The Breast to Bone (B2B) Cohort Study to Prevent, Detect and Improve Treatment of Metastatic Disease: Baseline Assessment, Description and Progress
Abstract
1. Introduction
2. Materials and Methods
B2B Cohort Creation
3. Results
3.1. B2B Cohort Description
3.2. Projects Utilizing Baseline Data and Biological Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.L.; Fitzgerald, N. Projected estimates of cancer in Canada in 2024. Can. Med. Assoc. J. 2024, 196, E615–E623. [Google Scholar]
- Canadian Cancer Society. Breast Cancer Statistics 2024. Available online: https://cancer.ca/en/cancer-information/cancer-types/breast/statistics (accessed on 20 October 2024).
- Efegoma, Y.C. Effect of Mammography Screening on Incidence and Mortality of Breast Cancer in Alberta; University of Calgary: Calgary, AB, Canada, 2020. [Google Scholar] [CrossRef]
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The lingering mysteries of metastatic recurrence in breast cancer. Br. J. Cancer 2021, 124, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Caswell-Jin, J.L.; Plevritis, S.K.; Tian, L.; Cadham, C.J.; Xu, C.; Stout, N.K.; Sledge, G.W.; Mandelblatt, J.S.; Kurian, A.W. Change in Survival in Metastatic Breast Cancer with Treatment Advances: Meta-Analysis and Systematic Review. JNCI Cancer Spectr. 2018, 2, pky062. [Google Scholar] [CrossRef] [PubMed]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative, G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar]
- Manders, K.; van de Poll-Franse, L.V.; Creemers, G.-J.; Vreugdenhil, G.; van der Sangen, M.J.C.; Nieuwenhuijzen, G.A.P.; Roumen, R.M.; Voogd, A.C. Clinical management of women with metastatic breast cancer: A descriptive study according to age group. BMC Cancer 2006, 6, 179. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Smith, P.; Rubens, R.D. Clinical course and prognostic factors following bone recurrence from breast cancer. Br. J. Cancer 1998, 77, 336–340. [Google Scholar] [CrossRef]
- Coleman, R.E.; Rubens, R.D. The clinical course of bone metastases from breast cancer. Br. J. Cancer 1987, 55, 61–66. [Google Scholar] [CrossRef]
- Dawood, S.; Broglio, K.; Gonzalez-Angulo, A.M.; Buzdar, A.U.; Hortobagyi, G.N.; Giordano, S.H. Trends in Survival Over the Past Two Decades Among White and Black Patients with Newly Diagnosed Stage IV Breast Cancer. J. Clin. Oncol. 2008, 26, 4891–4898. [Google Scholar] [CrossRef]
- Liede, A.; Jerzak, K.J.; Hernandez, R.K.; Wade, S.W.; Sun, P.; Narod, S.A. The incidence of bone metastasis after early-stage breast cancer in Canada. Breast Cancer Res. Treat. 2016, 156, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Body, J.-J.; Quinn, G.; Talbot, S.; Booth, E.; Demonty, G.; Taylor, A.; Amelio, J. Systematic review and meta-analysis on the proportion of patients with breast cancer who develop bone metastases. Crit. Rev. Oncol./Hematol. 2017, 115, 67–80. [Google Scholar] [CrossRef]
- Paget, S. The Distribution of Secondary Growths in Cancer of the Breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Ewing, J. Neoplastic Diseases: A Treatise on Tumours. Br. J. Surg. 1928, 16, 174–175. [Google Scholar] [CrossRef]
- Langley, R.R.; Fidler, I.J. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 2011, 128, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Price, T.T.; Burness, M.L.; Sivan, A.; Warner, M.J.; Cheng, R.; Lee, C.H.; Olivere, L.; Comatas, K.; Magnani, J.; Lyerly, H.K.; et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci. Transl. Med. 2016, 8, 340ra73. [Google Scholar] [CrossRef] [PubMed]
- Satcher, R.L.; Zhang, X.H.-F. Evolving cancer–niche interactions and therapeutic targets during bone metastasis. Nat. Rev. Cancer 2022, 22, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bado, I.L.; Hu, J.; Wan, Y.-W.; Wu, L.; Wang, H.; Gao, Y.; Jeong, H.-H.; Xu, Z.; Hao, X.; et al. The bone microenvironment invigorates metastatic seeds for further dissemination. Cell 2021, 184, 2471–2486.e20. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R.; Lippman, M.E.; Morrow, M.; Osborne, K. Diseases of the Breast, 2nd ed.; Lippincott William & Wilkins: Philadelphia, PA, USA, 2000; 1152p. [Google Scholar]
- Vincent-Salomon, A.; Bidard, F.C.; Pierga, J.Y. Bone marrow micrometastasis in breast cancer: Review of detection methods, prognostic impact and biological issues. J. Clin. Pathol. 2008, 61, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.F. Methods of reducing pain during bone marrow biopsy: A narrative review. Ann. Palliat. Med. 2015, 4, 184–193. [Google Scholar] [PubMed]
- Yazdani, A.; Dorri, S.; Atashi, A.; Shirafkan, H.; Zabolinezhad, H. Bone Metastasis Prognostic Factors in Breast Cancer. Breast Cancer 2019, 13, 1178223419830978. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Zhang, S.; Wang, X.; Yu, S. Factors associated with bone metastasis in breast cancer: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 4435–4452. [Google Scholar] [CrossRef]
- Brockton, N.T.; Gill, S.J.; Laborge, S.L.; Paterson, A.H.G.; Cook, L.S.; Vogel, H.J.; Shemanko, C.S.; Hanley, D.A.; Magliocco, A.M.; Friedenreich, C.M. The Breast Cancer to Bone (B2B) Metastases Research Program: A multi-disciplinary investigation of bone metastases from breast cancer. BMC Cancer 2015, 15, 512. [Google Scholar] [CrossRef] [PubMed]
- Diet History Questionnaire, 2nd ed.; National Institutes of Health, Epidemiology and Genomics Research Program, National Cancer Institute: Bethesda, MD, USA, 2010.
- Diet History Questionnaire, 1st ed.; National Institutes of Health, Epidemiology and Genomics Research Program, National Cancer Institute: Bethesda, MD, USA, 2007.
- Friedenreich, C.M.; Courneya, K.S.; Neilson, H.K.; Matthews, C.E.; Willis, G.; Irwin, M.; Troiano, R.; Ballard-Barbash, R. Reliability and validity of the Past Year Total Physical Activity Questionnaire. Am. J. Epidemiol. 2006, 163, 959–970. [Google Scholar] [CrossRef]
- Cook, L.S.; Moon, B.L.; Dong, Y.; Neilson, H.K. Reliability of self-reported sun exposure in Canadian women and estimation of lifetime exposure to vitamin D from sun and diet. Public Health Nutr. 2014, 17, 747–755. [Google Scholar] [CrossRef]
- Manocha, A.; Brockton, N.T.; Cook, L.; Kopciuk, K.A. Low Serum Vitamin D Associated With Increased Tumor Size and Higher Grade in Premenopausal Canadian Women With Breast Cancer. Clin. Breast Cancer 2023, 23, e368–e376. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.-C.; C Chen, T. The anti-cancer actions of vitamin D. Anti-Cancer Agents Med. Chem.-Anti-Cancer Agents 2013, 13, 126–139. [Google Scholar] [CrossRef]
- Holick, M.F. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin. Proc. 2006, 81, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Zhu, Y.; Froicu, M.; Wittke, A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am. J. Clin. Nutr. 2004, 80 (Suppl. 6), 1717S–1720S. [Google Scholar] [CrossRef]
- Bollard, M.E.; Stanley, E.G.; Lindon, J.C.; Nicholson, J.K.; Holmes, E. NMR-based metabonomic approaches for evaluating physiological influences on biofluid composition. NMR Biomed. 2005, 18, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, A.; Forsyth, A.; Cong, Y.; Grant, L.; Juan, T.-H.; Lee, J.K.; Klimowicz, A.; Petrillo, S.K.; Hu, J.; Chan, A.; et al. The role of prolactin in bone metastasis and breast cancer cell–mediated osteoclast differentiation. J. Natl. Cancer Inst. 2016, 108, djv338. [Google Scholar] [CrossRef]
- Zhang, Q.; Shemanko, C.S.; (University of Calgary, Calgary, AB, Canada). Novel biomarkers for bone metastases. Unpublished observations, 2020. [Google Scholar]
- You, B.; Mercier, F.; Assenat, E.; Langlois-Jacques, C.; Glehen, O.; Soulé, J.; Payen, L.; Kepenekian, V.; Dupuy, M.; Belouin, F.; et al. The oncogenic and druggable hPG80 (Progastrin) is overexpressed in multiple cancers and detected in the blood of patients. EBioMedicine 2020, 51, 102574. [Google Scholar] [CrossRef] [PubMed]
- Prieur, A.; Kepenekian, V.; Mazard, T.; Payen, L.; Maucourt-Boulch, D.; Assenat, E.; Mariani, O.M.; Liaud, P.; Flacelière, M.; Soulé, J.; et al. Progastrin, a New Blood Biomarker for Multiple Cancers Allowing a New Strategy for Screening, Early Detection and Monitoring. J. Glob. Oncol. 2018, 4 (Suppl. 2), 211s. [Google Scholar] [CrossRef]
- Dupuy, M.; Iltache, S.; Rivière, B.; Prieur, A.; Pageaux, G.P.; Bedoya, J.U.; Assenat, E. Plasma hPG(80) (Circulating Progastrin) as a Novel Prognostic Biomarker for Hepatocellular Carcinoma. Cancers 2022, 14, 402. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.; Tan, W.; Vire, B.; Liaud, P.; Blairvacq, M.; Berthier, F.; Rouison, D.; Garnier, G.; Payen, L.; Cousin, T.; et al. Prognostic Value of Plasma hPG(80) (Circulating Progastrin) in Metastatic Renal Cell Carcinoma. Cancers 2021, 13, 375. [Google Scholar] [CrossRef]
- Prieur, A.; Harper, A.; Khan, M.; Vire, B.; Joubert, D.; Payen, L.; Kopciuk, K. Plasma hPG80 (Circulating Progastrin) as a Novel Prognostic Biomarker for early-stage breast cancer in a breast cancer cohort. BMC Cancer 2023, 23, 305. [Google Scholar] [CrossRef] [PubMed]
- Dumas, E.; Laot, L.; Coussy, F.; Grandal Rejo, B.; Daoud, E.; Laas, E.; Kassara, A.; Majdling, A.; Kabirian, R.; Jochum, F.; et al. The French Early Breast Cancer Cohort (FRESH): A Resource for Breast Cancer Research and Evaluations of Oncology Practices Based on the French National Healthcare System Database (SNDS). Cancers 2022, 14, 2671. [Google Scholar] [CrossRef]
- Caan, B.; Sternfeld, B.; Gunderson, E.; Coates, A.; Quesenberry, C.; Slattery, M.L. Life After Cancer Epidemiology (LACE) Study: A cohort of early stage breast cancer survivors (United States). Cancer Causes Control 2005, 16, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.L.; Crumley, D.; McTiernan, A.; Bernstein, L.; Baumgartner, R.; Gilliland, F.D.; Kriska, A.; Ballard-Barbash, R. Physical activity levels before and after a diagnosis of breast carcinoma: The Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer 2003, 97, 1746–1757. [Google Scholar] [CrossRef]
- Kwan, M.L.; Ambrosone, C.B.; Lee, M.M.; Barlow, J.; Krathwohl, S.E.; Ergas, I.J.; Ashley, C.H.; Bittner, J.R.; Darbinian, J.; Stronach, K.; et al. The Pathways Study: A prospective study of breast cancer survivorship within Kaiser Permanente Northern California. Cancer Causes Control. 2008, 19, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Summary Tables: Alberta’s Tomorrow Project 2022. Available online: https://myatpresearch.ca/data-summary/ (accessed on 25 November 2022).
- Friedenreich, C.M.; Vallance, J.K.; McNeely, M.L.; Culos-Reed, S.N.; Matthews, C.E.; Bell, G.J.; Mackey, J.R.; Kopciuk, K.A.; Dickau, L.; Wang, Q.; et al. The Alberta moving beyond breast cancer (AMBER) cohort study: Baseline description of the full cohort. Cancer Causes Control. 2022, 33, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.N.; Ng, C.; Ellison, L.F.; Seely, J.M. Breast cancer incidence and mortality, by age, stage and molecular subtypes, by race/ethnicity in Canada. Oncologist 2024, oyae283. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Demographic Estimates by Age and Gender, Provinces and Territories: Interactive Dashboard 2024. Available online: https://www150.statcan.gc.ca/n1/pub/71-607-x/71-607-x2020018-eng.htm (accessed on 9 December 2024).
- Copson, E.; Maishman, T.; Gerty, S.; Eccles, B.; Stanton, L.; Cutress, R.I.; Altman, D.G.; Durcan, L.; Simmonds, P.; Jones, L. Ethnicity and outcome of young breast cancer patients in the United Kingdom: The POSH study. Br. J. Cancer 2014, 110, 230–241. [Google Scholar] [CrossRef]
- Gathani, T.; Reeves, G.; Broggio, J.; Barnes, I. Ethnicity and the tumour characteristics of invasive breast cancer in over 116,500 women in England. Br. J. Cancer 2021, 125, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Robbins, A.S.; Lin, C.C.; Flanders, W.D.; DeSantis, C.E.; Ward, E.M.; Freedman, R.A. Factors That Contributed to Black-White Disparities in Survival Among Nonelderly Women With Breast Cancer Between 2004 and 2013. J. Clin. Oncol. 2018, 36, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, M.L.; White, M.C.; Wu, M.; Weir, H.K.; Romieu, I. Differences in breast cancer incidence among young women aged 20–49 years by stage and tumor characteristics, age, race, and ethnicity, 2004–2013. Breast Cancer Res. Treat. 2018, 169, 595–606. [Google Scholar] [CrossRef]
- Lofters, A.K.; McBride, M.L.; Li, D.; Whitehead, M.; Moineddin, R.; Jiang, L.; Grunfeld, E.; Groome, P.A.; CanIMPACT Team. Disparities in breast cancer diagnosis for immigrant women in Ontario and BC: Results from the CanIMPACT study. BMC Cancer 2019, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Duran, N.; Norris, K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am. J. Public. Health 2014, 104, e16–e31. [Google Scholar] [CrossRef] [PubMed]
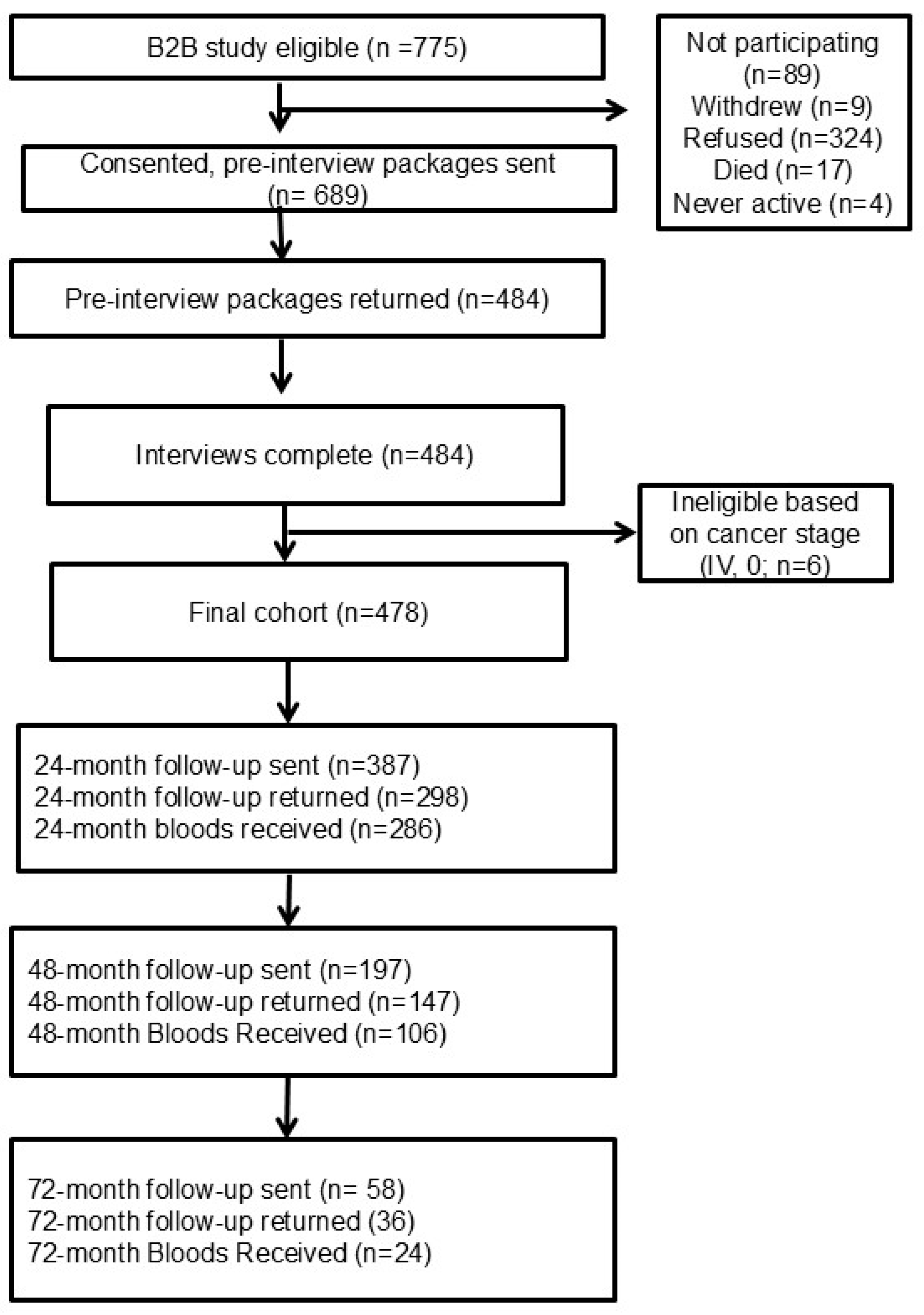
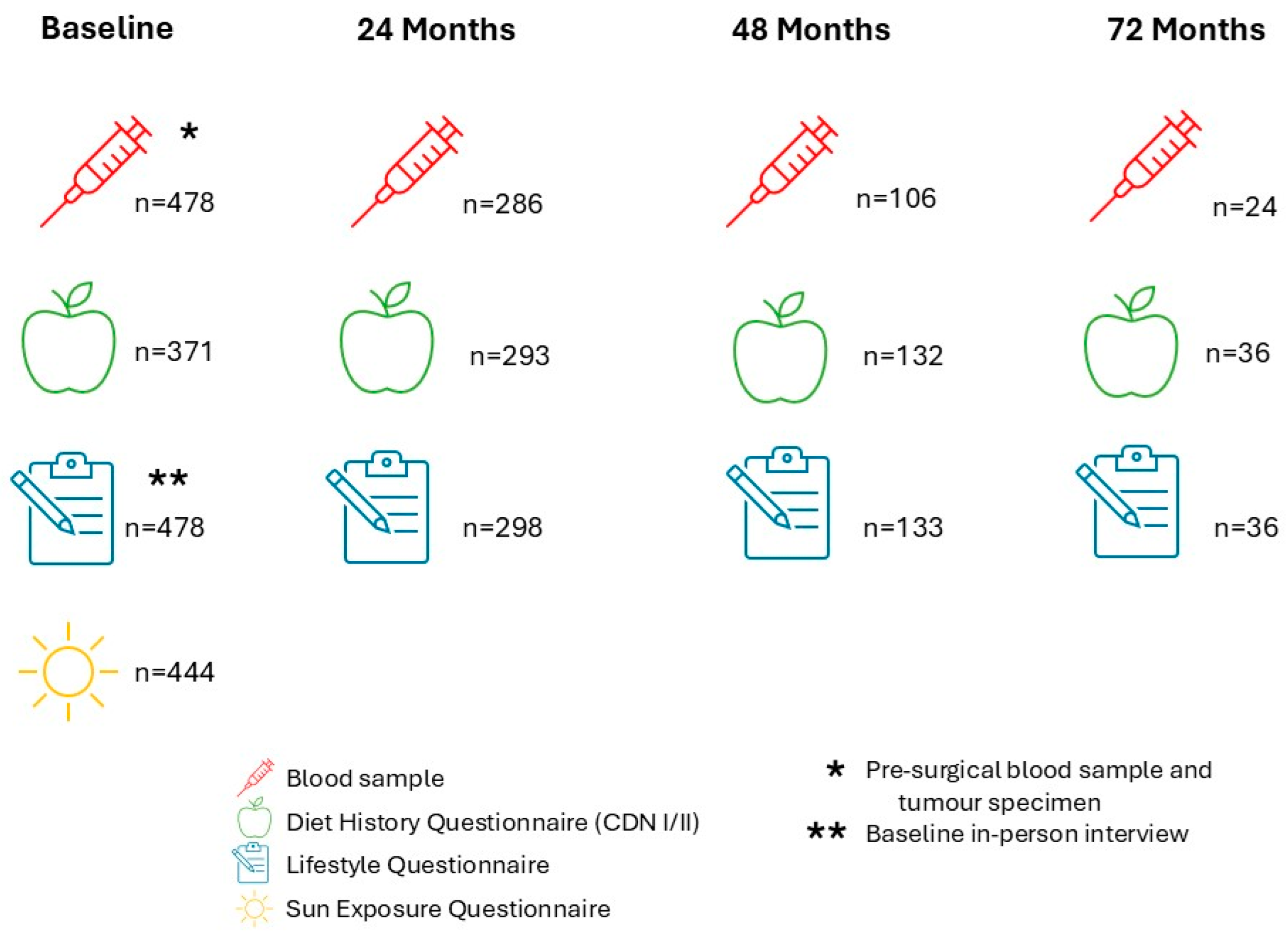
| Characteristic | N | Percentage (%) |
|---|---|---|
| Age at diagnosis (years) | ||
| <40 | 29 | 6.1 |
| 40–49 | 86 | 18.0 |
| 50–59 | 145 | 30.3 |
| 60–69 | 117 | 24.5 |
| >70 | 91 | 19.0 |
| Missing | 10 | 2.1 |
| Education | ||
| Less than high school | 19 | 4.0 |
| High school | 79 | 16.5 |
| Vocational or technical | 43 | 9.0 |
| Some college or university | 75 | 15.7 |
| University undergraduate degree or college diploma | 188 | 39.3 |
| University graduate degree | 41 | 8.6 |
| Missing | 33 | 6.9 |
| Marital Status | ||
| Married/Common Law | 326 | 68.2 |
| Widowed | 27 | 5.6 |
| Divorced/Separated | 56 | 11.7 |
| Other/Missing | 69 | 14.4 |
| Ethnicity | ||
| European | 261 | 54.6 |
| Aboriginal | 5 | 1.0 |
| Asian | 16 | 3.3 |
| South American | 6 | 1.3 |
| African | 1 | 0.2 |
| Missing | 189 | 39.5 |
| Income (CAD) | ||
| <50,000 | 58 | 12.1 |
| 50,000–100,000 | 146 | 30.5 |
| 100,000–200,000 | 126 | 26.4 |
| >200,000 | 69 | 14.4 |
| Missing | 79 | 16.5 |
| Characteristic | N | Percentage (%) |
|---|---|---|
| AJCC * 7 stage | ||
| I | 247 | 51.7 |
| II | 194 | 40.6 |
| III | 37 | 7.7 |
| Bloom–Richardson (BR) grade | ||
| Low | 70 | 14.6 |
| Medium | 197 | 41.2 |
| High | 194 | 40.6 |
| Unknown | 5 | 1.0 |
| Missing | 12 | 2.5 |
| Lymph nodes | ||
| N0 | 328 | 68.6 |
| N1mi | 115 | 24.1 |
| N1 | 19 | 4.0 |
| N2 | 1 | 0.2 |
| N3a | 2 | 0.4 |
| N3c | 1 | 0.2 |
| Missing | 12 | 2.5 |
| HER2 receptor | ||
| Positive | 84 | 17.6 |
| Negative | 372 | 77.8 |
| Indeterminate | 4 | 0.8 |
| Not done | 5 | 1.0 |
| Missing | 13 | 2.7 |
| Estrogen receptor | ||
| Positive | 408 | 85.4 |
| Negative | 56 | 11.7 |
| Not done | 2 | 0.4 |
| Missing | 12 | 2.5 |
| Progesterone receptor | ||
| Positive | 377 | 78.9 |
| Negative | 86 | 18.0 |
| Not done | 3 | 0.6 |
| Missing | 12 | 2.5 |
| Characteristic | N | Percentage (%) |
|---|---|---|
| Smoking Status | ||
| Occasional smoker | 7 | 1.5 |
| Ex-occasional smoker | 14 | 2.9 |
| Current smoker | 34 | 7.1 |
| Ex-smoker | 147 | 30.8 |
| Missing | 276 | 57.7 |
| Ever had 6 drinks of beer, wine or liquor in any given year? | ||
| No | 60 | 12.6 |
| Yes | 384 | 80.3 |
| Missing | 34 | 7.1 |
| Family history of breast cancer | ||
| No | 349 | 73.0 |
| Yes | 95 | 19.9 |
| Missing | 34 | 7.1 |
| Number of pregnancies | ||
| 0 | 65 | 13.6 |
| 1 | 51 | 10.7 |
| 2 | 143 | 29.9 |
| 3 | 102 | 21.3 |
| 4 | 48 | 10.0 |
| >4 | 36 | 7.5 |
| Missing | 33 | 6.9 |
| Menopausal status | ||
| pre-menopausal | 116 | 24.3 |
| post-menopausal | 329 | 68.9 |
| missing | 33 | 6.9 |
| Both ovaries removed | ||
| No | 416 | 87.0 |
| Yes | 29 | 6.1 |
| Missing | 33 | 6.9 |
| Hysterectomy | ||
| No | 217 | 45.4 |
| Yes | 95 | 19.9 |
| Missing | 166 | 34.7 |
| Birth control used a | ||
| No | 70 | 14.6 |
| Yes | 375 | 78.5 |
| Missing | 33 | 6.9 |
| Hours sitting at work b | ||
| 0 | 156 | 32.6 |
| 0.1–2 | 77 | 16.1 |
| 2.1–6 | 133 | 27.8 |
| >6 | 78 | 16.3 |
| Missing | 34 | 7.1 |
| Hours sleeping c | ||
| <5 | 50 | 10.5 |
| 5–7 | 222 | 46.4 |
| >7 | 140 | 29.3 |
| Missing | 66 | 13.8 |
| Ever used a sun lamp, or gone to a tanning salon or solarium | ||
| No | 297 | 62.13 |
| Yes | 147 | 30.75 |
| Missing | 34 | 7.11 |
| BMI category | ||
| Underweight (BMI < 18.5) | 10 | 2.1 |
| Normal (18.5 ≤ BMI < 25) | 158 | 33.1 |
| Overweight (25 ≤ BMI < 30) | 144 | 30.1 |
| Obese class I (30 ≤ BMI < 35) | 67 | 14.0 |
| Obese class I (30 ≤ BMI < 35) | 30 | 6.3 |
| Obese class III (BMI ≥ 40) | 23 | 4.8 |
| Missing | 46 | 9.6 |
| Waist-to-hip ratio category (cm) | ||
| <0.8 | 95 | 19.9 |
| 0.8 to 0.85 | 100 | 20.9 |
| >0.85 | 236 | 49.4 |
| Missing | 47 | 9.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brockton, N.T.; Cook, L.S.; Magliocco, A.M.; Shemanko, C.S.; Vogel, H.J.; Khan, M.; Kopciuk, K.A. The Breast to Bone (B2B) Cohort Study to Prevent, Detect and Improve Treatment of Metastatic Disease: Baseline Assessment, Description and Progress. Int. J. Environ. Res. Public Health 2025, 22, 242. https://doi.org/10.3390/ijerph22020242
Brockton NT, Cook LS, Magliocco AM, Shemanko CS, Vogel HJ, Khan M, Kopciuk KA. The Breast to Bone (B2B) Cohort Study to Prevent, Detect and Improve Treatment of Metastatic Disease: Baseline Assessment, Description and Progress. International Journal of Environmental Research and Public Health. 2025; 22(2):242. https://doi.org/10.3390/ijerph22020242
Chicago/Turabian StyleBrockton, Nigel T., Linda S. Cook, Anthony M. Magliocco, Carrie S. Shemanko, Hans J. Vogel, Momtafin Khan, and Karen A. Kopciuk. 2025. "The Breast to Bone (B2B) Cohort Study to Prevent, Detect and Improve Treatment of Metastatic Disease: Baseline Assessment, Description and Progress" International Journal of Environmental Research and Public Health 22, no. 2: 242. https://doi.org/10.3390/ijerph22020242
APA StyleBrockton, N. T., Cook, L. S., Magliocco, A. M., Shemanko, C. S., Vogel, H. J., Khan, M., & Kopciuk, K. A. (2025). The Breast to Bone (B2B) Cohort Study to Prevent, Detect and Improve Treatment of Metastatic Disease: Baseline Assessment, Description and Progress. International Journal of Environmental Research and Public Health, 22(2), 242. https://doi.org/10.3390/ijerph22020242







