Parental Low Level of Education and Single-Parent Families as Predictors of Poor Control of Type 1 Diabetes in Children Followed in French Guiana
Abstract
1. Introduction
2. Methods
2.1. Study Setting
2.2. Study Design
2.2.1. Data Collected
2.2.2. Data Analysis
2.3. Ethics and Consent
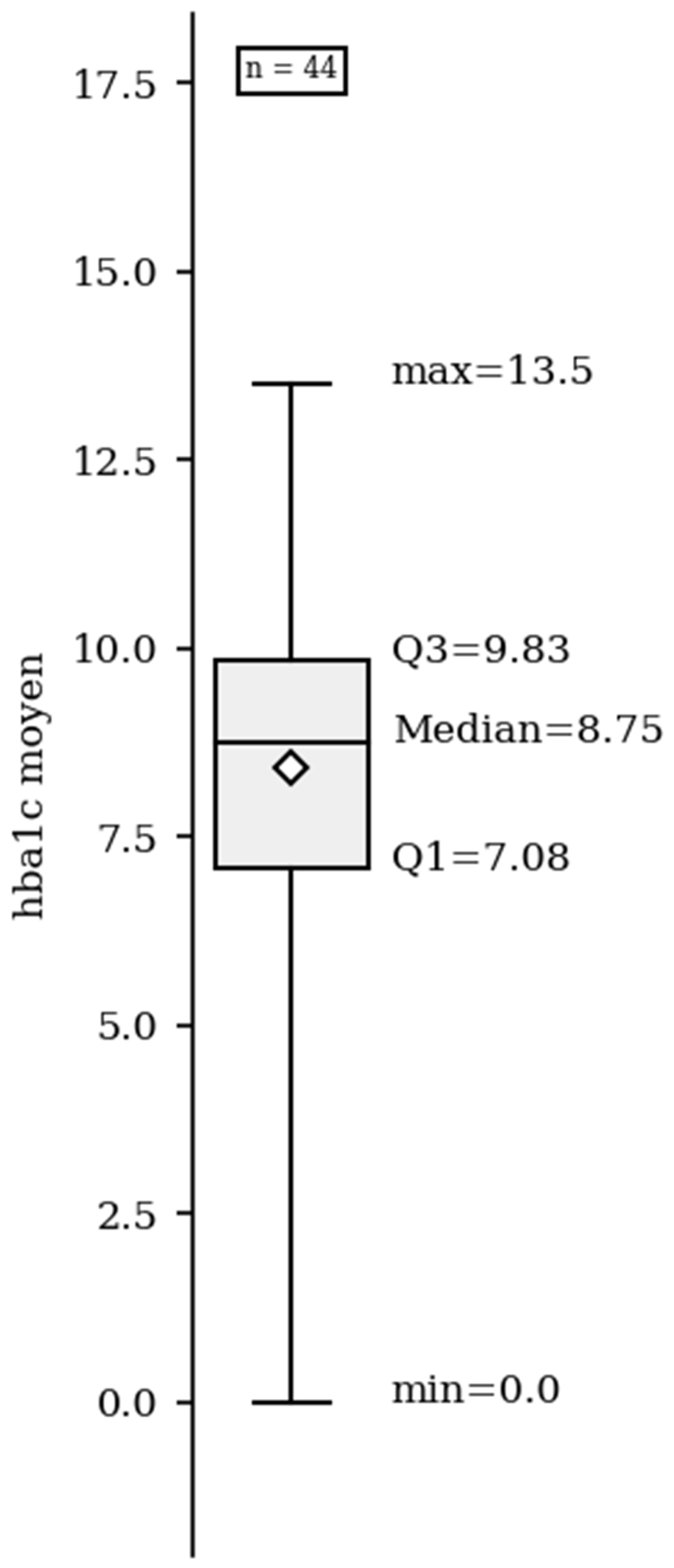
3. Results
3.1. Epidemiological Data
3.2. Social Data
3.3. Clinical Data
4. Discussion
Limitations of Our Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T1DM | Type 1 diabetes mellitus |
| DKA | Diabetes ketoacidosis |
| HbA1c | Glycemic hemoglobin |
References
- Subramanian, S.; Khan, F.; Hirsch, I.B. New advances in type 1 diabetes. BMJ 2024, 384, e075681, Erratum in: BMJ 2024, 385, q1224. https://doi.org/10.1136/bmj.q1224. [Google Scholar] [CrossRef] [PubMed]
- Piffaretti, C.; Mandereau-Bruno, L.; Guilmin-Crepon, S.; Choleau, C.; Coutant, R.; Fosse-Edorh, S. Trends in childhood type 1 diabetes incidence in France, 2010–2015. Diabetes Res. Clin. Pract. 2019, 149, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Njuieyon, F.; Buende Eyenga, R.S.; Elenga, N. Incidence du diabète chez les enfants de moins de 15 ans en Guyane française: 2011–2013. In Annales d’Endocrinologie; Elsevier Masson: Issy-les-Moulineaux, France, 2014; Volume 75, 374p. [Google Scholar] [CrossRef]
- Geffner, M.E. Editorial: Childhood diabetes in low- and middle-income countries: Progress, challenges, and actions needed, volume II. Front. Endocrinol. 2023, 14, 1304716. [Google Scholar] [CrossRef]
- Erikson, E.H. Childhood and Society, 2nd ed.; Norton: New York, NY, USA, 1963. [Google Scholar]
- Mayer-Davis, E.J.; Kahkoska, A.R.; Jefferies, C.; Dabelea, D.; Balde, N.; Gong, C.X.; Aschner, P.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2018, 19 (Suppl. S27), 7–19. [Google Scholar] [CrossRef] [PubMed]
- Pironetti, R.; Saha, M.T.; Luukkaala, T.; Keskinen, P. Sociodemographic factors affecting glycaemic control in Finnish paediatric patients with type 1 diabetes. Endocrinol. Diabetes Metab. 2023, 6, e452. [Google Scholar] [CrossRef]
- Ogugua, C.F.; Chikani, U.N.; Okiche, C.Y.; Ibekwe, U.M. Sociodemographic determinants of glycaemic control among children with type 1 diabetes in South Eastern Nigeria. Pan Afr. Med. J. 2021, 38, 250. [Google Scholar] [CrossRef]
- Donbaloğlu, Z.; Barsal Çetiner, E.; Tuhan, H.; Parlak, M. The Association of Sociodemographic Factors and Utilization of Diabetes Technologies with Diabetes Management: An Investigation in Children and Adolescents with Type 1 Diabetes. Turk. Arch. Pediatr. 2024, 59, 454–460. [Google Scholar] [CrossRef]
- Sudre, C.; Duplan, H.; Bukasakakamba, J.; Nacher, M.; Peyre-Costa, P.; Sabbah, N. Diabetes Care in French Guiana: The Gap Between National Guidelines and Reality. Front. Endocrinol. 2021, 12, 789391. [Google Scholar] [CrossRef]
- Insee Analyses Guyane. La Guyane, Une Région Jeune et Cosmopolite—35 (2015). Available online: https://www.insee.fr/fr/statistiques/3695893 (accessed on 12 December 2021).
- Insee Analyses Guyane. Niveaux de vie et Pauvreté en Guyane en 2017: La Moitié des Guyanais Vivent Sous le Seuil de Pauvret—46 (2017). Available online: https://www.insee.fr/fr/statistiques/4623886 (accessed on 25 March 2021).
- Osei, L.; Basurko, C.; Nacher, M.; Vignier, N.; Elenga, N. About the need to address pediatric health inequalities in French Guiana: A scoping review. Arch. Pediatr. 2022, 29, 340–346. [Google Scholar] [CrossRef]
- Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Relating to the Implementation of Good Clinical Practice in the Conduct of Clinical Trials on Medicinal Products for Human Use. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02001L0020-20090807 (accessed on 1 April 2025).
- Toulouse, E.; Masseguin, C.; Lafont, B.; McGurk, G.; Harbonn, A.; ARoberts, J.; Granier, S.; Dupeyron, A.; Bazin, J.E. French legal approach to clinical research. Anaesth. Crit. Care Pain. Med. 2018, 37, 607–614. [Google Scholar] [CrossRef]
- Ruiz-Grao, M.C.; Díez-Fernández, A.; Mesas, A.E.; Martínez-Vizcaíno, V.; Sequí-Domínguez, I.; Sebastián-Valles, F.; Garrido-Miguel, M. Trends in the Incidence of Type 1 Diabetes in European Children and Adolescents from 1994 to 2022: A Systematic Review and Meta-Analysis. Pediatr Diabetes 2024, 2024, 2338922. [Google Scholar] [CrossRef] [PubMed]
- Karvonen, M.; Viik-Kajander, M.; Moltchanova, E.; Libman, I.; LaPorte, R.; Tuomilehto, J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care 2000, 23, 1516–1526. [Google Scholar] [CrossRef]
- Donaghue, K.C.; Marcovecchio, M.L.; Wadwa, R.P.; Chew, E.Y.; Wong, T.Y.; Calliari, L.E.; Zabeen, B.; Salem, M.A.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Microvascular and macrovascular complications in children and adolescents. Pediatr. Diabetes 2018, 19 (Suppl. S27), 262–274. [Google Scholar] [CrossRef]
- Zabeen, B.; Khaled, M.Z.; Husain, L.; Aktar, A.; Huda, K.; Kamal, Y.A.; Choudhury, N.; Azad, K. Risk factors associated with retinopathy in young people with type 1 diabetes in Bangladesh. Endocrinol. Diabetes Metab. 2020, 4, e00197. [Google Scholar] [CrossRef] [PubMed]
- Negrato, C.A.; Lauris, J.R.P.; Saggioro, I.B.; Corradini, M.C.M.; Borges, P.R.; Crês, M.C.; Junior, A.L.; Guedes, M.F.S.; Gomes, M.B. Increasing incidence of type 1 diabetes between 1986 and 2015 in Bauru, Brazil. Diabetes Res. Clin. Pract. 2017, 127, 198–204. [Google Scholar] [CrossRef]
- Niechciał, E.; Michalak, M.; Skowrońska, B.; Fichna, P. Increasing trend of childhood type 1 diabetes incidence: 20-year observation from Greater Poland Province, Poland. Acta Diabetol. 2024, 61, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Casu, A.; Kanapka, L.G.; Foster, N.C.; Hirsch, I.B.; Laffel, L.M.; Shah, V.N.; DeSalvo, D.J.; Lyons, S.K.; Vendrame, F.; Aleppo, G.; et al. Characteristics of adult- compared to childhood-onset type 1 diabetes. Diabet. Med. 2020, 37, 2109–2115. [Google Scholar] [CrossRef]
- Sehgal, M.; Batra, M.; Jha, P.; Sanchez, O. Risk Factors and Laboratory Findings Associated With Diabetic Ketoacidosis in Hospitalized Pediatric Patients. Cureus 2022, 14, e25410. [Google Scholar] [CrossRef]
- Choleau, C.; Maitre, J.; Filipovic Pierucci, A.; Elie, C.; Barat, P.; Bertrand, A.M.; de Kerdanet, M.; Letallec, C.; Levy-Marchal, C.; Nicolino, M.; et al. Ketoacidosis at diagnosis of type 1 diabetes in French children and adolescents. Diabetes Metab. 2014, 40, 137–142. [Google Scholar] [CrossRef]
- Große, J.; Hornstein, H.; Manuwald, U.; Kugler, J.; Glauche, I.; Rothe, U. Incidence of Diabetic Ketoacidosis of New-Onset Type 1 Diabetes in Children and Adolescents in Different Countries Correlates with Human Development Index (HDI): An Updated Systematic Review, Meta-Analysis, and Meta-Regression. Horm. Metab. Res. 2018, 50, 209–222, Erratum in: Horm. Metab. Res. 2018, 50, e2. https://doi.org/10.1055/a-0584-6211. [Google Scholar] [CrossRef]
- Gunn, E.R.; Albert, B.B.; Hofman, P.L.; Cutfield, W.S.; Gunn, A.J.; Jefferies, C.A.; Starbase Diabetes Working Group, Paediatric Diabetes Service, Starship Children’s Hospital, Auckland, New Zealand. Pathways to reduce diabetic ketoacidosis with new onset type 1 diabetes: Evidence from a regional pediatric diabetes center: Auckland, New Zealand, 2010 to 2014. Pediatr. Diabetes 2017, 18, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Simba, S.; Von Oettingen, J.E.; Rahme, E.; Ladd, J.M.; Nakhla, M.; Li, P. Socioeconomic Disparities in Glycemic Management in Children and Youth With Type 1 Diabetes: A Retrospective Cohort Study. Can. J. Diabetes 2023, 47, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Hershey, J.A.; Morone, J.; Lipman, T.H.; Hawkes, C.P. Social Determinants of Health, Goals and Outcomes in High-Risk Children With Type 1 Diabetes. Can. J. Diabetes 2021, 45, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Alassaf, A.; Gharaibeh, L.; Odeh, R.; Ibrahim, S.; Ajlouni, K. Predictors of glycemic control in children and adolescents with type 1 diabetes at 12 months after diagnosis. Pediatr. Diabetes 2022, 23, 729–735. [Google Scholar] [CrossRef]
- Full Report-Department of French Guiana (973)|Insee. Available online: https://www.insee.fr/fr/statistiques/2011101?geo=DEP-97 (accessed on 1 January 2025).
- Fantahun, B.; Leulseged, T.W. Glycemic control among children with type 1 diabetes mellitus and its determinants in a resource-limited setting. J. Pediatr. Endocrinol. Metab. 2022, 35, 813–817. [Google Scholar] [CrossRef]
- Gomes, M.B.; Rodacki, M.; Pavin, E.J.; Cobas, R.A.; Felicio, J.S.; Zajdenverg, L.; Negrato, C.A. The impact of ethnicity, educational and economic status on the prescription of insulin therapeutic regimens and on glycemic control in patients with type 1 diabetes. A nationwide study in Brazil. Diabetes Res. Clin. Pract. 2017, 134, 44–52. [Google Scholar] [CrossRef]
- Campas-Lebecque, M.N.; Pochelu, S.; Vautier Vet coll Bacheré, N.; Beau, C.; Benoit, M.; Cammas, B.; Carré, M.; Chevrel, J.; Compain, F.; Fargeot-Espaliat, A.; et al. Do children and adolescents with type 1 diabetes suffer from a lack of resources in France? Results from a benchmark study in the New Aquitaine region. Arch. Pediatr. 2021, 28, 301–306. [Google Scholar] [CrossRef]
- American Diabetes Association. 13. Children and Adolescents: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. S1), S163–S182. [Google Scholar] [CrossRef] [PubMed]
- Beato-Víbora, P.I.; Quirós-López, C.; Lázaro-Martín, L.; Martín-Frías, M.; Barrio-Castellanos, R.; Gil-Poch, E.; Arroyo-Díez, F.J.; Giménez-Álvarez, M. Impact of Sensor-Augmented Pump Therapy with Predictive Low-Glucose Suspend Function on Glycemic Control and Patient Satisfaction in Adults and Children with Type 1 Diabetes. Diabetes Technol. Ther. 2018, 20, 738–743. [Google Scholar] [CrossRef]
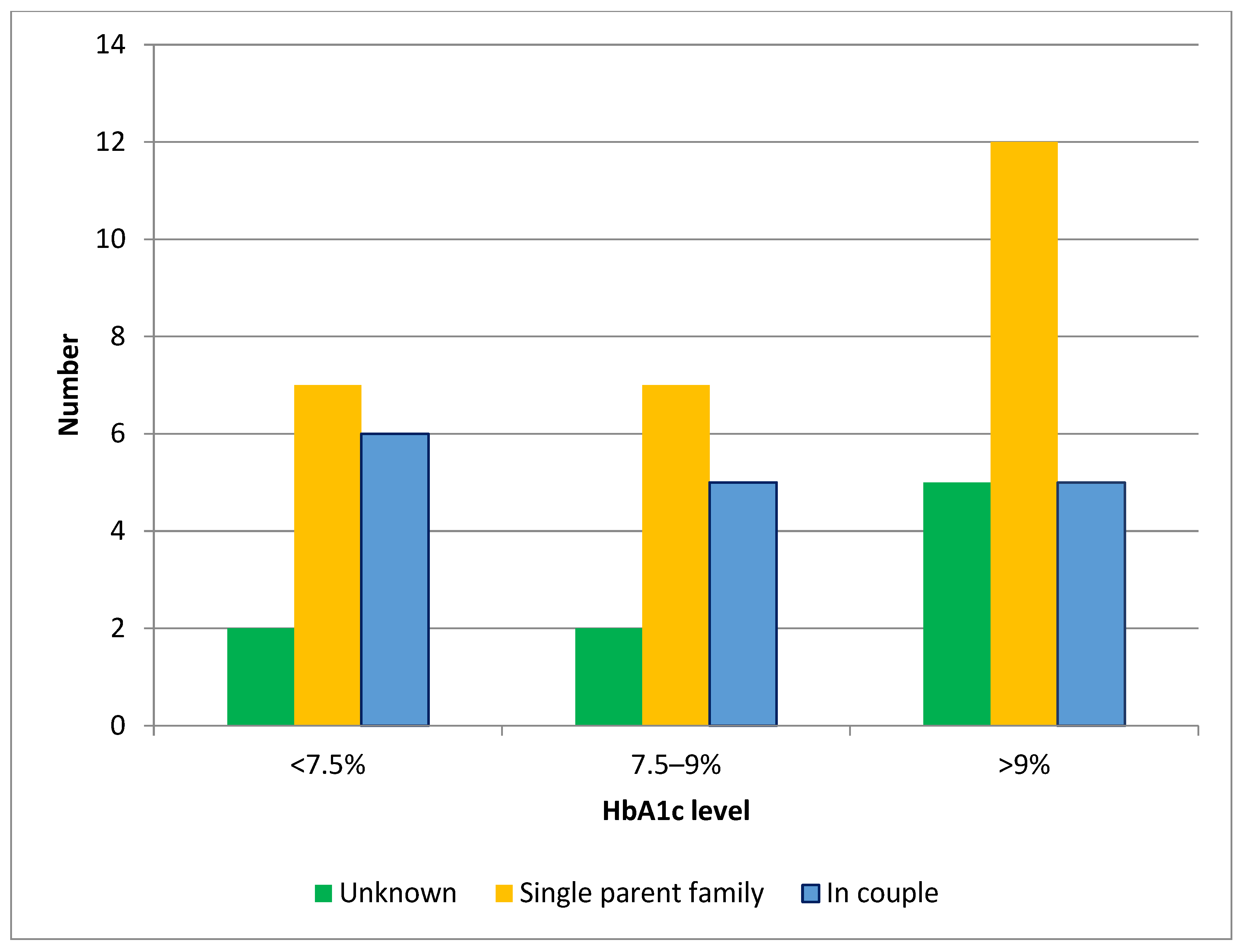
| Variables | HbA1c ≤ 7.5 | HbA1c > 7.5 | OR [95% CI] | p |
|---|---|---|---|---|
| N = 12 | N = 36 | |||
| Age at diagnosis (Median, IQR) | 8.4 [6–11] | 8.72 [6–13] | 0.4 | |
| Gender, Female, n (%) | 7 (58) | 19 (53) | 0.5 | |
| Origin | ||||
| African Caribbean | 9 (75) | 27 (75) | 0.5 | |
| Hispanic | 2 (17) | 6 (17) | 0.5 | |
| Caucasian | 1 (8) | 3 (8) | 0.5 | |
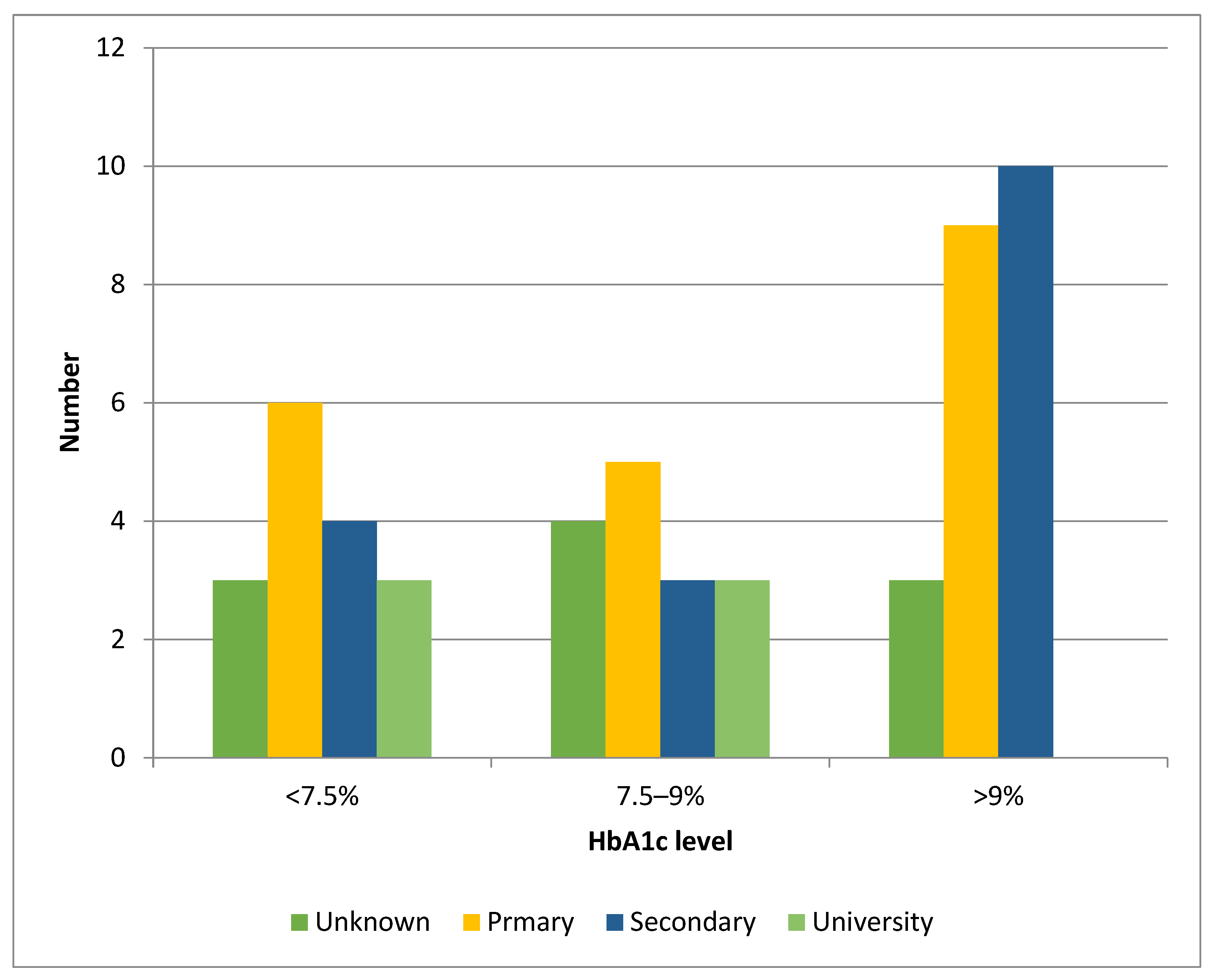
| Parental level of education | ||||
| Primary education | 2 (17) | 10 (28) | 2.1 [1.8–4.3] | <0.001 |
| Secondary | 3 (25) | 16 (44) | 0.1 | |
| University | 7 (58) | 8 (22) | 0.7 [0.5–0.9] | <0.001 |
| Unknown | 0 | 2 (6) | ||
| Parental marital status | ||||
| Single parent family | 5 (42) | 25 (69) | 3.5 [2.5–4.6] | <0.001 |
| In couple | 7 (58) | 11 (31) | 0.6 [0.6–0.85] | <0.001 |
| Poor parental financial status | 4 (33) | 10 (28) | 0.4 | |
| Social security coverage | 12 (100) | 17 (47) | 0.4 |
| Variables | HbA1c ≤ 7.5 | HbA1c > 7.5 | OR [95% CI] | p |
|---|---|---|---|---|
| N = 12 | N = 36 | |||
| Circumstances of diagnosis | ||||
| Polydipsia | 5 (42) | 6 (17) | 0.5 [0.4–0.7] | <0.001 |
| Ketoacidosis | 7 (82) | 21 (58) | 0.1 | |
| Unknown | 0 | 9 (25) | ||
| Follow-up | ||||
| Regular | 12 (100) | 20 (56) | 0.6 [0.4–0.86] | <0.001 |
| Irregular | 0 | 14 (39) | ||
| Unknown | 0 | 2 (5) | ||
| Chronic complications | ||||
| Retinopathy | 0 | 1 (3) | ||
| Diabetic nephropathy | 0 | 1 (3) | ||
| Treatment | ||||
| Insulin regimen | 11 (92) | 33 (92) | 0.1 | |
| Insulin pump | 1 (8) | 3 (8) | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samou-Fantcho, C.B.; Njuieyon, F.; Aigoun, N.; Elenga, N. Parental Low Level of Education and Single-Parent Families as Predictors of Poor Control of Type 1 Diabetes in Children Followed in French Guiana. Int. J. Environ. Res. Public Health 2025, 22, 1051. https://doi.org/10.3390/ijerph22071051
Samou-Fantcho CB, Njuieyon F, Aigoun N, Elenga N. Parental Low Level of Education and Single-Parent Families as Predictors of Poor Control of Type 1 Diabetes in Children Followed in French Guiana. International Journal of Environmental Research and Public Health. 2025; 22(7):1051. https://doi.org/10.3390/ijerph22071051
Chicago/Turabian StyleSamou-Fantcho, Christelle Boyom, Falucar Njuieyon, Nadjia Aigoun, and Narcisse Elenga. 2025. "Parental Low Level of Education and Single-Parent Families as Predictors of Poor Control of Type 1 Diabetes in Children Followed in French Guiana" International Journal of Environmental Research and Public Health 22, no. 7: 1051. https://doi.org/10.3390/ijerph22071051
APA StyleSamou-Fantcho, C. B., Njuieyon, F., Aigoun, N., & Elenga, N. (2025). Parental Low Level of Education and Single-Parent Families as Predictors of Poor Control of Type 1 Diabetes in Children Followed in French Guiana. International Journal of Environmental Research and Public Health, 22(7), 1051. https://doi.org/10.3390/ijerph22071051







