Dirty Utility Rooms of Hospitals in Saudi Arabia: A Multi-Regional Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Area/Setting
2.3. Study Subjects
2.4. Sample Size and Sampling Technique
2.5. Data Collection Methods, Tools, and Measurement Instructions
2.6. Data Management and Analysis Plan
2.7. Data Sharing Management
2.8. Ethical Considerations
3. Results
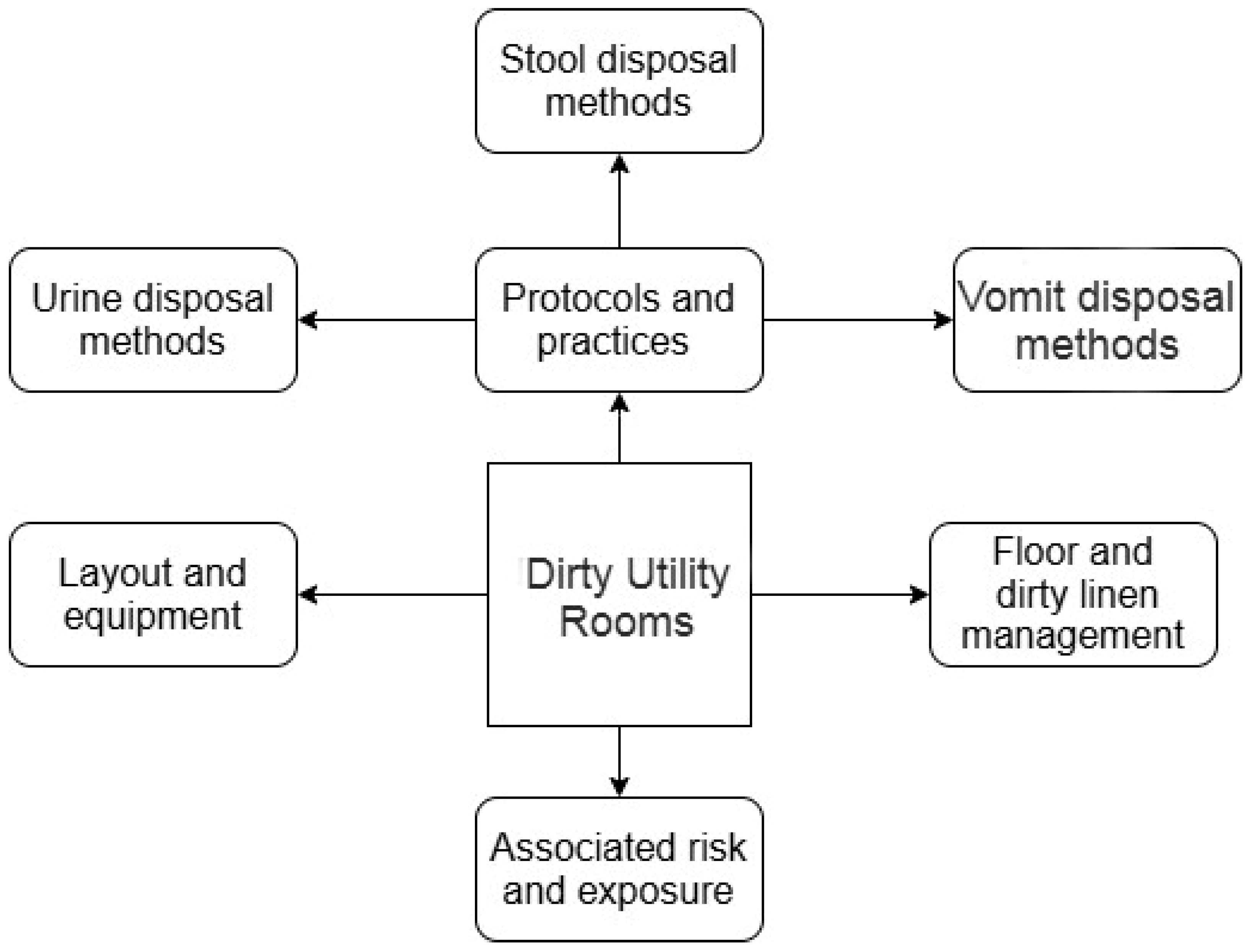
3.1. Main Themes
3.2. DUR Layout and Equipment
3.3. Protocols and Practices
3.3.1. Urine Disposal Methods
3.3.2. Stool Disposal Methods
3.3.3. Vomit Disposal Methods
3.4. Floor and Dirty Linen Management
3.5. Associated Risk and Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joseph, J. Facility Design and Process Utilities; Biopharmaceutical Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 933–986. [Google Scholar]
- Sehulster, L.; Chinn, R.Y.; Arduino, M.J.; Carpenter, J.; Donlan, R.; Ashford, D.; Besser, R.; Fields, B.; McNeil, M.M.; Whitney, C.; et al. Guidelines for environmental infection control in health-care facilities. In Morbidity and Mortality Weekly Report Recommendations and Reports RR; Centers for Disease Control and Preventio: Atlanta, GA, USA, 2003; Volume 52. [Google Scholar]
- King, M. Soiled Instrument Handling in Outpatient Clinics—Our process to compliance. Am. J. Infect. Control. 2019, 47, S20. [Google Scholar] [CrossRef]
- Dheda, K.R.; Centner, C.M.; Wilson, L.; Pooran, A.; Grimwood, S.; Ghebrekristos, Y.T.; Oelofse, S.; A Joubert, I.; Esmail, A.; Tomasicchio, M. Intensive Care Unit Sluice Room Sinks as Reservoirs and Sources of Potential Transmission of Carbapenem-Resistant Bacteria in a South African Tertiary Care Hospital. Infect. Drug Resist. 2023, 16, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Bates, J. A sluice by any other name. Nurs. Stand. Through 2013 2002, 16, 23. [Google Scholar] [CrossRef]
- Kim, E.S.; Yang, N.W. A Study on the Systematic Construction of the Utility Space in General Hospital. J. Korea Inst. Healthc. Archit. 2017, 23, 77–84. [Google Scholar]
- Gammon, J.; Morgan-Samuel, H.; Gould, D. A review of the evidence for suboptimal compliance of healthcare practitioners to standard/universal infection control precautions. J. Clin. Nurs. 2008, 17, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Theodore, D. “Dirty Dirty Dirt”: Automating Segregation in the Friesen Concept Hospital; Tracing Hospital Boundaries: Brill, The Netherlands, 2020; pp. 171–190. [Google Scholar]
- Hamer, G. Solid waste treatment and disposal: Effects on public health and environmental safety. Biotechnol. Adv. 2003, 22, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Okweso, J.A. Report on the Review of the Kenya National Guidelines for Safe Management of Health Care Waste, Injection Safety and Safe Disposal of Medical Waste National Communication Strategy and Health Care Waste Management Standard Operating Procedures; Ministry of Environment and Natural Resources: Nairobi, Kenya, 2016; pp. 1–181. [Google Scholar]
- WHO. Infection, Prevention and Control Excellence in the Kingdom of Saudi Arabia: Reducing Central Line-Associated Bloodstream Infections. 2024. Available online: https://www.who.int/news-room/feature-stories/detail/infection--prevention-and-control-excellence-in-the-kingdom-of-saudi-arabia--reducing-central-line-associated-bloodstream-infections (accessed on 18 March 2025).
- Alshamrani, M.M.; El-Saed, A.; Alsaedi, A.; El Gammal, A.; Al Nasser, W.; Nazeer, S.; Balkhy, H.H. Burden of healthcare-associated infections at six tertiary-care hospitals in Saudi Arabia: A point prevalence survey. Infect. Control. Hosp. Epidemiol. 2019, 40, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Alslamah, T.; Abalkhail, A. The National Strategies for and Challenges in Infection Prevention and Control of the Healthcare System in the Kingdom of Saudi Arabia (Review Study). Vaccines 2022, 10, 1302. [Google Scholar] [CrossRef] [PubMed]
- Alshamrani, M.M.; El-Saed, A.; Farahat, F.M. Challenges of infection control capacity in the Middle Eastern countries; time to be actively involved. J. Infect. Public Health 2022, 15, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.M.; Andersen, B.M. Cleaning of Rooms in Wards. In Prevention and Control of Infections in Hospitals: Practice and Theory; Springer: Berlin/Heidelberg, Germany, 2019; pp. 883–895. [Google Scholar]
- Braun, V.; Clarke, V. Thematic analysis. In Encyclopedia of Quality of Life and Well-Being Research; Springer: Berlin/Heidelberg, Germany, 2024; pp. 7187–7193. [Google Scholar]
- Blog OHaS. SLUICE ROOM. Available online: https://www.hseblog.com/sluice-room (accessed on 18 March 2025).
- IHFG. International Health Facility Guidelines 2019. Available online: https://www.healthfacilityguidelines.com/ (accessed on 7 February 2025).
- Haley, R.W. CDC guidelines on infection control. Infect. Control Hosp. Epidemiol. 1981, 2, 117–124. [Google Scholar] [CrossRef] [PubMed]
- NHS. Clinical and Clinical Support Space. 2023. Available online: https://www.england.nhs.uk/estates/health-building-notes/ (accessed on 18 March 2025).
- Ely, G. ISO 14698 or EN 17141: Is There a Choice for Cleanroom Compliance? Biomed. Instrum. Technol. 2023, 57 (Suppl. S1), 15–17. [Google Scholar] [CrossRef] [PubMed]
- WHO. Laboratory Biosafety Manual: World Health Organization; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Bartley, J.M. APIC state-of-the-art report: The role of infection control during construction in health care facilities. Am. J. Infect. Control 2000, 28, 156–169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Transplumb. MediWash: Hospitals, Health & Aged Care Facilities. Available online: https://transplumb.com/products/mediwash (accessed on 18 March 2025).
- Shepard, J.; Frederick, J.; Wong, F.; Madison, S.; Tompkins, L.; Hadhazy, E. Could the prevention of health care–associated infections increase hospital cost? The financial impact of health care–associated infections from a hospital management perspective. Am. J. Infect. Control 2020, 48, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Guidelines AHF. Dirty Utility 2021. Available online: https://healthfacilityguidelines.com.au/component/dirty-utility-12m2-2 (accessed on 10 February 2025).
- Health Service Executive. National Cleaning Standards Manual. 2005. Available online: https://www.hse.ie/eng/services/publications/hospitals/hse-national-cleaning-standards-manual.html (accessed on 10 February 2025).
- Management TIoHEaE. Dirt Utilite Rooms. 2024. Available online: https://www.iheem.org.uk/ (accessed on 18 March 2025).
- Hospodsky, D.; Qian, J.; Nazaroff, W.W.; Yamamoto, N.; Bibby, K.; Rismani-Yazdi, H.; Peccia, J. Human occupancy as a source of indoor airborne bacteria. PLoS ONE 2012, 7, e34867. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Pittet, D. Guideline for hand hygiene in health-care settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect. Control Hosp. Epidemiol. 2002, 23 (Suppl. S12), S3–S40. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Gefen, A.; Ousey, K. Update to device-related pressure ulcers: SECURE prevention. COVID-19, face masks and skin damage. J. Wound Care 2020, 29, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Glampedakis, E.; Snoussi, M.-C.; Sobgoui, B.; Battistella, F.; Iglesias, P.C.; Riccio, C.; Qalla-Widmer, L.; Cassini, A.; Tessemo, M.I.N. Bodily waste management and related hygiene practices in nursing homes of Vaud: Findings from a multicentre cross-sectional survey as a basis for targeted interventions. Antimicrob Resist. Infect Control 2025, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.W. Economic burden of healthcare-associated infections: An American perspective. Expert Rev. Pharmacoecon Outcomes Res. 2009, 9, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Health Facility Management. Managing the Cost of Specialized Rooms in Hospitals: Construction and Equipment Considerations. 2025. Available online: https://www.hfmmagazine.com (accessed on 18 March 2025).
- Fixr. How Much Does It Cost to Build a Hospital? 2022. Available online: https://www.fixr.com/costs/build-hospital (accessed on 18 March 2025).
- Nguemeleu, E.T.; Beogo, I.; Sia, D.; Kilpatrick, K.; Séguin, C.; Baillot, A.; Jabbour, M.; Parisien, N.; Robins, S.; Boivin, S. Economic analysis of healthcare-associated infection prevention and control interventions in medical and surgical units: Systematic review using a discounting approach. J. Hosp. Infect. 2020, 106, 134–154. [Google Scholar] [CrossRef] [PubMed]
- WHO. Environmental Cleaning and Infection Prevention and Control in Health Care Facilities in Low- and Middle-Income Countries: Modules and Resources; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
| Variables | Number of Wards | Number of ICUs |
|---|---|---|
| N (%) | N (%) | |
| Hospital Location | ||
| Central | 4 (20.0) | 4 (20.0) |
| Southern | 2 (10.0) | 2 (10.0) |
| Northern | 2 (10.0) | 2 (10.0) |
| Western | 2 (10.0) | 2 (10.0) |
| Eastern | 2 (10.0) | 2 (10.0) |
| Total | 20 (100) | 20 (100) |
| Hospital type | ||
| Primary | 0 (0.0) | 0 (0.0) |
| Secondary | 6 (30.0) | 6 (30.0) |
| Tertiary | 14 (70.0) | 14 (70.0) |
| Total | 20 (100) | 20 (100) |
| Bed Size | ||
| Small (<100 beds) | 4 (20.0) | 4 (20.0) |
| Medium (100–300 beds) | 6 (30.0) | 6 (30.0) |
| Large (>300 beds) | 10 (50.0) | 10 (50.0) |
| Total | 20 (100) | 20 (100) |
| Dirty Utility Rooms | Description | Essential Equipment | Central | North | West | East | South |
|---|---|---|---|---|---|---|---|
| General Sluice Room | Commonly found in hospitals and nursing homes, this type of room is equipped with basic necessities such as flushers disinfectors, macerators, sinks, and storage areas. It caters to inpatients’ regular needs, handling solid and liquid waste. | Bedpan washer disinfectors, medical waste macerators, handwashing basin, clinical waste bins, handwashing sinks with elbow/pedal control, storage cabinets, and shelving units | ☑ | ☑ | ☑ | ☑ | ☑ |
| Maternity Sluice Room | In maternity wards, these rooms are designed to handle postnatal waste. They might have specialized equipment to deal with items like placenta buckets. | Bedpan washer disinfectors, medical waste macerators, handwashing basins, clinical waste bins, handwashing sinks with elbow/pedal control, storage cabinets, and shelving units | ⊠ | ⊠ | ⊠ | ⊠ | ⊠ |
| Isolation Sluice Room | These are attached to isolation wards where patients with highly infectious diseases are housed. The design and equipment in these rooms ensure that waste is handled and disposed of to minimize the risk of disease transmission. | Bedpan washer disinfectors, medical waste macerators, handwashing basins, clinical waste bins, handwashing sinks with elbow/pedal control, storage cabinets, shelving units, automated touchless disinfection dispensers, washer disinfector machines, air filtration, and UV-C light disinfection | ☑ | ⊠ | ☑ | ☑ | ☑ |
| Pediatric Sluice Room | Located in children’s wards, these rooms may contain smaller equipment or items tailored to pediatric care needs. These DURs are essential for infection control in vulnerable children. | Bedpan washer disinfectors, medical waste macerators, handwashing basins, clinical waste bins, handwashing sinks with elbow/pedal control, storage cabinets, shelving units, diaper disposal units (odor-controlled), and infant pulp product macerators | ⊠ | ⊠ | ⊠ | ⊠ | ⊠ |
| Portable or Mobile Sluice Room | A relatively new concept, these modular units can be quickly set up in emergencies or during outbreaks where a rapid response is needed. They are often deployed in field hospitals or disaster zones. | Compact bedpan washer-disinfectors, portable macerators, slop hopper alternatives, sealed foot-operated waste bins, portable hand hygiene stations, and battery-operated or solar-powered ventilation | ⊠ | ⊠ | ⊠ | ⊠ | ⊠ |
| Specialized Sluice Rooms for Outpatient Procedures | In settings like dialysis centers, where there is a need to handle specific types of waste, specialized sluice rooms might be set up. Particularly in high-turnover outpatient clinics and diagnostic units | Bedpan washer disinfectors, medical waste macerators, handwashing basins, clinical waste bins, handwashing sinks with elbow/pedal control, storage cabinets, shelving units high-capacity disposable suction liner systems, automated disinfection units, and automated waste compactors | ⊠ | ⊠ | ⊠ | ⊠ | ⊠ |
| Elderly Care Sluice Room | Often found in long-term care facilities or nursing homes, these rooms might have equipment tailored to the needs of elderly patients, focusing especially on incontinence management and the vulnerability of elderly patients, those with weak immune systems, and mobility issues. | Bedpan washer disinfectors, medical waste macerators, handwashing basins, clinical waste bins, handwashing sinks with elbow/pedal control, storage cabinets, shelving units, adult diaper and incontinence pad disposal units, macerators for adult hygiene products, and odor-controlled waste bins with automatic sealing | ⊠ | ⊠ | ⊠ | ⊠ | ⊠ |
| Mortuary Sluice Room | Situated in mortuaries or post-mortem rooms, these sluice rooms handle the specific needs related to deceased patients, such as biological waste and fluids associated with deceased patients. | Bedpan washer disinfectors, medical waste macerators, handwashing basins, clinical waste bins, handwashing sinks with elbow/pedal control, storage cabinets, shelving units, pathological waste disposal units, and heavy-duty macerators for mortuary-specific waste | ⊠ | ⊠ | ⊠ | ⊠ | ⊠ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhurayji, K.; Alsuhaimi, A.; Alshathri, D.; Almazrou, D. Dirty Utility Rooms of Hospitals in Saudi Arabia: A Multi-Regional Case Study. Int. J. Environ. Res. Public Health 2025, 22, 604. https://doi.org/10.3390/ijerph22040604
Alkhurayji K, Alsuhaimi A, Alshathri D, Almazrou D. Dirty Utility Rooms of Hospitals in Saudi Arabia: A Multi-Regional Case Study. International Journal of Environmental Research and Public Health. 2025; 22(4):604. https://doi.org/10.3390/ijerph22040604
Chicago/Turabian StyleAlkhurayji, Khalid, Abdulmunim Alsuhaimi, Dalal Alshathri, and Dlal Almazrou. 2025. "Dirty Utility Rooms of Hospitals in Saudi Arabia: A Multi-Regional Case Study" International Journal of Environmental Research and Public Health 22, no. 4: 604. https://doi.org/10.3390/ijerph22040604
APA StyleAlkhurayji, K., Alsuhaimi, A., Alshathri, D., & Almazrou, D. (2025). Dirty Utility Rooms of Hospitals in Saudi Arabia: A Multi-Regional Case Study. International Journal of Environmental Research and Public Health, 22(4), 604. https://doi.org/10.3390/ijerph22040604






