Abstract
Background: There is a significant knowledge gap and limited studies have been carried out to evaluate the effect of type 2 diabetes (T2D) on bone quality and skeletal fragility. Previous reviews have tended to focus primarily on bone mineral density (BMD) as a measure of bone quality. However, BMD does not fully reflect the risk of fracture, cannot distinguish between cortical and trabecular bone, and bone fragility in patients with T2D results not only from alterations in bone mineralisation, but also due to changes in bone microarchitecture. In this regard, assessment tools such as trabecular bone score (TBS) and trabecular microarchitectural parameters could be useful and practical tools for examining bone status in people with T2D. Aim: This review aims to examine the effect of type 2 diabetes on bone quality based on a variety of assessment tools. Method: The PRISMA checklist and PICOS framework were relied on for this systematic review and meta-analysis. Two researchers conducted the searches from database inception until 24/02/25. Databases including Academic Search Premier, APA PsycArticles, APA PsycInfo, CINAHL Plus with Full Text, MEDLINE, and the Psychology & Behavioral Sciences Collection were searched for relevant articles. The reference lists of articles were also searched. The Review Manager 5.4.1 software was used to carry out the meta-analysis. Results: Ten studies were included in the systematic review, while nine studies were included in the meta-analysis. Based on the narrative synthesis and meta-analysis, four distinct themes were established: bone mineral density, TBS and trabecular microarchitectural parameters, fracture risk, and body mass index (BMI). The meta-analysis of the effect of T2D on BMD showed that T2D significantly (p < 0.05) increased lumbar spine, total hip, femoral neck, and narrow neck BMD compared with controls. The mean differences (MDs) for the respective parameters were 0.04 (95% CI, 0.03, 0.05, p < 0.0001); 0.05 (95% CI, 0.02, 0.08, p = 0.002); 0.07 (95% CI, 0.04, 0.10, p < 0.0001); and 0.03 (95% CI, 0.01, 0.05, p = 0.0005). While there was a significant reduction (p < 0.0001) in the patients with T2D with respect to volumetric BMD, involving two studies and 1037 participants, with an MD of −12.36 (95% CI,−18.15, −6.57, p < 0.0001), T2D did not appear to have a significant effect (p > 0.05) on total BMD and area BMD compared to controls. In relation to TBS and trabecular microarchitectural parameters, the effect of T2D was not significant (p > 0.05) compared with controls. Furthermore, T2D did not have a significant effect (p > 0.05) on the incidence of hip fracture and non-spine fracture compared to controls. Following meta-analysis, it was found that the T2D significantly (p < 0.05) increased BMI compared to controls with an MD of 0.94 (95% CI, 0.74, 1.14, p < 0.0001). Conclusions: Type 2 diabetes significantly increased (p < 0.05) lumbar spine, total hip, femoral neck, narrow neck BMD, and body mass index compared with controls. However, type 2 diabetes did not appear to have a significant effect (p > 0.05) on TBS, trabecular microarchitectural parameters, and the incidence of hip and non-spine fracture.
1. Introduction
The prevalence of type 2 diabetes is on the increase globally, partly due to the adoption of a Western lifestyle and other environmental influences [1]. People who develop type 2 diabetes (T2D) are also at risk of developing complications including acute and chronic complications such as bone-related problems [2,3,4]. These diabetic problems have implications for the patients in terms of the cost of treatment, quality of life, and the risk of mortality [5]. What has become evident is that most research studies conducted to examine the acute and chronic conditions in people with T2D have tended to focus on hyperglycaemia, diabetic ketoacidosis, hyperosmolar hyperglycaemic state, neuropathy, nephropathy, and retinopathy [2]. However, T2D has also been shown to affect bone metabolism and increase the risk of bone-related complications, known as diabetic osteopathy, that may result from impaired cortical and trabecular microarchitectural parameters, despite preserved bone mineral density (BMD) [4,6].
Description of Bone Quality
Bone quality involves the geometric and material factors that contribute to fracture resistance [7]. On the other hand, bone strength encompasses both bone quantity and quality [7]. As BMD has limitations in predicting fracture risk, especially in people with T2D, scientific and clinical interests are now focused on other measures of bone quality that could improve fracture risk prediction [7]. The bone geometric parameters comprise the macroscopic geometry of the whole bone and the microscopic architecture of the trabeculae [7]. The material factors involve the material properties of the constituent tissue that are drawn from the composition of the primary microstructural constituents, collagen and minerals [7]. In relation to bone tissue, it can be broadly divided into two types: cortical bone (compact bone or dense bone) and trabecular bone (cancellous or spongy bone) [8]. These distinctions between the two types of bone tissues are based mainly on porosity [8]. The role of diabetes in the pathogenesis of bone fragility may be due to its effect in suppressing bone remodelling, including the impairment of the healing of microfractures in mechanically loaded bones which may predispose individuals with diabetes to fractures [9]. In particular, it has been reported that chronic hyperglycaemia and the related advanced glycation end products have been implicated in the process of increased bone fragility in people with type 2 diabetes [9]. Furthermore, chronic inflammation and oxidative stress could significantly affect osteogenesis and increase bone resorption [9].
Why It Is Important to Do This Review
It would appear that there is a significant knowledge gap and limited studies that have been carried out in order to evaluate the effect of type 2 diabetes on bone quality and skeletal fragility [10,11]. For example, Ma et al. [12] conducted a meta-analysis of observational studies to assess the association between BMD and type 2 diabetes. However, it has been reported that BMD and the World Health Organisation Fracture Risk Assessment Tool (FRAX) underestimate fracture risk in people with diabetes as the metabolic contributors to bone are complex and multifactorial [13,14,15]. Bone cells, structure, vasculature, bone quality, and fracture risk may be influenced by hyperglycaemia, hyperinsulinaemia, diabetes duration, and glucose management [15]. BMD does not adequately reflect the tendency of patients with T2D to develop bone fragility [15,16]. BMD does not fully reflect the risk of fracture, cannot distinguish between cortical and trabecular bone, and the information it provides on bone quality is limited [17]. Furthermore, bone fragility in patients with T2D results not only from alterations in bone mineralisation, but also due to changes in bone microarchitecture [17]. In this regard, the trabecular bone score (TBS) which indicates a reduced number of trabeculae, less connectivity, and impaired bone microarchitecture is one of the most practical tools for examining bone status in people with T2D [17].
Therefore, it will be useful to evaluate bone quality as an indication of the risk of skeletal fragility in people with type 2 diabetes using a variety of bone quality measurement tools.
Aim: The current review aims to examine the effect of type 2 diabetes on bone quality based on a variety of assessment tools.
Research Question: What are the effects of type 2 diabetes on bone quality?
2. Method
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA—Supplementary Table S1) [18] was relied on for this review. The protocol for the systematic review and meta-analysis was registered with PROSPERO (Registration Number: CRD420250654178).
Participants of Interest: People with T2D were the population of interest.
Outcome Measures: These included BMD, TBS, and trabecular microarchitectural parameters, fracture risk assessment, and body mass index (BMI).
Search Strategy
Searches were carried out in EBSCOHost and the databases included Academic Search Premier, APA PsycArticles, APA PsycInfo, CINAHL Plus with Full Text, MEDLINE, and the Psychology & Behavioral Sciences Collection, which were searched for relevant articles. The reference lists of articles were also searched. The search terms and the research question were based on the Population, Intervention, Comparator, Outcomes and Study (PICOS) framework. The searches were carried out from database inception until 24/02/25 and involved two researchers (OO and OO); one carrying out the initial search and the other repeating the process to confirm the result of the searches. The search terms are outlined in Table 1, and these were combined using Boolean operators (AND/OR). The duplicates were removed in EndNote (Analytics, Philadelphia, PA, USA).

Table 1.
Search Strategy.
Collection of Data
The screening of articles for eligibility and inclusion was carried out by two researchers (OO and OO) who worked independently (Figure 1). Differences between the researchers were resolved through discussion.
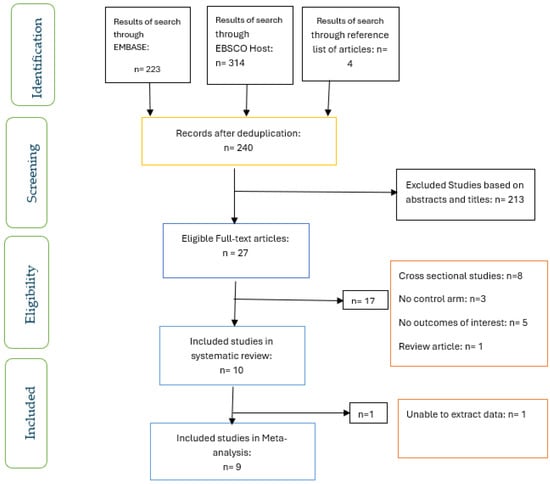
Figure 1.
PRISMA flow chart of studies included.
Study Selection
Inclusion Criteria: Cohort studies and patients with type 2 diabetes were included in the review. In addition, articles written in English were included.
Exclusion Criteria: Patients with type 1 diabetes, gestational diabetes, and articles not written in English were excluded from the review. Animal and in vivo studies were also excluded from the review.
Data Extraction and Management
Two researchers (YO and OO) extracted the qualitative data from the studies included in the review and three researchers (VA, IK, and YO) extracted the quantitative data for meta-analysis and this was cross-checked by a fourth researcher (OO). The characteristics of the studies included such as citation, the research method, the aim of the study, the mean age, the sample size, and outcomes were extracted as part of the qualitative data. The narrative synthesis of the findings of the articles included was also conducted.
Quality Assessment of Studies.
The studies included were evaluated for quality using the Critical Appraisal Skills Programme (CASP) checklist for cohort study [19]. Two researchers (OO and VA) worked independently to assess the risk of bias/quality of the included studies.
Meta-Analysis
The Review Manager 5.4.1 software was used to carry out the meta-analysis [20]. The measure of heterogeneity relied on the use of the I2 statistic [21], and p < 0.10 was the statistical significance of heterogeneity. The sensitivity analysis involved the removal of one study at a time from the meta-analysis. The means ± SEM and confidence intervals reported in some studies were converted to means ± SD for the meta-analysis.
Some of the studies [22,23,24] reported results separately for male and female participants. Therefore, those findings were analysed separately and subgroup analysis was carried out for these studies.
Measures of Effects: The fixed effects model and mean difference were used for the meta-analysis, except in the analysis of trabecular number, when the standardised mean difference was used.
Effect Size
Forest plots were used to present the meta-analysis and p < 0.05 was used to assess the overall effect of the intervention and the level of statistical significance.
3. Results
Figure 1 shows the screening for eligibility of the ten studies that were included in the systematic review, and the nine studies included in the meta-analysis. The characteristics of the studies included are shown in Table 2. Two studies each were conducted in the USA [25,26] and Japan [23,27], while one study each was carried out in the UK [22], the United Arab Emirates [28], the Netherlands [16], Canada [29], China [24], and Denmark [30]. All of the participants had T2D and were compared with those without diabetes.

Table 2.
The description and characteristics of included studies.
The Evaluation of the Quality/Risk of Bias of the Studies Included
All the studies included in this systematic review and meta-analysis effectively addressed most of the questions on the CASP [19] checklist, including whether the study addressed a clearly focused issue, if the outcomes were accurately measured to minimise bias, whether the results could be applied to the local population, and if the results of the study fit with other available evidence. However, although data in the Van Hulten et al. [30] study appeared to have been collected in an acceptable way, we could not find any statement relating to the ethical approval of the study. Furthermore, the Jawhar et al. [28] study did not appear to have clearly identified all important confounding factors. The potential publication bias of the included studies was assessed by examining whether the results reported were due to reporting bias. Based on the evaluation of the included studies, it was clear that there was no publication bias as the authors of this review believed in all the results of the studies.
Information regarding potential confounding variables (duration of diabetes, glycaemic control measures, and other medications) captured in the studies included are presented in Table 3.

Table 3.
Potential Confounding Variables of Included Studies.
Based on the narrative synthesis and meta-analysis, four distinct themes were established: bone mineral density, trabecular bone score and Microarchitectural parameters, fracture risk, and body mass index.
Bone Mineral Density (BMD)
In the recently diagnosed diabetic subjects in Dennison et al.’s [22] study, BMD was identified as higher with stronger relationships in women (p < 0.001) than men (p < 0.05). These findings were weakened by BMI adjustments. In addition, the BMD was notably higher (unadjusted and adjusted for lifestyle and BMI) in men who had a recent diagnosis of diabetes. Between the overall femur and femoral neck BMD, positive links were identified with insulin resistance measures (r = 0.17–0.22) in both men and women.
Oei et al.’s [16] findings concluded that the subjects with diabetes were older and had a higher BMI. They also had increased serum insulin levels, as well as raised creatinine levels, and were taking diuretics more regularly than the non-diabetes group [16]. A higher BMD, thicker cortices, and narrower femoral necks were seen in the inadequately controlled diabetes (ICD) group compared to the adequately controlled diabetes (ACD) and no diabetes (ND) groups, respectively [16]. In T2D, poor glycaemic control was linked to a higher BMD, as were thicker femoral cortices in bones that are narrower [16].
Wang et al. [24] identified that both men and women with diabetes were significantly older (p < 0.001). In addition, both sexes who had a diagnosis of diabetes had a lower volumetric bone mineral density (vBMD) compared to the non-diabetic subjects [24]. However, the vBMD figures were non-significant after they were adjusted according to age. In addition, after adjusting the findings for age in the dual-energy X-ray absorptiometry (DXA) sub-cohort, the results showed a significant rise in areal bone mineral density (aBMD) in men with diabetes [24].
In Heilmeier et al.’s [26] study, a significant decrease in the total BMD was noted in the control group (−3.8%, Tt.BMD, p = 0.001) and cortical area (Ct.Ar) (−3.9% Ct.Ar, p = 0.007) with a significant increase in the cortical pore diameter (control: +8.8% Ct.Po.Dm, p = 0.007) [26]. Both patients with T2D and control participants similarly demonstrated significant losses in cortical BMD (controls: −5.7% Ct.BMD, p < 0.001, T2D subjects: −3.9% Ct.BMD, p= 0.003) [26]. For women with diabetes in Pritchard et al.’s [29] study, lumbar spine was greater (p < 0.05) compared to those without diabetes at both the baseline and follow-up points [29].
In Jawhar et al.’s [28] study, the prevalence of osteoporosis was notably higher in the patients in the diabetes group (p ≤ 0.01) with the Z-score lumbar spine values, L1 and L3, also significantly higher (p ≤ 0.05) than the control group. In the diabetic and control groups, BMD and T-score values were identical. There were notably higher values of BMD, T-score, and Z-score in the left femur total hip in younger (age range of 40–49 years) diabetic patients (p ≤ 0.05) [28]. In the L3 region of the spine, patients with diabetes in the age range of 50–59 years had a very noticeably higher BMD value and Z-score [28].
Iki et al. [27] reported a significantly higher weight and areal BMD (aBMD) observed in men with T2DM in comparison with those without T2DM. Independently of bone turnover and pentosidine levels, hyperglycaemia and increased insulin resistance were seen to be associated with a low TBS [27]. Mitama et al. [23] found spine BMD to be significantly increased (p < 0.05) in men and women with diabetes.
The meta-analysis of the effect of T2D on BMD showed that T2D significantly (p < 0.05) increased lumbar spine, total hip, femoral neck, and narrow neck BMD compared with controls (Figure 2, Figure 3, Figure 4 and Figure 5). With respect to lumbar spine BMD, seven studies involving 8904 participants were analysed and the mean difference (MD) was 0.04 (95% CI, 0.03, 0.05, p < 0.0001) (Figure 2). The subgroup analysis still showed that T2D significantly increased (p < 0.05) lumbar spine BMD in the different subgroups.
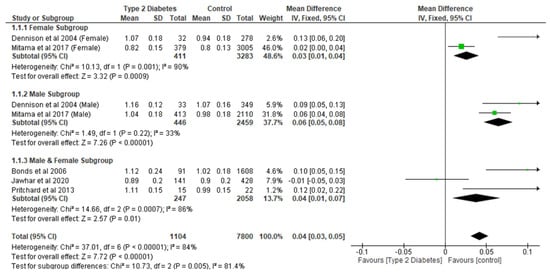
Figure 2.
The effect of type 2 diabetes on lumbar spine bone mineral density (g/cm2) [22,23,25,28,29].
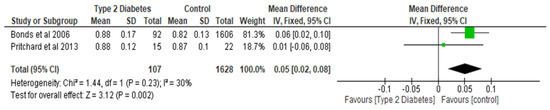
Figure 3.
The effect of type 2 diabetes on total hip bone mineral density (g/cm2) [25,29].
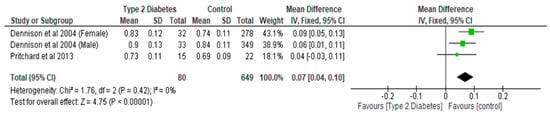
Figure 4.
The effect of type 2 diabetes on femoral neck bone mineral density (g/cm2) [22,29].
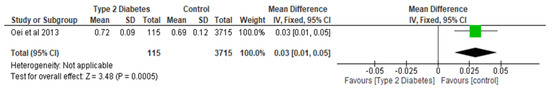
Figure 5.
The effect of type 2 diabetes on narrow neck BMD (g/cm2) [16].
Total hip BMD involved two studies and 1735 participants in the analysis, and the MD was 0.05 (95% CI,0.02, 0.08, p = 0.002) (Figure 3), while the femoral neck BMD had three studies and 729 participants in the analysis, with an MD of 0.07 (95% CI, 0.04, 0.10 p < 0.0001) (Figure 4). The analysis of the narrow neck BMD involved one study and 3830 subjects, with an MD of 0.03 (95% CI, 0.01, 0.05, p = 0.0005) (Figure 5). Following the sensitivity analysis, the results with respect to lumbar spine and femoral neck remained consistent (p < 0.05). However, the result was not significant (p > 0.05) when the Bonds et al. [25] study was removed from the analysis, with respect to the total hip BMD.
While there was a significant reduction (p < 0.0001) in the patients with T2D group with respect to volumetric BMD, involving two studies and 1037 participants, with an MD of −12.36 (95% CI,−18.15, −6.57, p < 0.0001) (Figure 6), T2D did not appear to have a significant effect (p > 0.05) on total BMD and areal BMD compared to controls (Table 4).
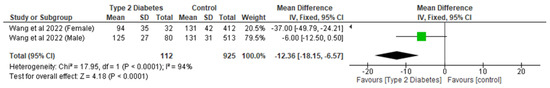
Figure 6.
The effect of type 2 diabetes on volumetric bone mineral density [24].

Table 4.
Results of the meta-analysis of the effect of Type 2 diabetes on bone quality.
Trabecular Microarchitectural Parameters
Heilmeier et al. [26] reported significant changes in most trabecular microarchitectural parameters for T2D- postmenopausal women without a history of fragility fractures and non-diabetic postmenopausal female control groups. For both T2D and controls, there was a significant decrease in trabecular number followed by a significant increase in trabecular thickness and trabecular spacing (controls: +8.8% trabecular thickness, p = 0.024, + 7.5% trabecular separation, p = 0.017; DM: +8.4% trabecular thickness, p = 0.039, 6.0% trabecular separation, p = 0.032) [26]. With regard to the ultradistal tibia, annualised percentage changes in the density parameters (total BMD, trabecular BMD, and cortical BMD) and in the cortical bone parameters, including cortical porosity, cortical pore volume, and cortical tissue mineral density, were similar between all three groups (p > 0.05) [26].
There was a higher percentage increase in the number of trabecular bone holes after adjusting for ethnicity in comparison to the controls [29]. However, after adjustment for multiple comparisons there was no significance (p = 0.090). Between the groups, there were no differences in the change in other bone microarchitecture variables [29]. Iki et al.’s [27] study did not identify measurable differences in TBS and the frequency of past osteoporotic fractures between the T2D group and control group.
In relation to trabecular microarchitectural parameters, the meta-analysis of the effect of T2D was not significant (p > 0.05) with respect to trabecular thickness, trabecular separation, trabecular number, and trabecular BMD (Figure 7, Figure 8 and Figure 9; Table 4). After the sensitivity analysis, the results with respect to trabecular thickness, trabecular separation, and trabecular number remained consistent (p > 0.05).
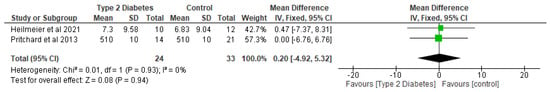
Figure 7.
The effect of type 2 diabetes on trabecular thickness (µm) [26,29].
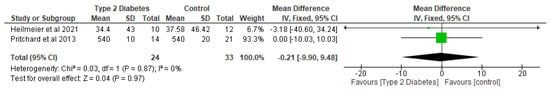
Figure 8.
The effect of type 2 diabetes on trabecular separation (µm) [26,29].
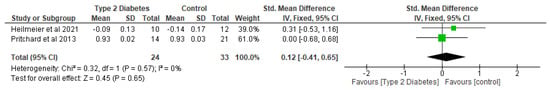
Figure 9.
The effect of type 2 diabetes on trabecular number (standardised mean difference) [26,29].
Furthermore, the effect of T2D on hole size, the number of holes, and bone volume fraction were also not significant (p > 0.05) compared with the control group (Table 4).
Fracture Risk
Bonds et al. [25] identified a significantly increased risk of any fracture in women with diabetes compared with non-diabetic women (p < 0.0001) after follow-up for 7 years. Women with diabetes had a higher fracture rate, with fracture by location identified at multiple sites, such as the hip, pelvis, upper leg; lower leg, ankle, knee; foot; upper arm shoulder, elbow; and spine and tailbone (p < 0.0001) [25]. The results also showed an equal rate of fractures to the lower arm, wrist, and hand in both groups [25]. However, among black women with diabetes, a higher risk of fracture at multiple sites such as the hip, pelvis, upper leg; foot; spine and tailbone was also reported (RR 1.33, 95% CI 1.00 –1.75) in comparison to non-Hispanic white (NHW) diabetic women (RR 1.18, 95% CI 1.08–1.29) [25].
Among the Japanese cohort, Mitama et al. [23] found a significantly higher risk of fracture among T2D men and women with high C-reactive protein (CRP) compared with the non-diabetes group with a low CRP (in men, hazard ratio [HR] 1.47, 95% CI: 1.02–1.98; in women HR 1.41, 95% CI: 1.04–1.92). Likewise, after age, BMD, and previous fractures adjustments, CRP was associated with a higher fracture risk in both sexes (in men, HR 1.04, 95% CI: 1.003–1.06; in women HR 1.07, 95% CI: 1.03–1.13) [23]. Oei et al. [16] also reported an increased fracture risk in participants with ICD compared to non-diabetic individuals.
In women, crude incidence rates (IRs) for hip fractures, non-spine fractures, and major osteoporotic fracture (MOF) were not significantly different (age > 30) in patients with T2D that are not using insulin (IR hip: 8.7; 95% CI 6.8–11.0) or using insulin (IR hip: 11.1; 95% CI 6.8–18.0) compared with women without T2D (IR hip: 7.0; 95% CI 6.6–7.4) [30]. Also, in men with T2D not taking insulin, IRs were not significantly different (IR hip: 4.6; 95% CI 2.6–8.0), compared to those using insulin (IR hip: 11.5; 95% CI 6.2–21.4) and men without T2D (IR hip: 6.3; 95% CI 5.5–7.1) for all three fracture types [30].
The meta-analysis of the risk of fracture showed that type 2 diabetes did not have a significant effect (p > 0.05) on the incidence of hip fracture and non-spine fracture compared to controls (Figure 10, Table 4). With respect to the incidence of hip fracture, two studies involving 41,121 participants were analysed and the MD was 0.46 (95% CI, −1.25, 2.16, p = 0.60) (Figure 10). After the sensitivity analysis, the results continued to show that the difference between the two groups was not significant (p > 0.05).
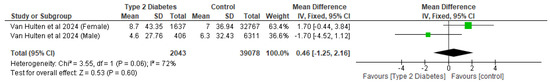
Figure 10.
The effect of type 2 diabetes on the incidence of hip fracture (Incidence Rate) (1000 PYs—Person Years) [30].
Body Mass Index
Wang et al. [24] noted that both men and women with diabetes were much older (p < 0.001), and their BMI was higher than that of women who did not have diabetes. Similarly, Oei et al. [16] showed that those with diabetes were older and had a higher BMI and higher serum insulin and creatinine levels. Oei et al. [16] also noted that these subjects used diuretics more regularly than the non-diabetes group.
Following the meta-analysis, it was found that the T2D group had a significantly (p < 0.05) increased BMI compared to controls. The analysis involved eight studies and 11,504 participants, with an MD of 0.94 (95% CI,0.74, 1.14, p < 0.0001) (Figure 11). Following the sensitivity analysis, the effect of T2D on BMI compared with controls remained significantly different (p < 0.05). The subgroup analysis showed that T2D significantly increased (p < 0.05) BMI in the different subgroups.
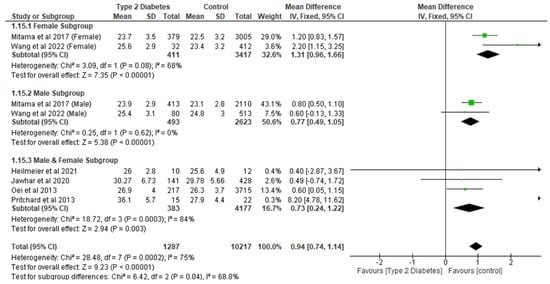
Figure 11.
The effect of type 2 diabetes on body mass index (kg/m2) [16,23,24,26,28,29].
4. Discussion
The results of this systematic review and meta-analysis show that T2D significantly increases (p < 0.05) lumbar spine, total hip, femoral neck, narrow neck BMD, and BMI compared with controls. However, T2D did not appear to have a significant effect (p > 0.05) on total BMD, areal BMD, TBS, trabecular microarchitectural parameters, and the incidence of hip and non-spine fracture compared to controls.
The findings of this review with respect to most of the parameters are in line with the results of previous primary research studies and reviews. For example, the meta-analysis of observational studies conducted by Ma et al. [12] found that patients with diabetes had a significantly higher (p < 0.05) BMD at the femoral neck, hip, and spine compared with those without diabetes. Furthermore, Sosa et al. [31] noted that, while studies that are small-scale may have reported either unchanged or decreased BMD in people with diabetes, all large epidemiological studies have now unanimously identified an increase in bone mass [31]. The clinical relevance of increased BMD in diabetes is that a high BMD in people with inadequately controlled diabetes may be a reflection of skeletal complications of the disease. Therefore, the use of BMD alone may not be adequate to predict or diagnose the risk of fracture in these people [16].
Sihota et al. [32] did not find significant differences in areal BMD between the groups, which was also confirmed in the result of the current review. However, in contrast to our results, Sosa et al. [31] did not find significant differences between patients with diabetes and controls with respect to femoral neck BMD, and suggested the differences reported in other studies may be due to differences in the weight of the patients between those with diabetes and controls, a well-known determinant of femoral bone density.
The mechanism of how diabetes influences bone quality including bone mineral density and trabecular microarchitectural parameters remains unclear and it is an evolving area of research [33]. However, several pathways, such as obesity, hyperglycaemia, hyperinsulinemia, growth factor deficiency, and neuropathy, have been proposed as causes of abnormal bone physiology in patients with diabetes [12,31,33]. The mechanism through which obesity increases BMD may be through the release of a broad range of adipokines from the adipose tissue, and these adipokines have been implicated either directly or indirectly in the regulation of bone remodelling [12]. For example, leptin has been found to be higher in men with diabetes compared with control, and that leptin may induce bone growth by stimulating osteoblast proliferation and differentiation in vitro, and inhibiting osteoclastogenesis [12].
With respect to the role of hyperinsulinaemia in influencing increased BMD, it has been shown that insulin resistance and excess insulin are common features in people with type 2 diabetes, and that insulin has an anabolic effect on bone due to its structural homology to Insulin-like Growth Factor-1 (IGF-1) by interacting with the IGF-1 receptor which is present on osteoblasts [12]. Based on this, it has been suggested that hyperinsulinaemia may have a mitogenic effect on osteoblasts and their differentiation by stimulating the IGF-1 signalling pathway [12].
According to La Fontaine et al. [33], a possible mechanism by which neuropathy affects bone turnover may be via the neuropeptide calcitonin gene-related peptide, that has been reported to be downregulated in patients with neuropathy. In women with T2D, increased androgen levels have been implicated in the alteration of bone quality [31].
In the present review, BMI was significantly (p < 0.05) increased in patients with T2D compared with controls. People with a high BMI are often associated with higher body fat content, which could be converted into fat-related hormones [34]. There is evidence body fatness may have an effect on the accuracy of dual-energy X-ray absorptiometry (DXA)-based BMD measurements in obese patients with diabetes [12].
Chronic hyperglycaemia has also been implicated as one of the pathways through which diabetes may impact bone quality [34]. In patients with T2D, prolonged disease duration may lead to a gradual decline in insulin production and function, and long-term insulin deficiency can lead to chronic hyperglycaemia and decreased bone turnover, affecting osteoclast activity and promoting bone resorption [34].
Chronic hyperglycaemia has also been reported to affect osteoblast function, leading to decreased bone formation and mineralization [21,29]. In addition, advanced glycation end-products (AGEs) and bone turnover are intermediate factors which play a considerable role in the association between hyperglycaemia and impaired bone microarchitecture [27].
The study by Sihota et al. [32] provides evidence of the negative effects of hyperglycaemia on trabecular bone quality that could lead to lower energy absorption and toughness and may explain the increased bone fragility in patients with T2D. Therefore, bone quality and increased bone fragility in patients with T2D cannot be explained by BMD alone [32]. In particular, traditional techniques for measuring bone fragility, such as DXA, do not perform well in patients with T2D and the Fracture Risk Assessment Tool (FRAX) usually underestimates fracture risk in this population [5]. However, new techniques for the assessment of trabecular microarchitecture in patients with T2D, such as TBS and high-resolution peripheral quantitative computed tomography (HR-pQCT), are emerging, although (HR-pQCT) involves significant costs and exposure to radiation [5]. Therefore, conflicting data may exist in relation to trabecular bone quality in patients with T2D depending on the applied assessment method [35]. For example, studies using standard HR-pQCT measures identified that trabecular microarchitecture was preserved in patients with T2D, while studies relying on other tools suggested that it may be impaired [35].
In line with our review, an earlier study by Patsch et al. [36] also found no significant differences in the microarchitectural parameters of the trabecular bone between patients with T2D for ≥10 years and controls [27]. Therefore, trabecular plate qualities, which suggest a normal or improved microstructure, may not explain the increased fracture risk in patients with inadequately controlled T2D [16,35]. Furthermore, the difference in the findings of this review with respect to TBS compared with some previous studies may be due to the fact participants in this review were community-dwelling volunteers and may have included fewer patients with severe illnesses, such as uncontrolled T2D [27].
In the present review, the incidence of hip and non-spine fracture did not differ significantly between patients with T2D and controls. In an earlier study by Wallander et al. [37], it was observed that only for individuals with T2D using insulin was fracture risk significantly increased.
Limitations
The small numbers of articles included in the systematic review and meta-analysis are limitations of this review. In addition, the number of articles included in the meta-analysis on some of the outcomes of interest was limited, and this would suggest that those results should be interpreted with caution. The high heterogeneity in some of the analyses conducted is also a limitation of this review, although sub-group analysis was conducted in the meta-analysis with large studies. The studies included in this review were granted ethical approval by the various ethics review boards and committees, except for Jawhar et al. [28] and Van Hulten et al. [30], both retrospective cohort studies that did not include any statements about the ethical approval of their studies.
5. Conclusions
The findings of this review revealed that T2D significantly increased (p < 0.05) lumbar spine, total hip, femoral neck, narrow neck BMD, and BMI compared with controls. However, T2D did not appear to have a significant effect (p > 0.05) on total BMD, areal BMD, TBS, trabecular microarchitectural parameters, and the incidence of hip and non-spine fractures compared to controls. We recommend that future research and clinical practice involving the assessment of bone quality in patients with T2D should not be limited only to BMD, but should include a variety of assessment tools, such as TBS and trabecular microarchitectural parameters.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph22060910/s1, Table S1: PRISMA 2020 Checklist.
Author Contributions
Conceptualization, O.O. (Omorogieva Ojo); methodology, O.O. (Omorogieva Ojo), Y.O., V.A., I.K. and O.O. (Osarhumwese Ojo); validation, O.O. (Omorogieva Ojo); formal analysis, O.O. (Omorogieva Ojo); writing—original draft preparation, O.O. (Omorogieva Ojo), Y.O., V.A., I.K., O.O. (Osarhumwese Ojo) and J.B.; writing—review and editing, O.O. (Omorogieva Ojo), Y.O., V.A., I.K., O.O. (Osarhumwese Ojo) and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ojo, O.; Kalocsányiová, E.; McCrone, P.; Elliott, H.; Milligan, W.; Gkaintatzi, E. Non-Pharmacological Interventions for Type 2 Diabetes in People Living with Severe Mental Illness: Results of a Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2024, 21, 423. [Google Scholar] [CrossRef] [PubMed]
- Cirovic, A.; Vujacic, M.; Petrovic, B.; Cirovic, A.; Zivkovic, V.; Nikolic, S.; Djonic, D.; Bascarevic, Z.; Djuric, M.; Milovanovic, P. Vascular Complications in Individuals with Type 2 Diabetes Mellitus Additionally Increase the Risk of Femoral Neck Fractures Due to Deteriorated Trabecular Microarchitecture. Calcif. Tissue Int. 2022, 110, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.; Boateng, J.; Pacella, R.; Hanrahan, A.; Essex, R.; Dibley, L. Factors Influencing the Care and Management of Diabetic Foot Ulcers: A Scoping Review. Endocr. Pract. 2024, 31, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.; Liu, X.; Chen, B.; Lei, L. Effect of type 2 diabetes on biochemical markers of bone metabolism: A meta-analysis. Front. Physiol. 2024, 15, 1330171. [Google Scholar] [CrossRef]
- Martínez-Montoro, J.I.; García-Fontana, B.; García-Fontana, C.; Muñoz-Torres, M. Evaluation of Quality and Bone Microstructure Alterations in Patients with Type 2 Diabetes: A Narrative Review. J. Clin. Med. 2022, 11, 2206. [Google Scholar] [CrossRef]
- Sheu, A.; Blank, R.D.; Tran, T.; Bliuc, D.; Greenfield, J.R.; White, C.P.; Center, J.R. Associations of Type 2 Diabetes, Body Composition, and Insulin Resistance with Bone Parameters: The Dubbo Osteoporosis Epidemiology Study. JBMR Plus 2023, 7, e10780. [Google Scholar] [CrossRef]
- Donnelly, E. Methods for assessing bone quality: A review. Clin. Orthop. Relat. Res. 2011, 469, 2128–2138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Napoli, N.; Incalzi, R.A.; De Gennaro, G.; Marcocci, C.; Marfella, R.; Papalia, R.; Purrello, F.; Ruggiero, C.; Tarantino, U.; Tramontana, F.; et al. Bone fragility in patients with diabetes mellitus: A consensus statement from the working group of the Italian Diabetes Society (SID), Italian Society of Endocrinology (SIE), Italian Society of Gerontology and Geriatrics (SIGG), Italian Society of Orthopaedics and Traumatology (SIOT). Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1375–1390. [Google Scholar] [CrossRef]
- Weber, D.R.; Long, F.; Zemel, B.S.; Kindler, J.M. Glycemic Control and Bone in Diabetes. Curr. Osteoporos. Rep. 2022, 20, 379–388. [Google Scholar] [CrossRef]
- Misof, B.M.; Blouin, S.; Andrade, V.F.C.; Roschger, P.; Borba, V.Z.C.; Hartmann, M.A.; Zwerina, J.; Recker, R.R.; Moreira, C.A. No evidence of mineralization abnormalities in iliac bone of premenopausal women with type 2 diabetes mellitus. J. Musculoskelet. Neuronal Interact. 2022, 22, 305–315. [Google Scholar] [PubMed]
- Ma, L.; Oei, L.; Jiang, L.; Estrada, K.; Chen, H.; Wang, Z.; Yu, Q.; Zillikens, M.C.; Gao, X.; Rivadeneira, F. Association between bone mineral density and type 2 diabetes mellitus: A meta-analysis of observational studies. Eur. J. Epidemiol. 2012, 27, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.V. Epidemiology of fractures in type 2 diabetes. Bone 2016, 82, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, J.; Xiao, L.; Liu, D.; Yan, W.; Hu, T.; Li, K.; Hua, X.; Zeng, X. Comparison of FRAX in postmenopausal Asian women with and without type 2 diabetes mellitus: A retrospective observational study. J. Int. Med. Res. 2020, 48, 300060519879591. [Google Scholar] [CrossRef] [PubMed]
- Sheu, A.; White, C.P.; Center, J.R. Bone metabolism in diabetes: A clinician’s guide to understanding the bone-glucose interplay. Diabetologia 2024, 67, 1493–1506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oei, L.; Zillikens, M.C.; Dehghan, A.; Buitendijk, G.H.S.; Castaño-Betancourt, M.C.; Estrada, K.; Stolk, L.; Oei, E.H.G.; van Meurs, J.B.J.; Janssen, J.A.M.J.L.; et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: The Rotterdam Study. Diabetes Care 2013, 36, 1619–1628. [Google Scholar] [CrossRef]
- Trandafir, A.-I.; Sima, O.-C.; Gheorghe, A.-M.; Ciuche, A.; Cucu, A.-P.; Nistor, C.; Carsote, M. Trabecular Bone Score (TBS) in Individuals with Type 2 Diabetes Mellitus: An Updated Review. J. Clin. Med. 2023, 12, 7399. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme. CASP Cohort Study Checklist. 2018. Available online: https://casp-uk.net/casp-tools-checklists/cohort-study-checklist/ (accessed on 1 March 2025).
- The Nordic Cochrane Centre. Review Manager (RevMan) [Computer Program]; Version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2014. [Google Scholar]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Dennison, E.M.; Syddall, H.E.; Aihie Sayer, A.; Craighead, S.; Phillips, D.I.W.; Cooper, C. Type 2 diabetes mellitus is associated with increased axial bone density in men and women from the Hertfordshire Cohort Study: Evidence for an indirect effect of insulin resistance? Diabetologia 2004, 47, 1963–1968. [Google Scholar] [CrossRef]
- Mitama, Y.; Fujiwara, S.; Yoneda, M.; Kira, S.; Kohno, N. Association of type 2 diabetes and an inflammatory marker with incident bone fracture among a Japanese cohort. J. Diabetes Investig. 2017, 8, 709–715. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Zhao, K.; Zha, X.; Ran, L.; Su, H.; Yang, Y.; Shuang, Q.; Liu, Y.; Xu, L.; Blake, G.M.; et al. Hyperglycemia Is Not Associated With Higher Volumetric BMD in a Chinese Health Check-up Cohort. Front. Endocrinol. 2022, 12, 794066. [Google Scholar] [CrossRef] [PubMed]
- Bonds, D.E.; Larson, J.C.; Schwartz, A.V.; Strotmeyer, E.S.; Robbins, J.; Rodriguez, B.L.; Johnson, K.C.; Margolis, K.L. Risk of fracture in women with type 2 diabetes: The Women’s Health Initiative Observational Study. J. Clin. Endocrinol. Metab. 2006, 91, 3404–3410. [Google Scholar] [CrossRef] [PubMed]
- Heilmeier, U.; Joseph, G.B.; Pasco, C.; Dinh, N.; Torabi, S.; Darakananda, K.; Youm, J.; Carballido-Gamio, J.; Burghardt, A.J.; Link, T.M.; et al. Longitudinal Evolution of Bone Microarchitecture and Bone Strength in Type 2 Diabetic Postmenopausal Women With and Without History of Fragility Fractures-A 5-Year Follow-Up Study Using High Resolution Peripheral Quantitative Computed Tomography. Front. Endocrinol. 2021, 12, 599316. [Google Scholar] [CrossRef]
- Iki, M.; Fujita, Y.; Kouda, K.; Yura, A.; Tachiki, T.; Tamaki, J.; Winzenrieth, R.; Sato, Y.; Moon, J.-S.; Okamoto, N.; et al. Hyperglycemia is associated with increased bone mineral density and decreased trabecular bone score in elderly Japanese men: The Fujiwara-kyo osteoporosis risk in men (FORMEN) study. Bone 2017, 105, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Jawhar, D.S.; Hassan, N.A.; Shamssain, M.H. Dual-energy x-ray absorptiometry scan (DXA) findings in diabetic and non-diabetic female: A retrospective cohort study. Med. J. Malays. 2020, 75, 47–51. [Google Scholar]
- Pritchard, J.M.; Giangregorio, L.M.; Atkinson, S.A.; Beattie, K.A.; Inglis, D.; Ioannidis, G.; Gerstein, H.; Punthakee, Z.; Adachi, J.D.; Papaioannou, A. Changes in trabecular bone microarchitecture in postmenopausal women with and without type 2 diabetes: A two year longitudinal study. BMC Musculoskelet. Disord. 2013, 14, 114. [Google Scholar] [CrossRef]
- Van Hulten, V.; Driessen, J.H.M.; Andersen, S.; Kvist, A.; Viggers, R.; Bliuc, D.; Center, J.R.; Brouwers, M.C.J.G.; Vestergaard, P.; van den Bergh, J.P. Fracture risk revisited: Bone mineral density T-score and fracture risk in type 2 diabetes. Diabetes Obes. Metab. 2024, 26, 5325–5335. [Google Scholar] [CrossRef]
- Sosa, M.; Saavedra, P.; Jódar, E.; Lozano-Tonkin, C.; Quesada, J.M.; Torrijos, A.; Pérez-Cano, R.; Nogués, X.; Díaz-Curiel, M.; Moro, M.J.; et al. Bone mineral density and risk of fractures in aging, obese post-menopausal women with type 2 diabetes. The GIUMO Study. Aging Clin. Exp. Res. 2009, 21, 27–32. [Google Scholar] [CrossRef]
- Sihota, P.; Yadav, R.N.; Dhaliwal, R.; Bose, J.C.; Dhiman, V.; Neradi, D.; Karn, S.; Sharma, S.; Aggarwal, S.; Goni, V.G.; et al. Investigation of Mechanical, Material, and Compositional Determinants of Human Trabecular Bone Quality in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, e2271–e2289. [Google Scholar] [CrossRef]
- La Fontaine, J.; Shibuya, N.; Sampson, H.W.; Valderrama, P. Trabecular quality and cellular characteristics of normal, diabetic, and charcot bone. J. Foot Ankle Surg. 2011, 50, 648–653. [Google Scholar] [CrossRef]
- Li, T.; Hu, L.; Yin, X.-L.; Zou, Y.; Fu, H.-Y.; Li, H.-L. Prevalence and Risk Factors of Osteoporosis in Patients with Type 2 Diabetes Mellitus in Nanchang (China): A Retrospective Cohort Study. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 3039–3048. [Google Scholar] [CrossRef] [PubMed]
- Starr, J.F.; Bandeira, L.C.; Agarwal, S.; Shah, A.M.; Nishiyama, K.K.; Hu, Y.; McMahon, D.J.; Guo, X.E.; Silverberg, S.J.; Rubin, M.R. Robust Trabecular Microstructure in Type 2 Diabetes Revealed by Individual Trabecula Segmentation Analysis of HR-pQCT Images. J. Bone Miner. Res. 2018, 33, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Patsch, J.M.; Burghardt, A.J.; Yap, S.P.; Baum, T.; Schwartz, A.V.; Joseph, G.B.; Link, T.M. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J. Bone Miner. Res. 2013, 28, 313–324. [Google Scholar] [CrossRef]
- Wallander, M.; Axelsson, K.F.; Nilsson, A.G.; Lundh, D.; Lorentzon, M. Type 2 diabetes and risk of hip fractures and non-skeletal fall injuries in the elderly: A study from the fractures and fall injuries in the elderly cohort (FRAILCO). J. Bone Miner. Res. 2016, 32, 449–460. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).