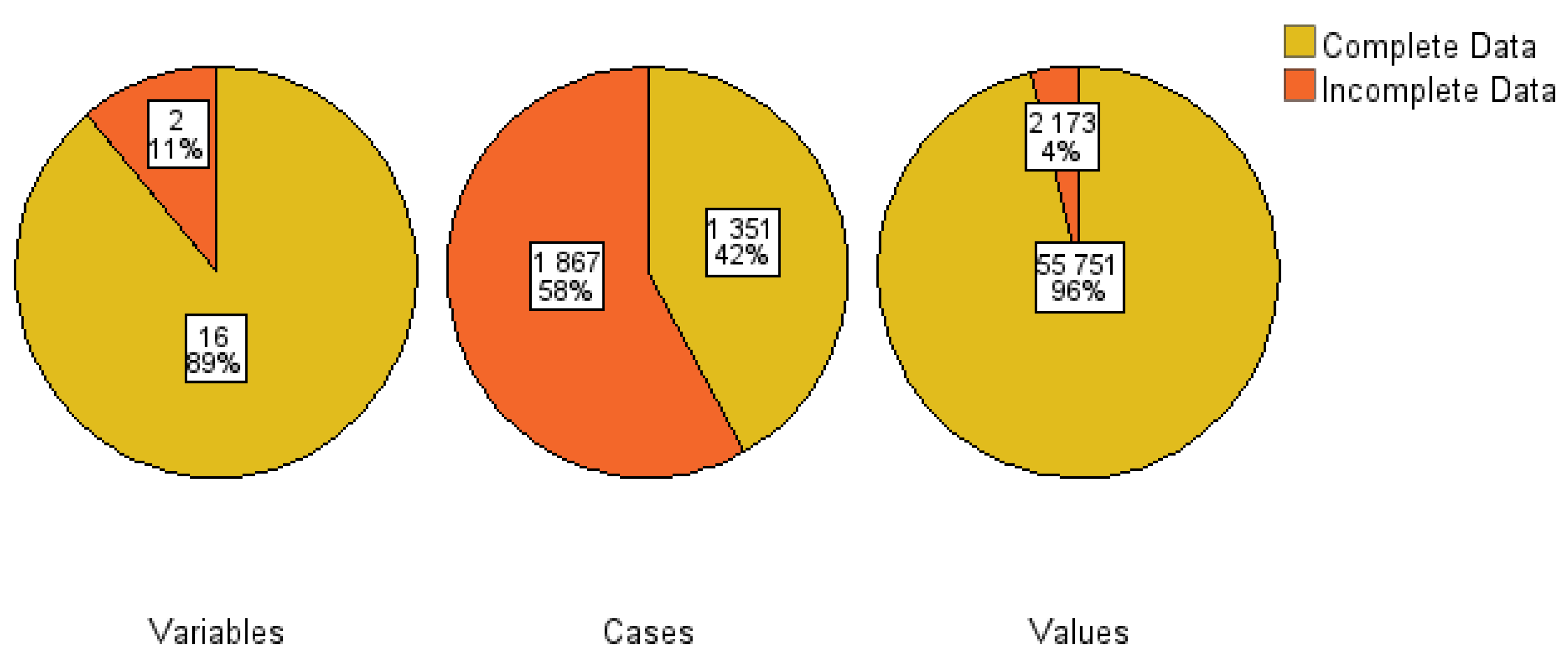
2.2. Missing Data Management and Multicollinearity Assessment
2.3. Model Formulation
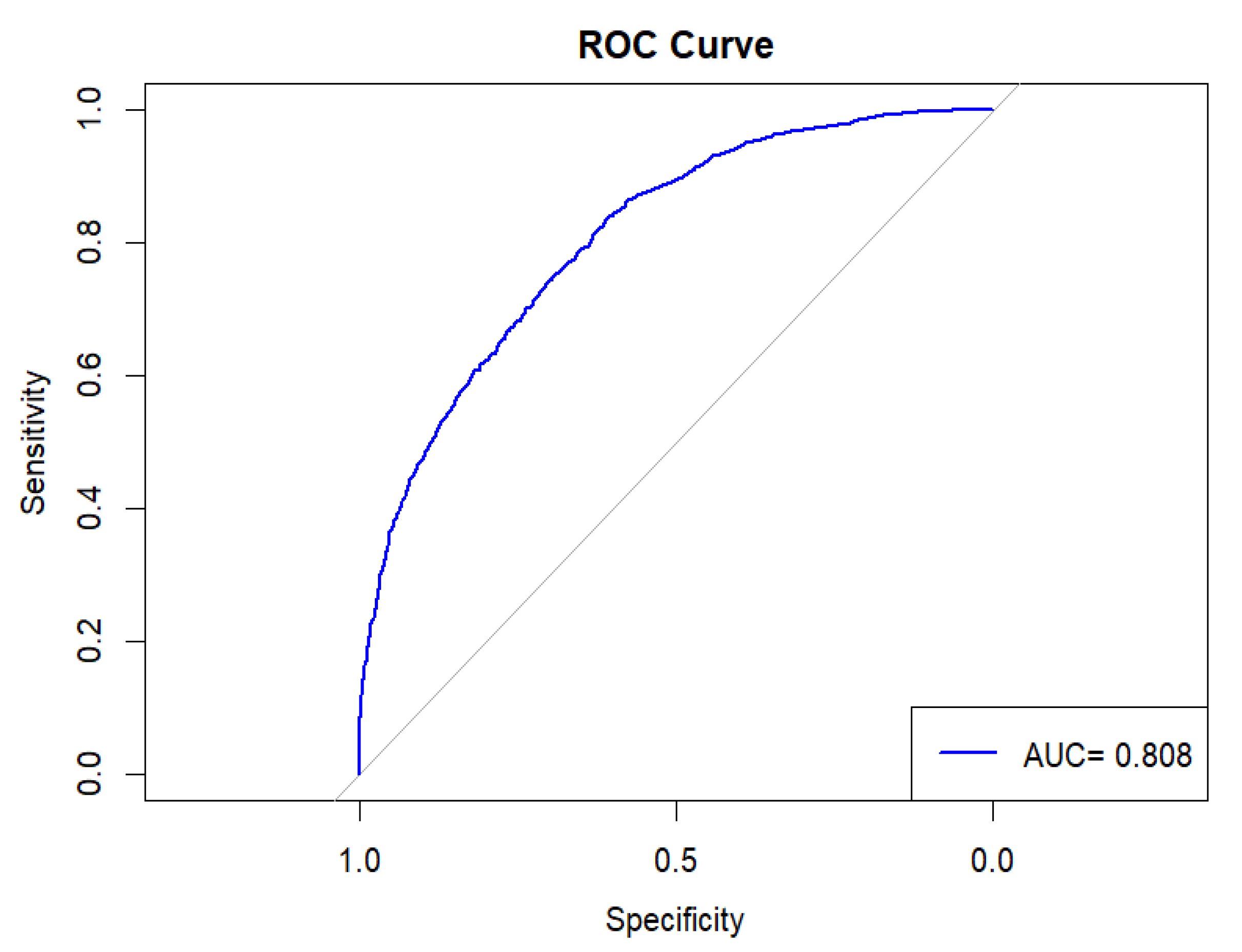
2.4. Statistical Analysis
2.5. Ethics Consideration
3. Result
3.1. Sociodemographic Characteristics of the Study Participants
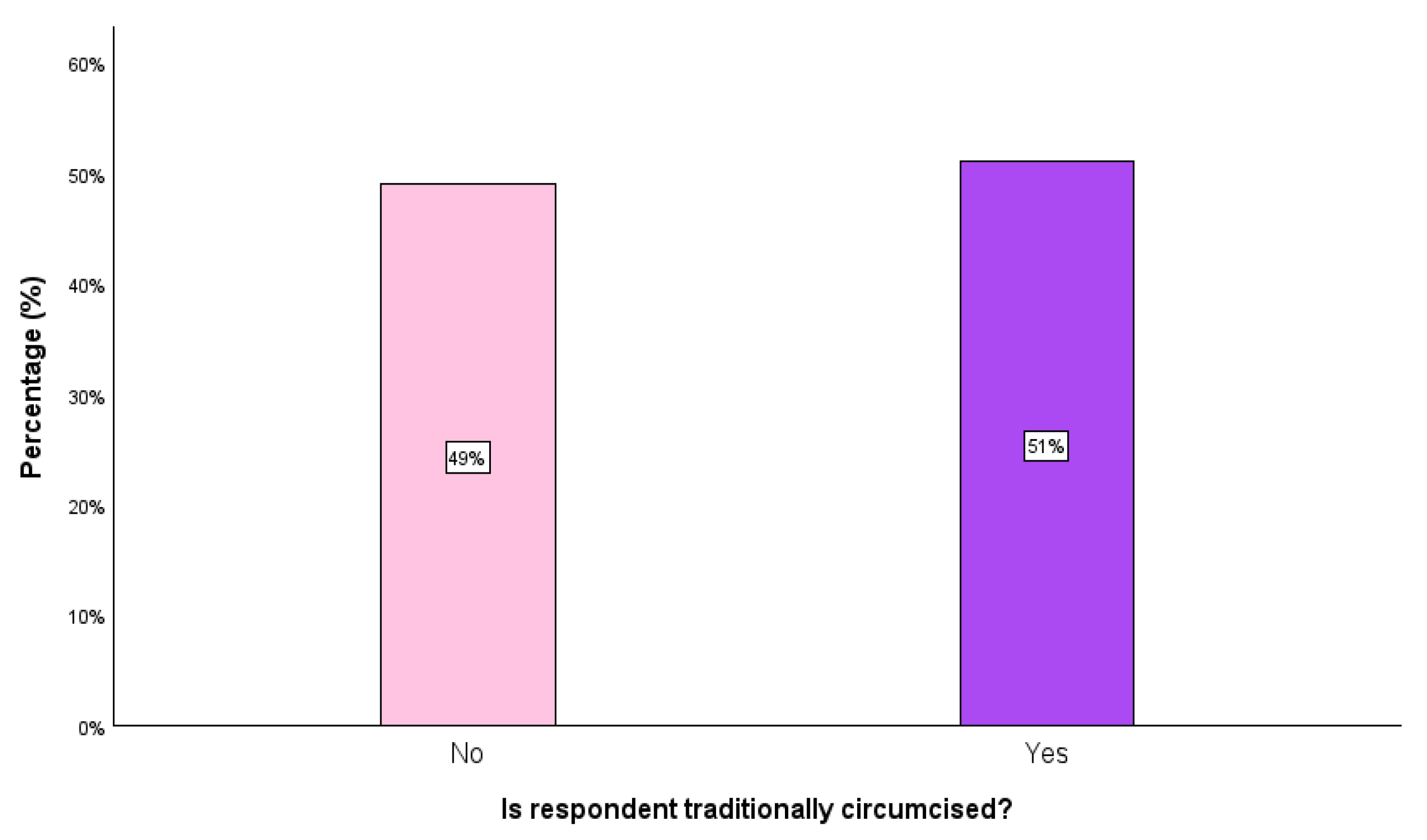
3.2. The Prevalence of Traditional Circumcision (TC)
4. Discussion
Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandova, E.; Mutonhori, T.; Mudzanire, S. The cultural significance and relevance of the Shangani rite of male circumcision in light of HIV and AIDS mitigation in Zimbabwe. Int. J. Asian Soc. Sci. 2013, 3, 584–589. [Google Scholar]
- Tusa, B.S.; Weldesenbet, A.B.; Tefera, T.K.; Kebede, S.A. Spatial distribution of traditional male circumcision and associated factors in Ethiopia; using multilevel generalized linear mixed effects model. BMC Public Health 2021, 21, 1423. [Google Scholar] [CrossRef] [PubMed]
- Palmer, E.; Rau, A.; Engelbrecht, M. Changing cultural practices: A case study of male circumcision in South Africa. Am. J. Men′s Health 2020, 14, 1557988320927285. [Google Scholar] [CrossRef]
- Cox, G.; Morris, B.J. Why circumcision: From prehistory to the twenty-first century. Surg. Guide Circumcision 2012, 17, 243–259. [Google Scholar]
- Meissner, O.; Buso, D.L. Traditional male circumcision in the Eastern Cape-scourge or blessing? S. Afr. Med. J. 2007, 97, 371–373. [Google Scholar]
- Meel, B.L. Traditional male circumcision-related fatalities in the Mthatha area of South Africa. Med. Sci. Law 2010, 50, 189–191. [Google Scholar] [CrossRef]
- Bailey, R.C.; Egesah, O.; Rosenberg, S. Male circumcision for HIV prevention: A prospective study of complications in clinical and traditional settings in Bungoma, Kenya. Bull. World Health Organ. 2008, 86, 669–677. [Google Scholar] [CrossRef]
- Frisch, M.; Earp, B.D. Circumcision of male infants and children as a public health measure in developed countries: A critical assessment of recent evidence. Glob. Public Health 2018, 13, 626–641. [Google Scholar] [CrossRef]
- Shi, C.; Li, M.; Dushoff, J. Traditional male circumcision is associated with sexual risk behaviours in sub-Saharan countries prioritized for male circumcision. AIDS Behav. 2020, 24, 951–959. [Google Scholar] [CrossRef]
- Asa, G.A.; Fauk, N.K.; Gesesew, H.A.; Ward, P.R. Knowledge and attitude towards traditional male circumcision and the risk of HIV transmission in Indonesia: A cross-sectional study. arXiv 2024. [Google Scholar] [CrossRef]
- Ntshiqa, T.; Musekiwa, A.; Manesen, R.; Mdose, H.; Ngoma, N.; Kuonza, L.; Dlamini, T.; Reddy, C.; Williams, S. Knowledge, Attitudes, Practices, and Acceptability of Medical Male Circumcision among Males in Traditionally Circumcising Rural Communities of Alfred Nzo District, Eastern Cape, South Africa. Int. J. Environ. Res. Public Health 2023, 20, 7091. [Google Scholar] [CrossRef] [PubMed]
- Matlala, T.F. Knowledge, attitude and perception of university students regarding Medical Male Circumcision at the University of Venda, South Africa. Ph.D. Thesis, University of Venda, Durban, South Africa, 2021. [Google Scholar]
- Kang’ethe, S.M. The panacea and perfidy of cultural rites of circumcision in African countries: Examples from Kenya, Botswana and South Africa. East. Afr. Soc. Sci. Res. Rev. 2013, 29, 107–123. [Google Scholar] [CrossRef]
- Maughan-Brown, B.; Venkataramani, A.S.; Nattrass, N.; Seekings, J.; Whiteside, A.W. A cut above the rest: Traditional male circumcision and HIV risk among Xhosa men in Cape Town, South Africa. JAIDS J. Acquir. Immune Defic. Syndr. 2011, 58, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Greely, P.; Maharaj, P.; Letsoalo, T.; Miti, A. Traditional male circumcision for reducing the risk of HIV infection: Perspectives of young people in South Africa. Cult. Health Sex. 2013, 15, 148–159. [Google Scholar] [CrossRef]
- Maffioli, E.M. Is traditional male circumcision effective as an HIV prevention strategy? Evidence from Lesotho. PLoS ONE 2017, 12, e0177076. [Google Scholar] [CrossRef]
- Baduro, J.; Mccabe, K.C.; Cavele, N.; José, A.; Mulimela, A.; Jamnadas, M.; Manhiça, C.; Monjane, C.; Nhachungue, S.; Decroo, T.; et al. Understanding male circumcision: Insights from a peri-urban community in Maputo City, Mozambique. Int. Health 2024, 17, ihae042. [Google Scholar] [CrossRef]
- Dent, J.; Gaspar, N.; Njeuhmeli, E.; Kripke, K. Age targeting and scale-up of voluntary medical male circumcision in Mozambique. PLoS ONE 2019, 14, e0211958. [Google Scholar] [CrossRef]
- Mpateni, A.; Kang’ethe, S.M. Susceptibility of contracting diseases during the Traditional Male Circumcision (TMC) rite of males in Alice, South Africa (Eastern Cape): Implication to social work. E-J. Humanit. Arts Soc. Sci. 2025, 6, 97–107. [Google Scholar] [CrossRef]
- Dlamini, N. Metamorphosis of Xhosa masculinity in Thando Mgqolozana’s A man who is not a man. Safundi 2020, 21, 176–189. [Google Scholar] [CrossRef]
- Mpateni, A.; Kang’Ethe, S.M. Behaviours of traditional male circumcision initiates of Cala and Mdantsane, South Africa. Inkanyiso 2022, 14, 8. [Google Scholar] [CrossRef]
- Okyere, Y.M.; Aboagye, R.G.; Boateng, E.N.; Okyere, J.; Osborne, A.; Ahinkorah, B.O. Spatial distribution and factors associated with unmet need for contraception among women in Ghana. Reprod. Health 2025, 22, 31. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, P.A.; Adzigbli, L.A.; Atsu, P.; Ansong-Aggrey, S.K.; Adu, C.; Cadri, A.; Aboagye, R.G. Unmet need for contraception among women in Benin: A cross-sectional analysis of the Demographic and Health Survey. Int. Health 2024, 16, 302–312. [Google Scholar] [CrossRef]
- Agyekum, A.K.; Adde, K.S.; Aboagye, R.G.; Salihu, T.; Seidu, A.A.; Ahinkorah, B.O. Unmet need for contraception and its associated factors among women in Papua New Guinea: Analysis from the demographic and health survey. Reprod. Health 2022, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Ahinkorah, B.O. Predictors of unmet need for contraception among adolescent girls and young women in selected high fertility countries in sub-Saharan Africa: A multilevel mixed effects analysis. PLoS ONE 2020, 15, e0236352. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yang, W. Multivariate logistic regression analysis of complex survey data with application to BRFSS data. J. Data Sci. 2012, 10, 157–173. [Google Scholar] [CrossRef]
- Addila, A.E.; Azale, T.; Gete, Y.K.; Yitayal, M. Individual and community-level predictors of maternal alcohol consumption during pregnancy in Gondar town, Northwest Ethiopia: A multilevel logistic regression analysis. BMC Pregnancy Childbirth 2021, 21, 419. [Google Scholar] [CrossRef]
- Ba, D.M.; Ssentongo, P.; Agbese, E.; Yang, Y.; Cisse, R.; Diakite, B.; Traore, C.B.; Kamate, B.; Kassogue, Y.; Dolo, G.; et al. Prevalence and determinants of breast cancer screening in four sub-Saharan African countries: A population-based study. BMJ Open 2020, 10, e039464. [Google Scholar] [CrossRef]
- Antabe, R.; Kansanga, M.; Sano, Y.; Kyeremeh, E.; Galaa, Y. Utilization of breast cancer screening in Kenya: What are the determinants? BMC Health Serv. Res. 2020, 20, 228. [Google Scholar] [CrossRef]
- Lunani, L.L.; Abaasa, A.; Omosa-Manyonyi, G. Prevalence and factors associated with contraceptive use among Kenyan women aged 15–49 years. AIDS Behav. 2018, 22, 125–130. [Google Scholar] [CrossRef]
- Tibenderana, J.R.; Kessy, S.A.; Mlaponi, D.F.; Mwaitete, N.L.; Mtenga, J.E. Predictors of breast cancer screening among women of reproductive age in Tanzania: Evidence from DHS 2022. PLoS ONE 2024, 19, e0298996. [Google Scholar] [CrossRef]
- Mbona, S.V.; Mwambi, H.; Ramroop, S. Multiple imputation using chained equations for missing data in survival models: Applied to multidrug-resistant tuberculosis and HIV data. J. Public Health Afr. 2023, 14, 2388. [Google Scholar] [CrossRef] [PubMed]
- Van Buuren, S.; Boshuizen, H.C.; Knook, D.L. Multiple imputation of missing blood pressure covariates in survival analysis. Stat. Med. 1999, 18, 681–694. [Google Scholar] [CrossRef]
- Carle, A.C. Fitting multilevel models in complex survey data with design weights: Recommendations. BMC Med. Res. Methodol. 2009, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Tesema, G.A.; Tessema, Z.T.; Tamirat, K.S.; Teshale, A.B. Complete basic childhood vaccination and associated factors among children aged 12–23 months in East Africa: A multilevel analysis of recent demographic and health surveys. BMC Public Health 2020, 20, 1837. [Google Scholar] [CrossRef]
- Kianoush, F.; Masoomehni, K. Application REML model and determining cut off of ICC by multi-level model based on Markov Chains simulation in Health. Indian J. Fundam. Appl. Life Sci. 2015, 5, 1432–1448. [Google Scholar]
- Croft, T.N.; Allen, C.K.; Zachary, B.W. Guide to DHS Statistics, DHS-7; ICF: Rockville, MD, USA, 2023; Available online: https://www.dhsprogram.com/publications/publication-dhsg1-dhs-questionnaires-and-manuals.cfm (accessed on 28 March 2025).
- Deacon, H.; Thomson, K. The Social Penis. Traditional Male Circumcision and Initiation in Southern Africa, 1800–2000: A Literature Review; Centre for Social Science Research, University of Cape Town: Cape Town, South Africa, 2012. [Google Scholar]
- Mshana, G.; Wambura, M.; Mwanga, J.; Mosha, J.; Mosha, F.; Changalucha, J. Traditional male circumcision practices among the Kurya of North-eastern Tanzania and implications for national programmes. AIDS Care 2011, 23, 1111–1116. [Google Scholar] [CrossRef]
- Gray, R.H.; Wawer, M.J.; Kigozi, G. Programme science research on medical male circumcision scale-up in sub-Saharan Africa. Sex. Transm. Infect. 2013, 89, 345–349. [Google Scholar] [CrossRef]
- Nyembezi, A.; Sifunda, S.; Funani, I.; Ruiter, R.A.; Van Den Borne, B.; Reddy, P.S. Correlates of risky sexual behaviours in recently traditionally circumcised men from initiation lodges in the Eastern Cape, South Africa. Int. Q. Community Health Educ. 2010, 30, 97–114. [Google Scholar] [CrossRef]
- Meel, B.L. Community perception of traditional circumcision in a sub-region of the Transkei, Eastern Cape, South Africa. S. Afr. Fam. Pract. 2005, 47, 58–59. [Google Scholar] [CrossRef]
- Gwata, F. Traditional Male Circumcision: What Is Its Socio-Cultural Significance Among Young Xhosa Men? Centre for Social Science Research, University of Cape Town: Cape Town, South Africa, 2009. [Google Scholar]
- Rasmussen, D.N.; Wejse, C.; Larsen, O.; Da Silva, Z.; Aaby, P.; Sodemann, M. The when and how of male circumcision and the risk of HIV: A retrospective cross-sectional analysis of two HIV surveys from Guinea-Bissau. Pan Afr. Med. J. 2016, 23, 21. [Google Scholar] [CrossRef]
- Rogers, J.H.; Odoyo-June, E.; Jaoko, W.; Bailey, R.C. Time to complete wound healing in HIV-positive and HIV-negative men following medical male circumcision in Kisumu, Kenya: A prospective cohort study. PLoS ONE 2013, 8, e61725. [Google Scholar] [CrossRef] [PubMed]
- Peltzer, K.; Onoya, D.; Makonko, E.; Simbayia, L. Prevalence and acceptability of male circumcision in South Africa. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Nyembezi, A.; Ruiter, R.A.; van den Borne, B.; Sifunda, S.; Funani, I.; Reddy, P. Correlates of consistent condom use among recently initiated and traditionally circumcised men in the rural areas of the Eastern Cape Province, South Africa. BMC Public Health 2014, 14, 668. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yuan, T.; Zhan, Y.; Qian, H.Z.; Sun, Y.; Zheng, W.; Fu, L.; Liang, B.; Zhu, Z.; Ouyang, L.; et al. Association between medical male circumcision and HIV risk compensation among heterosexual men: A systematic review and meta-analysis. Lancet Glob. Health 2021, 9, e932–e941. [Google Scholar] [CrossRef]

| Variables | Definition and Coding |
|---|---|
| Is respondent traditionally circumcised | 0 = No; 1 = Yes |
| Age group in years | 1 = 15–24; 2 = 25–34; 3 = 35+ |
| Place of residence | 1 = Urban; 2 = Rural |
| District | 1 = Butha-Bothe; 2 = Leribe; 3 = Berea; 4 = Maseru; 5 = Mafeteng; 6 = Mohale′s Hoek; 7 = Quthing; 8 = Qacha′s Nek; 9 = Mokhotlong; 10 = Thaba-Tseka |
| Level of education | 0 = No education; 1 = Primary; 2 = Secondary; 3 = Higher |
| Sex of household head | 1 = Male; 2 = Female |
| Literacy | 0 = Cannot read at all; 1 = Able to read only parts of sentence; 2 = Able to read whole sentence; 3 = No card with required language |
| Wealth status | 1 = Poorer; 2 = Middle; 3 = Richer |
| Smoke cigarette | 0 = No; 1 = Yes |
| Covered by health insurance | 0 = No; 1 = Yes |
| Marital status | 0 = Never in union; 1 = Married; 2 = Living with partner; 3 = Windowed; 4 = Divorced; 5 = No longer living together/separated |
| Working | 0 = No; 1 = Yes |
| HIV test result | 1 = Positive; 2 = Negative; 3 = Indeterminate; 4 = Declined to answer |
| Would you marry a person with HIV | 0 = No; 1 = Yes; 2 = Don’t know/not sure/depends |
| Number of wives/partners | 0 = No wives/partners; 1 = one wife/partner |
| Self-reported health status | 1 = Good; 2 = Moderate; 3 = Bad |
| Partner currently pregnant | 0 = No; 1 = Yes; 2 = Unsure |
| Ever had sex | 0 = No; 1 = Yes |
| Variable | All Respondents, n (%) | TC | (p-Value) | |
|---|---|---|---|---|
| Yes (95% CI) | ||||
| Prevalence | 51.0 (49.3–52.7) | |||
| Individual-level variables | ||||
| Mean age (SD) in years | 12.3) | 11.7) | ||
| Age group (years) | 15–24 | 1119 (35.0) | 43.1 (40.2–46.0) | <0.001 |
| 25–34 | 729 (22.8) | 56.1 (52.5–59.7) | ||
| 35 + | 1354 (42.3) | 54.8 (52.1–57.5) | ||
| Level of education | No education | 201 (6.3) | 87.6 (83.0–92.2) | <0.001 |
| Primary | 1192 (37.2) | 71.9 (69.3–74.5) | ||
| Secondary | 1375 (42.9) | 38.9 (36.3–41.5) | ||
| Higher | 434 (13.6) | 15.0 (11.6–18.4) | ||
| Literacy | Cannot read at all | 381 (11.9) | 82.1 (78.3–85.9) | <0.001 |
| Able to read only parts of sentence | 436 (13.6) | 64.4 (59.9–68.9) | ||
| Able to read whole sentence | 2385 (74.5) | 43.6 (41.6–45.6) | ||
| Smoke cigarettes | No | 1755 (54.8) | 39.8 (37.5–42.1) | <0.001 |
| Yes | 1447 (45.2) | 64.6 (62.1–67.1) | ||
| Covered by health insurance | No | 2652 (82.8) | 53.4 (51.5–55.3) | <0.001 |
| Yes | 550 (17.2) | 39.3 (35.2–43.4) | ||
| Marital status | Never in union | 1514 (47.3) | 44.0 (41.5–46.5) | <0.001 |
| Married | 1356 (42.3) | 56.6 (54.0–59.2) | ||
| Living with partner | 73 (2.3) | 54.8 (43.4–66.2) | ||
| Widowed | 68 (2.1) | 64.7 (53.3–76.1) | ||
| Divorced | 37 (1.1) | 52.8 (36.7–68.9) | ||
| No longer living together/separated | 154 (4.8) | 62.3 (54.6–70.0) | ||
| Working | No | 1212 (37.9) | 53.5 (50.7–56.3) | 0.026 |
| Yes | 1990 (62.1) | 49.5 (47.3–51.7) | ||
| Result of HIV test | Positive | 507 (15.8) | 58.4 (54.1–62.7) | <0.001 |
| Negative | 2486 (77.6) | 50.8 (48.8–52.8) | ||
| Indeterminate | 99 (3.1) | 41.4 (31.7–51.1) | ||
| Declined to answer | 110 (3.4) | 31.8 (23.1–40.5) | ||
| Would you marry a person with HIV? | No | 1373 (42.9) | 53.6 (51.0–56.2) | <0.001 |
| Yes | 1679 (52.4) | 50.2 (47.8–52.6) | ||
| Don′t know/not sure/depends | 151 (4.7) | 36.4 (28.7–44.1) | ||
| Number of wives/partners | No wives/partners | 1773 (55.4) | 46.6 (44.3–48.9) | <0.001 |
| One wife/partner | 1429 (44.6) | 56.5 (53.9–59.1) | ||
| Variable | All Respondents, n (%) | TC | (p-Value) | |
| Yes (95% CI) | ||||
| Self-reported health status | Good | 1672 (52.2) | 48.4 (46.0–50.8) | <0.001 |
| Moderate | 1160 (36.2) | 51.0 (48.1–53.9) | ||
| Bad | 370 (11.6) | 63.0 (58.1–67.9) | ||
| Partner currently pregnant | No | 2069 (64.6) | 53.6 (51.5–55.7) | <0.001 |
| Yes | 906 (28.3) | 43.8 (40.6–47.0) | ||
| Unsure | 227 (7.1) | 56.4 (49.9–62.9) | ||
| Ever had sex | No | 254 (7.9) | 13.8 (9.6–18.0) | <0.001 |
| Yes | 2949 (92.1) | 54.2 (52.4–56.0) | ||
| Community-level variables | ||||
| Place of residence | Urban | 1319 (41.2) | 34.7 (32.1–37.3) | <0.001 |
| Rural | 1884 (58.8) | 62.4 (60.2–64.6) | ||
| District | Butha-Buthe | 195 (6.1) | 64.6 (57.9–71.3) | <0.001 |
| Leribe | 624 (19.5) | 58.7 (54.8–62.6) | ||
| Berea | 477 (14.9) | 40.4 (36.0–44.8) | ||
| Maseru | 1012 (31.6) | 35.1 (32.2–38.0) | ||
| Mafeteng | 215 (6.7) | 53.5 (46.8–60.2) | ||
| Mohale′s Hoek | 161 (5.0) | 62.1 (54.6–69.6) | ||
| Quthing | 122 (3.8) | 68.0 (59.7–76.3) | ||
| Qacha′s Nek | 90 (2.8) | 68.9 (59.3–78.5) | ||
| Mokhotlong | 123 (3.8) | 75.4 (67.8–83.0) | ||
| Thaba-Tseka | 184 (5.8) | 77.7 (71.7–83.7) | ||
| Sex of household head | Male | 2378 (74.3) | 50.1 (48.1–52.1) | 0.082 |
| Female | 824 (25.7) | 53.6 (50.2–57.0) | ||
| Wealth status | Poorer | 1127 (35.2) | 75.2 (72.7–77.7) | <0.001 |
| Middle | 705 (22.0) | 55.5 (51.8–59.2) | ||
| Richer | 1370 (42.8) | 28.8 (26.4–31.2) | ||
| Variables | COR | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|---|
| (95% CI) | (Null Model) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | |
| Individual-level variables | |||||
| Age group | |||||
| 15–24 | 1 | 1 | 1 | ||
| 25–34 | 2.44 (1.94–3.07) | 1.26 (0.95–1.67) | - | 1.21 (0.90–1.61) | |
| 35+ | 2.51 (2.07–3.04) | 1.61 (1.45–1.83) | - | 1.63 * (1.46–1.86) | |
| Level of education | |||||
| No education | 1 | 1 | 1 | ||
| Primary | 0.30 (0.19–0.46) | 0.49 (0.28–0.86) | - | 0.54 * (0.30–0.97) | |
| Secondary | 0.08 (0.05–0.13) | 0.14 (0.08–0.25) | - | 0.17 * (0.09–0.32) | |
| Higher | 0.03 (0.02–0.04) | 0.04 (0.02–0.07) | - | 0.06 * (0.03–0.12) | |
| Literacy | |||||
| Cannot read at all | 1 | 1 | 1 | ||
| Able to read only parts of sentence | 0.36 (0.25–0.52) | 0.56 (0.36–0.88) | - | 0.59 * (0.37–0.93) | |
| Able to read whole sentence | 0.17 (0.13–0.24) | 0.59 (0.39–0.90) | - | 0.68 (0.44–1.03) | |
| Smoke cigarettes | |||||
| No | 1 | 1 | 1 | ||
| Yes | 3.33 (2.79–3.97) | 1.99 (1.64–2.41) | - | 1.82 * (1.50–2.21) | |
| Covered by health insurance | |||||
| No | 1 | 1 | 1 | ||
| Yes | 0.64 (0.49–0.83) | 0.79 (0.60–1.03) | - | 0.93 (0.70–1.22) | |
| Marital status | |||||
| Never in union | 1 | 1 | 1 | ||
| Married | 2.40 (2.00–2.88) | 1.30 (0.98–1.72) | - | 1.43 * (1.07–1.91) | |
| Living with partner | 2.79 (1.48–5.27) | 1.40 (0.79–2.57) | - | 1.77 (0.97–3.23) | |
| Widowed | 2.28 (1.29–4.03) | 1.30 (0.62–2.71) | - | 1.55 (0.73–3.32) | |
| Divorced | 3.25 (1.55–6.84) | 1.92 (0.80–4.58) | - | 2.32 (0.95–5.63) | |
| No longer living together/separated | 2.46 (1.62–3.74) | 1.11 (0.69–1.78) | - | 1.16 (0.72–1.86) | |
| Working | |||||
| No | 1 | 1 | 1 | ||
| Yes | 0.94 (0.79–1.13) | 0.87 (0.70–1.06) | - | 0.96 (0.78–1.18) | |
| Result of HIV test | |||||
| Positive | 1 | 1 | 1 | ||
| Negative | 0.69 (0.55–0.87) | 0.90 (0.68–1.18) | - | 0.88 (0.67–1.17) | |
| Indeterminate | 0.34 (0.21–0.55) | 0.51 (0.28–0.93) | - | 0.50 * (0.27–0.93) | |
| Declined to answer | 0.34 (0.21–0.57) | 0.25 (0.14–0.45) | - | 0.26 * (0.14–0.46) | |
| Would you marry a person with HIV? | |||||
| No | 1 | 1 | 1 | ||
| Yes | 1.05 (0.88–1.25) | 0.99 (0.81–1.22) | - | 1.04 (0.84–1.27) | |
| Don’t know/not sure/depends | 0.49 (0.32–0.75) | 0.55 (0.35–0.88) | - | 0.59 * (0.37–0.95) | |
| Number of wives/partners | |||||
| No wives/partners | 1 | 1 | 1 | ||
| One wife/partner | 2.11 (1.77–2.50) | 2.24 (1.01–4.35) | - | 3.12 * (1.71–5.13) | |
| Self-reported health status | |||||
| Good | 1 | 1 | 1 | ||
| Moderate | 1.22 (1.01–1.47) | 1.11 (0.90–1.36) | - | 1.14 (0.92–1.41) | |
| Bad | 1.68 (1.26–2.25) | 0.97 (0.71–1.32) | - | 0.93 (0.68–1.28) | |
| Partner currently pregnant | |||||
| No | 1 | 1 | 1 | ||
| Yes | 0.63 (0.52–0.76) | 0.88 (0.69–1.13) | - | 0.90 (0.70–1.15) | |
| Unsure | 0.81 (0.59–1.11) | 0.95 (0.66–1.37) | - | 0.95 (0.65–1.38) | |
| Ever had sex | |||||
| No | 1 | 1 | 1 | ||
| Yes | 13.37 (9.03–19.80) | 10.91 (6.78–17.56) | - | 12.42 * (7.69–20.07) | |
| Community-level variables | |||||
| Place of residence | |||||
| Urban | 1 | 1 | 1 | ||
| Rural | 4.66 (3.61–6.02) | - | 1.52 (1.13–2.04) | 1.54 * (1.12–2.12) | |
| District | |||||
| Butha-Buthe | 1 | 1 | 1 | ||
| Leribe | 0.66 (0.40–1.11) | - | 0.90 (0.54–1.52) | 0.73 (0.41–1.28) | |
| Berea | 0.34 (0.20–0.58) | - | 0.46 (0.27–0.79) | 0.39 * (0.22–0.70) | |
| Maseru | 0.21 (0.12–0.35) | - | 0.40 (0.24–0.67) | 0.33 * (0.19–0.57) | |
| Mafeteng | 0.58 (0.34–1.00) | - | 0.69 (0.39–1.23) | 0.65 (0.35–1.22) | |
| Mohale’s Hoek | 0.93 (0.53–1.63) | - | 0.84 (0.45–1.55) | 0.64 (0.33–1.25) | |
| Quthing | 1.21 (0.69–2.13) | - | 0.84 (0.35–2.01) | 0.83 (0.33–2.12) | |
| Qacha’s Nek | 1.27 (0.70–2.27) | - | 1.12 (0.39–3.20) | 0.87 (0.28–2.67) | |
| Mokhotlong | 1.72 (0.96–3.06) | - | 1.18 (0.54–2.58) | 0.99 (0.42–2.35) | |
| Thaba-Tseka | 1.91 (1.08–3.36) | - | 1.06 (0.57–1.97) | 0.74 (0.38–1.47) | |
| Sex of household head | |||||
| Male | 1 | 1 | 1 | ||
| Female | 0.86 (0.71–1.04) | - | 0.90 (0.74–1.09) | 1.24 (0.99–1.55) | |
| Wealth status | |||||
| Poorer | 1 | 1 | 1 | ||
| Middle | 0.41 (0.33–0.52) | - | 0.57 (0.44–0.72) | 0.72 * (0.55–0.95) | |
| Richer | 0.14 (0.11–0.18) | - | 0.22 (0.17–0.29) | 0.39 * (0.28–0.52) | |
| Random effect | |||||
| Cluster variance | 1.32 | 0.94 | 0.76 | 0.21 | |
| ICC | 0.29 | 0.24 | 0.21 | 0.20 | |
| MOR | 1.88 | 1.82 | 1.74 | 1.69 | |
| PCV | Reff | 1.34 | 7.78 | 21.51 | |
| Model comparison | |||||
| Log-likelihood | −2011.701 | −1985.521 | −2001.632 | −1986.202 | |
| AIC | 4027.402 | 3851.922 | 4077.645 | 3804.416 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbona, S.V.; Ananth, A.; Chifurira, R. Investigating the Factors Influencing Traditional Male Circumcision and Its Contribution to HIV Transmission Amongst Men in Lesotho: A Multilevel Binary Logistic Regression Approach. Int. J. Environ. Res. Public Health 2025, 22, 993. https://doi.org/10.3390/ijerph22070993
Mbona SV, Ananth A, Chifurira R. Investigating the Factors Influencing Traditional Male Circumcision and Its Contribution to HIV Transmission Amongst Men in Lesotho: A Multilevel Binary Logistic Regression Approach. International Journal of Environmental Research and Public Health. 2025; 22(7):993. https://doi.org/10.3390/ijerph22070993
Chicago/Turabian StyleMbona, Sizwe Vincent, Anisha Ananth, and Retius Chifurira. 2025. "Investigating the Factors Influencing Traditional Male Circumcision and Its Contribution to HIV Transmission Amongst Men in Lesotho: A Multilevel Binary Logistic Regression Approach" International Journal of Environmental Research and Public Health 22, no. 7: 993. https://doi.org/10.3390/ijerph22070993
APA StyleMbona, S. V., Ananth, A., & Chifurira, R. (2025). Investigating the Factors Influencing Traditional Male Circumcision and Its Contribution to HIV Transmission Amongst Men in Lesotho: A Multilevel Binary Logistic Regression Approach. International Journal of Environmental Research and Public Health, 22(7), 993. https://doi.org/10.3390/ijerph22070993








