Impact of the COVID-19 Pandemic and Control Measures on Screening and Diagnoses of Type 2 Diabetes in British Columbia
Abstract
1. Introduction
2. Methods
- BC residents with a valid Personal Health Number (PHN). PHN is a unique identifier for British Columbia residents who are enrolled in the Medical Services Plan (MSP) and is used to access healthcare services;
- BC adults ≥ 40 years (for screening) and BC adults ≥ 18 years (for type 2 diabetes diagnosis);
- No prior diagnosis of diabetes.
2.1. Data Source
2.2. Study Exposure and Outcomes
2.3. Study Covariates
2.4. Statistical Analysis
3. Results
3.1. Diabetes Screening
3.2. Diabetes Diagnoses
4. Discussion
Strengths and Limitations
5. Conclusions
- Medical Services Plan (MSP)—ICD-9 billing/diagnostic codes;
- Discharge Abstract Database (DAD)—DAD1 contains ICD-9 coded hospitalization data and DAD2 contains ICD-10 coded hospitalization data;
- National Ambulatory Care Reporting System (NACRS)—Contains ICD-10 coded diagnostic codes;
- PharmaNet—Each medication is identified with a drug identification number (DINPIN);
- Provincial Lab Information System (PLIS);
- Canadian Census (2016);
- Vital Statistics (VS);
- Client Roster: for sociodemographic information on each client.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BC Government. Province Declares State of Emergency to Support COVID-19 Response. 2020. Available online: https://news.gov.bc.ca/releases/2020PSSG0017-000511 (accessed on 31 December 2023).
- Frank, K. Difficulties accessing health care in Canada during the COVID-19 pandemic: Comparing individuals with and without chronic conditions. Health Rep. 2022, 33, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Moynihan, R.; Sanders, S.; Michaleff, Z.A.; Scott, A.M.; Clark, J.; To, E.J.; Jones, M.; Kitchener, E.; Fox, M.; Johansson, M.; et al. Impact of COVID-19 pandemic on utilisation of healthcare services: A systematic review. BMJ Open 2021, 11, e045343. [Google Scholar] [CrossRef] [PubMed]
- Moin, J.S.; Troke, N.; Plumptre, L.; Anderson, G.M. Impact of the COVID-19 Pandemic on Diabetes Care for Adults With Type 2 Diabetes in Ontario, Canada. Can. J. Diabetes 2022, 46, 715–721. [Google Scholar] [CrossRef]
- Sandhu, J.; Demlow, E.; Claydon-Platt, K.; Gully, M.; Chong, M.; Oakey, M.; Chhokar, R.; Frosst, G.; Moustaqim-Barrette, A.; Shergill, S.; et al. British Columbia’s COVID-19 surveys on population experiences, action, and knowledge (SPEAK): Methods and key findings from two large cross-sectional online surveys. Can. J. Public Health 2023, 114, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, Y.V.; Gillies, C.L.; Zaccardi, F.; Coles, B.; Davies, M.J.; Seidu, S.; Khunti, K. Impact of COVID-19 on routine care for chronic diseases: A global survey of views from healthcare professionals. Diabetes Metab. Syndr. 2020, 14, 965–967. [Google Scholar] [CrossRef]
- Vamos, E.P.; Khunti, K. Indirect effects of the COVID-19 pandemic on people with type 2 diabetes: Time to urgently move into a recovery phase. BMJ Qual. Saf. 2022, 31, 483–485. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef]
- Lipscombe, L.; Booth, G.; Butalia, S.; Dasgupta, K.; Eurich, D.T.; Goldenberg, R. Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Pharmacologic Glycemic Management of Type 2 Diabetes in Adults. Can. J. Diabetes 2018, 42 (Suppl 1), S88–S103. [Google Scholar] [CrossRef]
- WHO. COVID-19 Significantly Impacts Health Servcies for Noncommunicable Diseses. 2020. Available online: https://www.who.int/news/item/01-06-2020-covid-19-significantly-impacts-health-services-for-noncommunicable-diseases (accessed on 7 July 2023).
- Carr, M.J.; Wright, A.K.; Leelarathna, L.; Thabit, H.; Milne, N.; Kanumilli, N.; Ashcroft, D.M.; Rutter, M.K. Impact of COVID-19 on diagnoses, monitoring, and mortality in people with type 2 diabetes in the UK. Lancet. Diabetes Endocrinol. 2021, 9, 413–415. [Google Scholar] [CrossRef]
- Carr, M.J.; Wright, A.K.; Leelarathna, L.; Thabit, H.; Milne, N.; Kanumilli, N.; Ashcroft, D.M.; Rutter, M.K. Impact of COVID-19 restrictions on diabetes health checks and prescribing for people with type 2 diabetes: A UK-wide cohort study involving 618 161 people in primary care. BMJ Qual. Saf. 2022, 31, 503–514. [Google Scholar] [CrossRef]
- Misra, S.; Barron, E.; Vamos, E.; Thomas, S.; Dhatariya, K.; Kar, P.; Young, B.; Khunti, K.; Valabhji, J. Temporal trends in emergency admissions for diabetic ketoacidosis in people with diabetes in England before and during the COVID-19 pandemic: A population-based study. Lancet Diabetes Endocrinol. 2021, 9, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Population Estimates: Quarterly. 2023. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000901 (accessed on 7 July 2023).
- Diabetes Canada. Diabetes in British Columbia. Estimated Prevalence and Cost of Diabetes. Available online: https://www.diabetes.ca/advocacy---policies/advocacy-reports/national-and-provincial-backgrounders/diabetes-in-british-columbia (accessed on 7 July 2023).
- Kaul, P.; Chu, L.M.; Dover, D.C.; Yeung, R.O.; Eurich, D.T.; Butalia, S. Disparities in adherence to diabetes screening guidelines among males and females in a universal care setting: A population-based study of 1,380,697 adults. Lancet Reg. Health Am. 2022, 14, 100320. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Canada Clinical Practice Guidelines Expert Committee; Ekoe, J.M.; Goldenberg, R.; Katz, P. Screening for Diabetes in Adults. Can. J. Diabetes 2018, 42 (Suppl. S1), S16–S19. [Google Scholar] [CrossRef]
- Leiter, L.A.; Barr, A.; Belanger, A.; Lubin, S.; Ross, S.A.; Tildesley, H.D.; Fontaine, N. Diabetes Screening in Canada (DIASCAN) Study: Prevalence of undiagnosed diabetes and glucose intolerance in family physician offices. Diabetes Care 2001, 24, 1038–1043. [Google Scholar] [CrossRef]
- De Faye, B.; Perrin, D.; Trumpy, C. COVID-19 Lessons Learned Review. Final Report. 2022. Available online: https://www2.gov.bc.ca/assets/gov/public-safety-and-emergency-services/emergency-preparedness-response-recovery/embc/reports/covid-19_lessons_learned_report.pdf (accessed on 31 December 2022).
- Chen, G.; Khan, N.; Walker, R.; Quan, H. Validating ICD coding algorithms for diabetes mellitus from administrative data. Diabetes Res. Clin. Pract. 2010, 89, 189–195. [Google Scholar]
- Jeong, D.; Karim, M.E.; Wong, S.; Wilton, J.; Butt, Z.A.; Binka, M.; Adu, P.A.; Bartlett, S.; Pearce, M.; Clementi, E.; et al. Impact of HCV infection and ethnicity on incident type 2 diabetes: Findings from a large population-based cohort in British Columbia. BMJ Open Diabetes Res. Care 2021, 9, e002145. [Google Scholar]
- Pinilla, J.; Negrín, M. Non-Parametric Generalized Additive Models as a Tool for Evaluating Policy Interventions. Mathematics 2021, 9, 299. [Google Scholar] [CrossRef]
- Jia, H.; Lubetkin, E.I. Time trends and seasonal patterns of health-related quality of life among U.S. adults. Public Health Rep. 2009, 124, 692–701. [Google Scholar]
- Holland, D.; Heald, A.H.; Stedman, M.; Hanna, F.; Wu, P.; Duff, C.; Green, L.; Robinson, S.; Halsall, I.; Gaskell, N.; et al. Assessment of the effect of the COVID-19 pandemic on UK HbA1c testing: Implications for diabetes management and diagnosis. J. Clin. Pathol. 2023, 76, 177–184. [Google Scholar]
- Fazli, G.; Moineddin, R.; Ling, V.; Booth, G. Changes to Diabetes Screening During the Early Waves of the COVID-19 Pandemic. Can. J. Diabetes 2022, 46, S13–S14. [Google Scholar] [CrossRef]
- Czeisler, M.E.; Barrett, C.E.; Siegel, K.R.; Weaver, M.D.; Czeisler, C.A.; Rajaratnam, S.M.W.; Howard, M.E.; Bullard, K.M. Health Care Access and Use Among Adults with Diabetes During the COVID-19 Pandemic—United States, February-March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Bertakis, K.D.; Azari, R.; Helms, L.J.; Callahan, E.J.; Robbins, J.A. Gender differences in the utilization of health care services. J. Fam. Pract. 2000, 49, 147–152. [Google Scholar] [PubMed]
- Pinkhasov, R.M.; Wong, J.; Kashanian, J.; Lee, M.; Samadi, D.B.; Pinkhasov, M.M.; Shabsigh, R. Are men shortchanged on health? Perspective on health care utilization and health risk behavior in men and women in the United States. Int. J. Clin. Pract. 2010, 64, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Bradfield, O.; Spittal, M.J.; King, T. Age and gender patterns in health service utilisation: Age-Period-Cohort modelling of linked health service usage records. BMC Health Serv. Res. 2023, 23, 480. [Google Scholar] [CrossRef]
- Cherry, D.K.; Woodwell, D.A.; Rechtsteiner, E.A. National Ambulatory Medical Care Survey: 2005 summary. Adv. Data 2007, 29, 386. [Google Scholar]
- Cameron, K.A.; Song, J.; Manheim, L.M.; Dunlop, D.D. Gender disparities in health and healthcare use among older adults. J. Womens Health 2010, 19, 1643–1650. [Google Scholar] [CrossRef]
- Williams, R.; Jenkins, D.A.; Ashcroft, D.M.; Brown, B.; Campbell, S.; Carr, M.J.; Cheraghi-Sohi, S.; Kapur, N.; Thomas, O.; Webb, R.T.; et al. Diagnosis of physical and mental health conditions in primary care during the COVID-19 pandemic: A retrospective cohort study. Lancet Public Health 2020, 5, e543–e550. [Google Scholar] [CrossRef]
- Ye, M.; Vena, J.E.; Shen-Tu, G.; Johnson, J.A.; Eurich, D.T. Reduced incidence of diabetes during the COVID-19 pandemic in Alberta: A time-segmented longitudinal study of Alberta’s Tomorrow Project. Diabetes Obes. Metab. 2024, 26, 1244–1251. [Google Scholar] [CrossRef]
- Lebrasseur, A.; Fortin-Bédard, N.; Lettre, J.; Raymond, E.; Bussières, E.L.; Lapierre, N.; Faieta, J.; Vincent, C.; Duchesne, L.; Ouellet, M.C.; et al. Impact of the COVID-19 Pandemic on Older Adults: Rapid Review. JMIR Aging 2021, 4, 26474. [Google Scholar] [CrossRef]
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why does COVID-19 disproportionately affect older people? Aging 2020, 12, 9959–9981. [Google Scholar] [CrossRef]
- Vigezzi, G.P.; Bertuccio, P.; Amerio, A.; Bosetti, C.; Gori, D.; Cavalieri d’Oro, L.; Iacoviello, L.; Stuckler, D.; Zucchi, A.; Gallus, S.; et al. Older Adults’ Access to Care during the COVID-19 Pandemic: Results from the LOckdown and LifeSTyles (LOST) in Lombardia Project. Int. J. Env. Res. Public Health 2022, 19, 11271. [Google Scholar]
- Izzo, R.; Pacella, D.; Trimarco, V.; Manzi, M.V.; Lombardi, A.; Piccinocchi, R.; Gallo, P.; Esposito, G.; Lembo, M.; Piccinocchi, G.; et al. Incidence of type 2 diabetes before and during the COVID-19 pandemic in Naples, Italy: A longitudinal cohort study. EClinicalMedicine 2023, 66, 102345. [Google Scholar] [CrossRef] [PubMed]
- Marks, B.E.; Khilnani, A.; Meyers, A.; Flokas, M.E.; Gai, J.; Monaghan, M.; Streisand, R.; Estrada, E. Increase in the Diagnosis and Severity of Presentation of Pediatric Type 1 and Type 2 Diabetes during the COVID-19 Pandemic. Horm. Res. Paediatr. 2021, 94, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Mefford, M.T.; Wei, R.; Lustigova, E.; Martin, J.P.; Reynolds, K. Incidence of Diabetes Among Youth Before and During the COVID-19 Pandemic. JAMA Netw. Open 2023, 6, 34953. [Google Scholar] [CrossRef]
- Modarelli, R.; Sarah, S.; Ramaker, M.E.; Bolobiongo, M.; Benjamin, R.; Gumus Balikcioglu, P. Pediatric Diabetes on the Rise: Trends in Incident Diabetes During the COVID-19 Pandemic. J. Endocr. Soc. 2022, 6, bvac024. [Google Scholar] [CrossRef]
- Diabetes, U.K. Reverse the Trend. Reducing Type 2 Diabetes in Young People. 2024. Available online: https://www.diabetes.org.uk/sites/default/files/2024-06/Reverse%20the%20Trend%20report%20V2.pdf? (accessed on 2 July 2024).
- International Diabetes Federation. IDF Diabetes Atlas. 2021. Available online: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf (accessed on 2 July 2024).
- Misra, S.; Ke, C.; Srinivasan, S.; Goyal, A.; Nyriyenda, M.; Florez, J.; Khunti, K.; Magliano, D.; Luk, A. Current insights and emerging trends in early-onset type 2 diabetes. Lancet Diabetes Endocrinol. 2023, 11, 768–782. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration. Life expectancy associated with different ages at diagnosis of type 2 diabetes in high-income countries: 23 million person-years of observation. Lancet Diabetes Endocrinol. 2023, 11, 731–742. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Leutner, M.; Harreiter, J. Sex differences in type 2 diabetes. Diabetologia 2023, 66, 986–1002. [Google Scholar] [CrossRef]
- Bai, F.; Tomasoni, D.; Falcinella, C.; Barbanotti, D.; Castoldi, R.; Mulè, G.; Augello, M.; Mondatore, D.; Allegrini, M.; Cona, A.; et al. Female gender is associated with long COVID syndrome: A prospective cohort study. Clin. Microbiol. Infect. 2022, 28, 611.e9–611.e16. [Google Scholar]
- Sylvester, S.V.; Rusu, R.; Chan, B.; Bellows, M.; O’Keefe, C.; Nicholson, S. Sex differences in sequelae from COVID-19 infection and in long COVID syndrome: A review. Curr. Med. Res. Opin. 2022, 38, 1391–1399. [Google Scholar]
- Ayoubkhani, D.; Khunti, K.; Nafilyan, V.; Maddox, T.; Humberstone, B.; Diamond, I.; Banerjee, A. Post-covid syndrome in individuals admitted to hospital with covid-19: Retrospective cohort study. Bmj 2021, 31, n693. [Google Scholar]
- Nabavi, N. Long covid: How to define it and how to manage it. Bmj 2020, 7, m3489. [Google Scholar]
- Oronsky, B.; Larson, C.; Hammond, T.C.; Oronsky, A.; Kesari, S.; Lybeck, M.; Reid, T.R. A Review of Persistent Post-COVID Syndrome (PPCS). Clin. Rev. Allergy Immunol. 2023, 64, 66–74. [Google Scholar] [PubMed]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar]
- Breyer, M.K.; Ofenheimer, A.; Altziebler, J.; Hartl, S.; Burghuber, O.C.; Studnicka, M.; Purin, D.; Heinzle, C.; Drexel, H.; Franssen, F.M.E.; et al. Marked differences in prediabetes- and diabetes-associated comorbidities between men and women-Epidemiological results from a general population-based cohort aged 6-80 years-The LEAD (Lung, hEart, sociAl, boDy) study. Eur. J. Clin. Investig. 2020, 50, 12. [Google Scholar]
- Claypool, K.T.; Chung, M.K.; Deonarine, A.; Gregg, E.W.; Patel, C.J. Characteristics of undiagnosed diabetes in men and women under the age of 50 years in the Indian subcontinent: The National Family Health Survey (NFHS-4)/Demographic Health Survey 2015-2016. BMJ Open Diabetes Res Care 2020, 8, e000965. [Google Scholar]
- Galaviz, K.I.; Narayan, K.M.V.; Lobelo, F.; Weber, M.B. Lifestyle and the Prevention of Type 2 Diabetes: A Status Report. Am. J. Lifestyle Med. 2015, 12, 4–20. [Google Scholar]
- Bell, M.; Duncan, M.J.; Patte, K.A.; Roy, B.D.; Ditor, D.S.; Klentrou, P. Changes in Body Mass, Physical Activity, and Dietary Intake during the COVID-19 Pandemic Lockdowns in Canadian University Students. Biology 2023, 12, 326. [Google Scholar] [CrossRef]
- Manuel, D.G.; Eddeen, A.B.; Colley, R.C.; Tjepkema, M.; Garner, R.; Bennett, C.; Bernier, J. Statistics Canada. The Effect of COVID-19 on Physical Activity Among Canadians and the Future Risk of Cardiovascular Disease. 2021. Available online: https://www150.statcan.gc.ca/n1/en/pub/45-28-0001/2021001/article/00019-eng.pdf?st=yTMw64ZP (accessed on 12 December 2023).
- Robinson, E.; Boyland, E.; Chisholm, A.; Harrold, J.; Maloney, N.G.; Marty, L.; Mead, B.R.; Noonan, R.; Hardman, C.A. Obesity, eating behavior and physical activity during COVID-19 lockdown: A study of UK adults. Appetite 2021, 156, 104853. [Google Scholar]
- Moyser, M. Gender Differences in Mental Health During the COVID-19 Pandemic; Statistics Canada: Ottawa, ON, Canada, 2020.
- Saldarriaga-Giraldo, C.I.; Ramirez-Ramos, C.F.; Lopez-Santi, R.; Lanas, F.; Valdés Martín, A.; Sotomayor Perales, J.L.; Juárez-Lloclla, J.P.; Ruise, M.; Carrión Arcela, J.P.; Flores de Espinal, E.H.; et al. Gender-related Differences in the Impact of COVID-19 Pandemic in Cardiometabolic Patients in Latin America: The CorCOVID LATAM Gender Sub-study. Curr. Probl. Cardiol. 2022, 47, 101075. [Google Scholar]
- Thomas, T.W.; Lindsey, R.; Yassin, M.; Rodriguez, L.A.; Heisler, M.; Schmittdiel, J.A. Effects of COVID-19 shelter-in-place confinement on diabetes prevention health behaviors among US adults with prediabetes: A cross-sectional survey. Prev. Med. Rep. 2023, 32, 102139. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.M.; Slaughter, J.C.; Duffus, S.H.; Smith, T.J.; LeStourgeon, L.M.; Jaser, S.S.; McCoy, A.B.; Luther, J.M.; Giovannetti, E.R.; Boeder, S.; et al. COVID-19 Severity Is Tripled in the Diabetes Community: A Prospective Analysis of the Pandemic’s Impact in Type 1 and Type 2 Diabetes. Diabetes Care 2021, 44, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Naveed, Z.; Velásquez García, H.A.; Wong, S.; Wilton, J.; McKee, G.; Mahmood, B.; Binka, M.; Rasali, D.; Janjua, N.Z. Association of COVID-19 Infection With Incident Diabetes. JAMA Netw. Open 2023, 6, e238866. [Google Scholar] [CrossRef]
- Sathish, T.; Kapoor, N.; Cao, Y.; Tapp, R.J.; Zimmet, P. Proportion of newly diagnosed diabetes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Obes Metab. 2021, 23, 870–874. [Google Scholar] [CrossRef]
- Wong, R.; Lam, E.; Bramante, C.T.; Johnson, S.G.; Reusch, J.; Wilkins, K.J.; Yeh, H.C. Does COVID-19 Infection Increase the Risk of Diabetes? Current Evidence. Curr. Diabetes Rep. 2023, 23, 207–216. [Google Scholar] [CrossRef]
- Zhang, T.; Mei, Q.; Zhang, Z.; Walline, J.H.; Liu, Y.; Zhu, H.; Zhang, S. Risk for newly diagnosed diabetes after COVID-19: A systematic review and meta-analysis. BMC Med. 2022, 20, 444. [Google Scholar]
- Xie, Y.; Al-Aly, Z. Risks and burdens of incident diabetes in long COVID: A cohort study. Lancet. Diabetes Endocrinol. 2022, 10, 311–321. [Google Scholar] [CrossRef]
- Diabetes Canada: The Impact of COVID-19 on Access to Diabetes Care, Management, and Related Complications. 2021. Available online: https://www.diabetes.ca/diabetescanadawebsite/media/campaigns/covid-19%20and%20diabetes/impactcovid.pdf (accessed on 20 February 2025).
- British Columbia Ministry of Health. Medical Services Plan (MSP) Payment Information File. British Columbia Ministry of Health: Data Extract. MOH (2021). Available online: https://www2.gov.bc.ca/gov/content/health/health-forms/online-services (accessed on 7 July 2023).
- Community Health Service Areas—CHSA—B.C. Health Region Master Table—Version 2022. Available online: https://open.canada.ca/data/en/dataset/68f2f577-28a7-46b4-bca9-7e9770f2f357 (accessed on 7 July 2023).
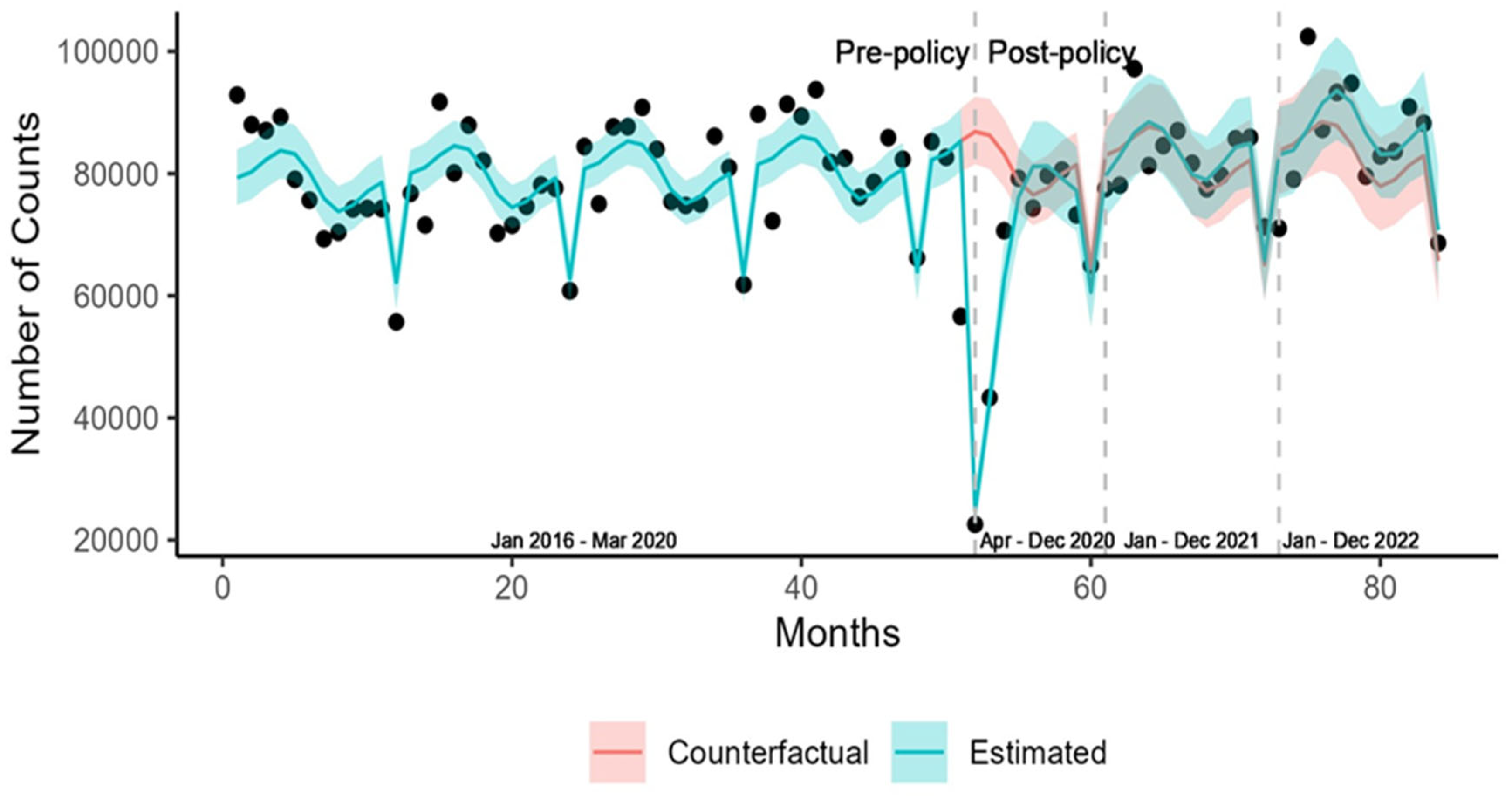
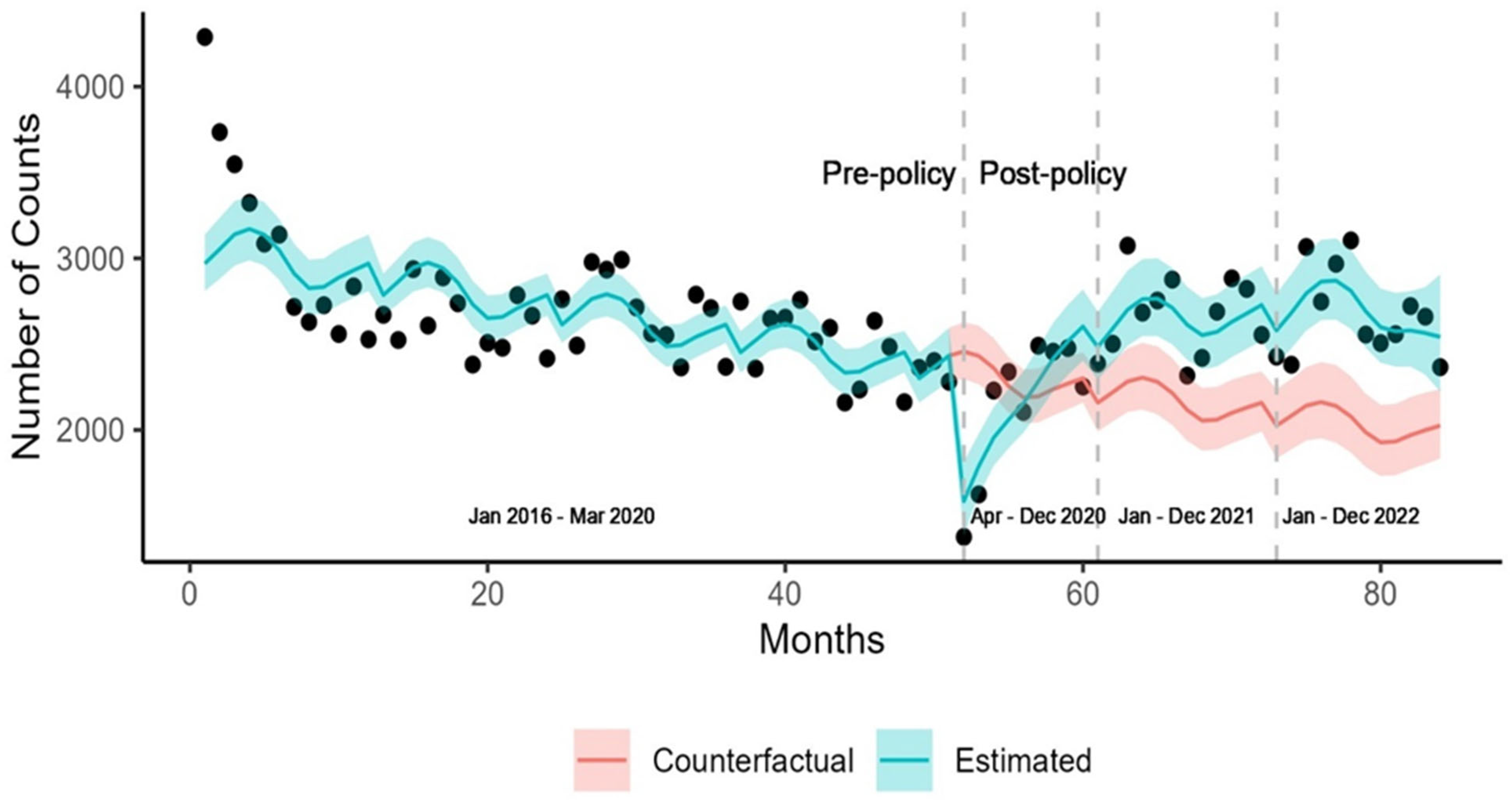
| Population | Number of Screenings (Pre-Policy) | Number of Screenings (Post-Policy) |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Total | 79,045 (9199) | 78,717 (14,663) |
| Sex | ||
| Female | 42,573 (5426) | 42,650 (8396) |
| Male | 36,472 (3943) | 36,067 (6352) |
| Age Group | ||
| 40–49 | 12,193 (2082) | 6846 (1373) |
| 50–59 | 22,514 (2369) | 21,679 (4106) |
| 60–69 | 23,191 (2778) | 23,597 (4728) |
| 70–79 | 14,724 (2344) | 17,777 (3522) |
| >79 | 6423 (1022) | 7818 (1613) |
| Urban/Rural Residence | ||
| Metropolitan | 35,769 (3899) | 36,401 (6884) |
| Large Urban | 14,182 (1660) | 13,988 (2480) |
| Medium Urban | 8006 (1070) | 7822 (1480) |
| Small Urban | 7415 (958) | 7241 (1401) |
| Rural Hub | 4138 (546) | 4010 (745) |
| Rural | 8880 (1187) | 8653 (1697) |
| Remote | 630 (87) | 596 (108) |
| Missing | 24 (36) | 6 (3) |
| Health Authority | ||
| Fraser | 26,608 (3000) | 26,911 (5038) |
| Interior | 13,371 (1875) | 12,904 (2470) |
| Northern | 4142 (510) | 3997 (815) |
| Vancouver Coastal | 19,557 (2130) | 19,919 (3803) |
| Vancouver Island | 15,343 (1868) | 14,981 (2656) |
| Missing | 24 (36) | 6 (3) |
| Population | Absolute Difference, n (95% CI) | Percentage Difference, % (95% CI) | ||||
|---|---|---|---|---|---|---|
| 1 April 2020–31 December 2020 | 1 January 2021–31 December 2021 | 1 January 2022–31 December 2022 | 1 April 2020–31 December 2020 | 1 January 2021–31 December 2021 | 1 January 2022–31 December 2022 | |
| Total | −129,346 (−180,717, −78,287) | −9381 (−72,360, 89,553) | 44,690 (−55,288, 141,268) | −18.0 (−24.2, −11.4) | 1.1 (−7.1, 9.7) | 4.8 (−5.1, 15.4) |
| Sex | ||||||
| Female | −65,620 (−95,029, −36,621) | 18,785 (−27,965, 64,588) | 35,325 (−21,114, 89,332) | −17.1 (−23.8, −10.0) | 3.8 (−5.1, 13.2) | 7.1 (−3.7, 18.7) |
| Male | −63,713 (−85,758, −41,726) | −9444 (−44,918, 25,495) | 9106 (−34,765, 51,373) | −19.1 (−24.8, −13.0) | −2.0 (−9.3, 5.9) | 2.1 (−7.0, 11.9) |
| Age Group | ||||||
| 40–49 | −22,430 (−28,051, −16,907) | −10,094 (−18,319, −2039) | −18,632 (−27,313, −10,296) | −27.0 (−32.3, −21.2) | −9.8 (−17.0, −2.1) | −20.1 (−27.6, −12.0) |
| 50–59 | −41,887 (−55,558, −28,285) | 3362 (−18,835, 25,003) | 10,009 (−16,721, 35,717) | −20.8 (−26.7, −14.7) | 1.3 (−6.6, 9.7) | 3.9 (−5.8, 14.1) |
| 60–69 | −38,633 (−54,254, −23,014) | 3293 (−21,640, 27,828) | 16,964 (−14,148, 47,046) | −17.5 (−23.7, −10.9) | 1.2 (−6.8, 9.7) | 5.7 (−4.2, 16.3) |
| 70–79 | −24,228 (−36,296, −12,178) | −3856 (−23,741, 15,638) | 3011 (−23,001, 27,643) | −15.6 (−22.6, −8.2) | −1.6 (−10.1, 7.5) | 1.5 (−8.9, 12.7) |
| >79 | −8353 (−13,716, −3016) | 2754 (−6276, 11,545) | 8881 (−2615, 19,989) | −12.9 (−20.5, −4.9) | 3.1 (−6.3, 13.2) | 9.3 (−2.5, 22.2) |
| Urban/Rural Residence | ||||||
| Metropolitan | −59,800 (−83,955, −35,893) | 6249 (−32,666, 44,466) | 18,308 (−29,431, 63,990) | −18.1 (−23.4, −11.3) | 1.5 (−6.8, 10.4) | 4.2 (−5.8, 15.1) |
| Large Urban | −20,778 (−29,722, −11,913) | 1466 (−12,722, 15,331) | 8050 (−9212, 24,594) | −16.2 (−22.5, −9.7) | 1.0 (−6.9, 9.4) | 4.9 (−4.8, 15.4) |
| Medium Urban | −13,787 (−19,059, −8545) | −2406 (−10,905, 5865) | 779 (−9545, 10,761) | −18.9 (−25.2, −12.2) | −2.3 (−10.3, 6.2) | 0.9 (−8.8, 11.4) |
| Small Urban | −12,828 (−17,539, −8115) | 1999 (−5592, 9447) | 7112 (−2113, 16,046) | −19.6 (−25.8, −13.0) | 2.3 (−6.0, 11.2) | 8.2 (−2.2, 19.4) |
| Rural Hub | −6710 (−9456, −3953) | 652 (−3758, 4978) | 2327 (−3032, 7442) | −18.3 (−24.8, −11.3) | 1.4 (−7.1, 10.5) | 4.9 (−5.5, 16.2) |
| Rural | −14,922 (−20,644, 9236) | 1048 (−8102, 10,017) | 7275 (−3923, 18,049) | −19.0 (−25.3, −12.3) | 1.1 (−7.2, 9.9) | 7.0 (−3.4, 18.2) |
| Remote | −816 (−1292, −342) | −104 (−860, 627) | 363 (−546, 1232) | −14.9 (−22.7, −6.5) | −1.2 (−10.7, 9) | 5.3 (−6.7, 18.3) |
| Health Authority | ||||||
| Fraser | −45,524 (−63,220, −28,008) | 2527 (−26,147, 30,456) | 11,864 (−22,867, 45,480) | −18.5 (−24.8, −11.9) | 0.9 (7.4, 9.7) | 3.7 (−6.3, 14.4) |
| Interior | −22,726 (−31,408, −14,063) | −666 (−14,579, 12,908) | 7140 (−9774, 23,352) | −19.1 (−25.4, −12.3) | −0.3 (−8.5, 8.5) | 4.7 (−5.5, 15.7) |
| Northern | −7585 (−10,171, −5023) | 873 (−3355, 5008) | 2867 (−2276, 7792) | −20.8 (−26.9, −14.3) | 1.9 (−6.4, 10.6) | 6.0 (−4.2, 16.9) |
| Vancouver Coastal | −31,049 (−44,310, −17,838) | 5824 (−15,391, 26,724) | 13,853 (−12,130, 38,875) | −17.3 (−23.8, −10.4) | 2.5 (−6.0, 11.6) | 5.8 (−4.6, 17.1) |
| Vancouver Island | −22,768 (−32,642, −12,950) | 397 (−15,208, 15,675) | 8412 (−10,411, 26,624) | −16.6 (−22.9, −9.9) | 0.3 (−7.7, 8.8) | 4.7 (−5.2, 15.4) |
| Population | Number of Individuals Diagnosed (Pre-Policy) | Number of Individuals Diagnosed (Post-Policy) |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Total | 2705 (386) | 2526 (367) |
| Sex | ||
| Female | 1269 (175) | 1261 (203) |
| Male | 1436 (219) | 1266 (178) |
| Age Group | ||
| 18–29 | 66 (16) | 61 (9) |
| 30–39 | 212 (28) | 223 (33) |
| 40–49 | 433 (67) | 406 (61) |
| 50–59 | 719 (115) | 640 (98) |
| 60–69 | 718 (112) | 637 (99) |
| 70–79 | 400 (54) | 397 (65) |
| >79 | 157 (20) | 162 (27) |
| Urban/Rural Residence | ||
| Metropolitan | 1430 (195) | 1330 (199) |
| Large Urban | 430 (63) | 416 (66) |
| Medium Urban | 272 (41) | 252 (39) |
| Small Urban | 221 (43) | 203 (35) |
| Rural Hub | 110 (23) | 100 (20) |
| Rural | 218 (39) | 206 (33) |
| Remote | 18 (5) | 17 (5) |
| Missing | 6 (3) | 3 (2) |
| Health Authority | ||
| Fraser | 1129 (159) | 1062 (156) |
| Interior | 370 (71) | 344 (62) |
| Northern | 152 (27) | 149 (23) |
| Vancouver Coastal | 633 (89) | 592 (92) |
| Vancouver Island | 414 (64) | 376 (60) |
| Missing | 6 (3) | 3 (2) |
| Population | Absolute Difference, n (95% CI) | Percentage Difference, % (95% CI) | ||||
|---|---|---|---|---|---|---|
| 1 April 2020–31 December 2020 | 1 January 2021–31 December 2021 | 1 January 2022–31 December 2022 | 1 April 2020–31 December 2020 | 1 January 2021–31 December 2021 | 1 January 2022–31 December 2022 | |
| Total | −1328 (−3034, 369) | 5716 (3117, 8255) | 7679 (4746, 10519) | −6.3 (−14.1, 1.7) | 22.0 (11.2, 33.6) | 31.6 (17.8, 46.6) |
| Sex | ||||||
| Female | −426 (−1272, 423) | 3451 (2164, 4726) | 4658 (3185, 6098) | −4.2 (−12.4, 4.4) | 28.1 (16.4, 40.7) | 40.3 (25.2, 56.7) |
| Male | −917 (−1815, −19) | 2267 (881, 3621) | 3010 (1462, 4512) | −8.4 (−16.1, −0.1) | 16.7 (6.1, 28.0) | 23.8 (10.4, 38.3) |
| Age Group | ||||||
| 18–29 | 103 (26, 181) | 163 (66, 254) | 190 (73, 300) | 22.5 (5.1, 41.8) | 29.2 (10.3, 50.4) | 37.8 (12.2, 67.4) |
| 30–39 | 26 (−145, 196) | 602 (348, 851) | 695 (404, 977) | 1.6 (−7.9, 11.9) | 27.6 (14.7, 41.7) | 33.3 (17.2, 50.9) |
| 40–49 | −115 (−410, 177) | 1103 (662, 1537) | 1413 (917, 1894) | −3.5 (−12.1, 5.7) | 27.5 (15.3, 40.7) | 38.0 (22.3, 55.2) |
| 50–59 | −460 (−924, −1) | 1518 (817, 2206) | 2055 (1275, 2811) | −8.6 (−16.6, −0.1) | 23.2 (11.7, 35.7) | 34.0 (19.1, 50.2) |
| 60–69 | −438 (−921, 45) | 1336 (604, 2052) | 1705 (892, 2487) | −8.0 (−16.3, 0.9) | 20.1 (8.4, 32.6) | 27.5 (13.0, 43.5) |
| 70–79 | −319 (−629, −14) | 724 (246, 1191) | 1136 (584, 1672) | −9.6 (−18.3, −0.4) | 17.4 (5.5, 30.2) | 28.4 (13.0, 45.3) |
| >79 | −150 (−286, −16) | 227 (17, 433) | 381 (128, 622) | −11.0 (−20.0, −1.2) | 13.1 (0.9 (26.3) | 22.2 (6.7, 39.5) |
| Urban/Rural Residence | ||||||
| Metropolitan | −837 (−1793, 114) | 3208 (1760, 4635) | 3664 (2039, 5248) | −7.5 (−15.5, 1.0) | 23.4 (11.8, 35.9) | 28.6 (14.3, 44.1) |
| Large Urban | −138 (−433, 160) | 751 (306, 1190) | 1340 (824, 1844) | −4.0 (−12.4, 5.0) | 17.6 (6.6, 29.5) | 33.0 (18.3, 49.2) |
| Medium Urban | −156 (−371, 59) | 376 (54, 693) | 567 (195, 923) | −7.2 (−16.5, 2.8) | 14.0 (1.9, 27.1) | 22.1 (6.7, 39.1) |
| Small Urban | −162 (−348, 24) | 480 (194, 760) | 780 (451, 1097) | −9.7 (−20.1, 1.5) | 23.7 (8.5, 40.3) | 41.4 (21.0, 64.4) |
| Rural Hub | −25 (−128, 79) | 353 (201, 502) | 422 (254, 583) | −3.0 (−15.6, 10.9) | 38.4 (19.5, 59.3) | 51.0 (26.6, 78.8) |
| Rural | −79 (−242, 86) | 545 (298, 787) | 790 (509, 1062) | −4.7 (−14.2, 5.6) | 27.0 (13.5, 41.6) | 42.1 (24.2, 61.6) |
| Remote | 13 (−18, 44) | 63 (20, 104) | 64 (15, 109) | 10.8 (−12.1, 37.9) | 40.9 (10.5, 77.2) | 46.0 (8.5, 92.5) |
| Health Authority | ||||||
| Fraser | −486 (−1236, 261) | 2590 (1455, 3706) | 3209 (1931, 4448) | −5.5 (−13.6, 3.2) | 24.1 (12.5, 36.4) | 31.8 (17.4, 47.4) |
| Interior | −133 (−393, 127) | 1082 (690, 1469) | 1706 (1262, 2139) | −5.0 (−14.3, 5.1) | 34.0 (19.9, 49.2) | 59.0 (39.2, 80.6) |
| Northern | −40 (−184, 103) | 284 (67, 498) | 333 (82, 573) | −3.1 (−14.2, 9.0) | 18.2 (3.7, 34.0) | 22.3 (4.7, 41.9) |
| Vancouver Coastal | −327 (−784, 131) | 1527 (829, 2213) | 1499 (724, 2249) | −6.6 (−15.3, 2.7) | 25.1 (12.5, 38.7) | 26.3 (11.3, 42.7) |
| Vancouver Island | −393 (−716, −74) | 260 (−235, 742) | 866 (279, 1427) | −11.8 (−20.6, −2.3) | 6.3 (−5.0, 18.6) | 21.5 (6.2, 38.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, B.; Li, G.; Li, J.; Wilton, J.; Tang, T.S.; Velásquez García, H.A.; Wong, S.; Jain, A.B.; Naveed, Z.; Garg, A.; et al. Impact of the COVID-19 Pandemic and Control Measures on Screening and Diagnoses of Type 2 Diabetes in British Columbia. Int. J. Environ. Res. Public Health 2025, 22, 519. https://doi.org/10.3390/ijerph22040519
Mahmood B, Li G, Li J, Wilton J, Tang TS, Velásquez García HA, Wong S, Jain AB, Naveed Z, Garg A, et al. Impact of the COVID-19 Pandemic and Control Measures on Screening and Diagnoses of Type 2 Diabetes in British Columbia. International Journal of Environmental Research and Public Health. 2025; 22(4):519. https://doi.org/10.3390/ijerph22040519
Chicago/Turabian StyleMahmood, Bushra, Gordon Li, Julia Li, James Wilton, Tricia S. Tang, Héctor Alexander Velásquez García, Stanley Wong, Akshay B. Jain, Zaeema Naveed, Arun Garg, and et al. 2025. "Impact of the COVID-19 Pandemic and Control Measures on Screening and Diagnoses of Type 2 Diabetes in British Columbia" International Journal of Environmental Research and Public Health 22, no. 4: 519. https://doi.org/10.3390/ijerph22040519
APA StyleMahmood, B., Li, G., Li, J., Wilton, J., Tang, T. S., Velásquez García, H. A., Wong, S., Jain, A. B., Naveed, Z., Garg, A., Nandra, A., Janjua, N. Z., & McKee, G. (2025). Impact of the COVID-19 Pandemic and Control Measures on Screening and Diagnoses of Type 2 Diabetes in British Columbia. International Journal of Environmental Research and Public Health, 22(4), 519. https://doi.org/10.3390/ijerph22040519






