Atrial Fibrillation as a Geriatric Syndrome: Why Are Frailty and Disability Often Confused? A Geriatric Perspective from the New Guidelines
Abstract
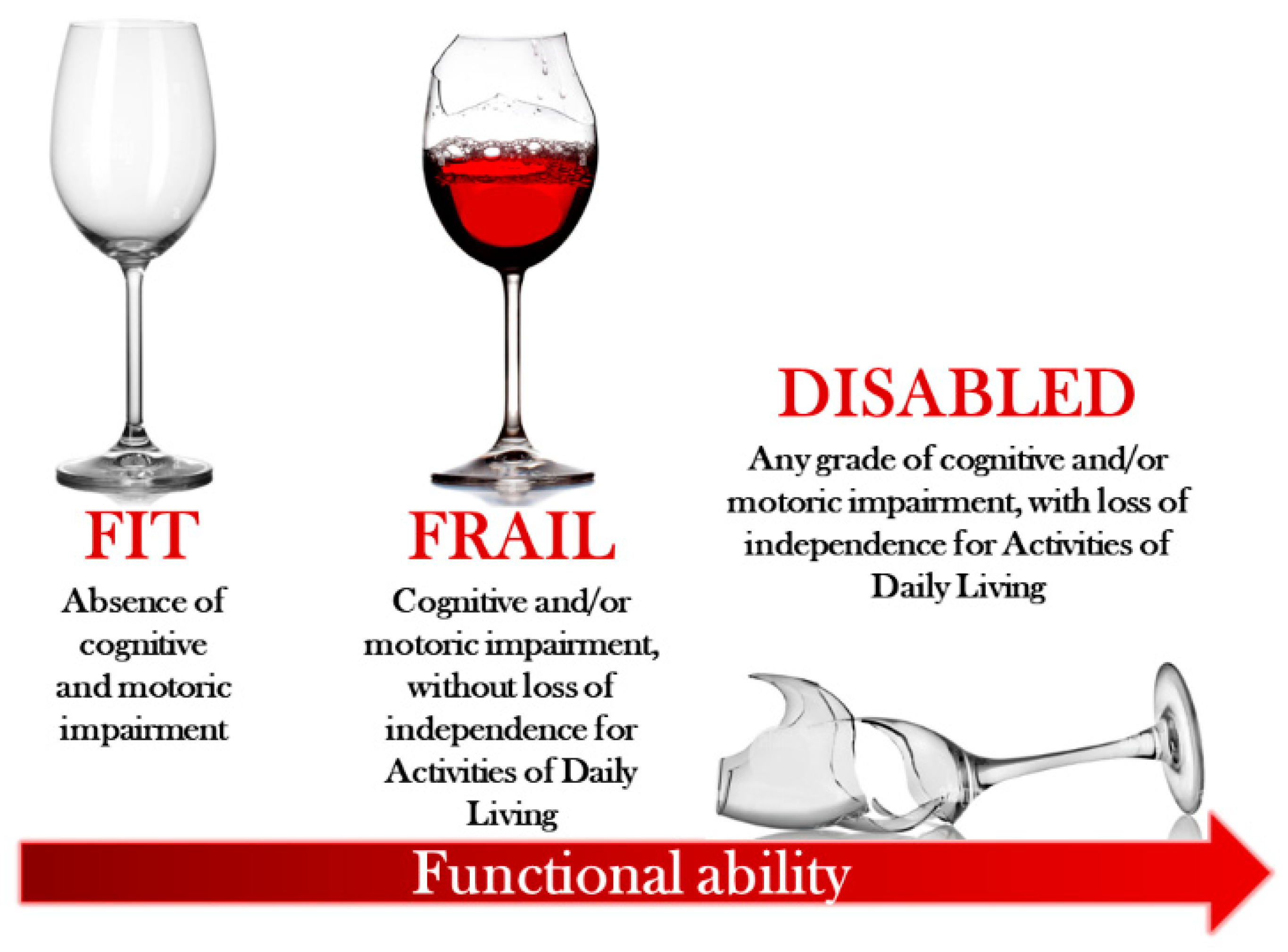
1. Introduction
- C: Comorbidity and risk factor management;
- A: Avoid stroke and thromboembolism;
- R: Reduce symptoms by rate and rhythm control;
- E: Evaluation and dynamic reassessment.
1.1. Comorbidity and Risk Factor Management
1.2. Avoid Stroke and Thromboembolism
1.3. Reduce Symptoms by Rate and Rhythm Control
1.4. Evaluation and Dynamic Reassessment
- Improved Patient Selection: by distinguishing between frailty and disability, these tools help identify patients who may benefit most from interventions, avoiding the risks of overtreatment or undertreatment [43].
- Enhanced Predictive Accuracy: multidimensional assessments provide a clearer picture of treatment risks and benefits, improving the reliability of trial outcomes.
- Alignment with Real-World Practice: these tools mirror the complexity of clinical settings, making trial findings more applicable to everyday patient care.
2. Benefits of Collaborative Models in Managing Frailty and Multimorbidity
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Inouye, S.K.; Studenski, S.; Tinetti, M.E.; Kuchel, G.A. Geriatric syndromes: Clinical, research, and policy implications of a core geriatric concept. J. Am. Geriatr. Soc. 2007, 55, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Maher, R.L.; Hanlon, J.; Hajjar, E.R. Clinical consequences of polypharmacy in elderly. Expert. Opin. Drug. Saf. 2014, 13, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Ferrucci, L.; Darer, J.; Williamson, J.D.; Anderson, G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E.; Kumar, C. The patient who falls: “It’s always a trade-off”. JAMA 2010, 303, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; He, J.; Han, Y.; Han, S.; Li, P.; Liao, H.; Guo, J. Global burden of atrial fibrillation/atrial flutter and its attributable risk factors from 1990 to 2021. Europace 2024, 26, euae195. [Google Scholar] [CrossRef]
- Proietti, M.; Romiti, G.F.; Raparelli, V.; Diemberger, I.; Boriani, G.; Dalla Vecchia, L.A.; Bellelli, G.; Marzetti, E.; Lip, G.Y.; Cesari, M. Frailty prevalence and impact on outcomes in patients with atrial fibrillation: A systematic review and meta-analysis of 1,187,000 patients. Ageing Res. Rev. 2022, 79, 101652. [Google Scholar] [CrossRef]
- Hire, A.J.; Franklin, B.D. Potentially inappropriate prescribing (PIP) in older people and its association with socioeconomic deprivation-a systematic review and narrative synthesis. BMC Geriatr. 2024, 24, 651. [Google Scholar] [CrossRef]
- Kotecha, D.; Ahmed, A.; Calvert, M.; Lencioni, M.; Terwee, C.B.; Lane, D.A. Patient-Reported Outcomes for Quality of Life Assessment in Atrial Fibrillation: A Systematic Review of Measurement Properties. PLoS ONE 2016, 11, e0165790. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar]
- Kim, D.H.; Rockwood, K. Frailty in Older Adults. N. Engl. J. Med. 2024, 391, 538–548. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pazan, F.; Wehling, M. Polypharmacy in older adults: A narrative review of definitions, epidemiology and consequences. Eur. Geriatr. Med. 2021, 12, 443–452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davies, L.E.; Spiers, G.; Kingston, A.; Todd, A.; Adamson, J.; Hanratty, B. Adverse Outcomes of Polypharmacy in Older People: Systematic Review of Reviews. J. Am. Med. Dir. Assoc. 2020, 21, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Adam, L.; Moutzouri, E.; Baumgartner, C.; Loewe, A.L.; Feller, M.; M’Rabet-Bensalah, K.; Schwab, N.; Hossmann, S.; Schneider, C.; Jegerlehner, S.; et al. Rationale and design of OPtimising thERapy to prevent Avoidable hospital admissions in Multimorbid older people (OPERAM): A cluster randomised controlled trial. BMJ Open 2019, 9, e026769. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samuel, M.J. By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2023, 71, 2052–2081. [Google Scholar] [CrossRef] [PubMed]
- Sallevelt, B.T.G.M.; Huibers, C.J.A.; Heij, J.M.J.O.; Egberts, T.C.G.; van Puijenbroek, E.P.; Shen, Z.; Spruit, M.R.; Jungo, K.T.; Rodondi, N.; Dalleur, O.; et al. Frequency and Acceptance of Clinical Decision Support System-Generated STOPP/START Signals for Hospitalised Older Patients with Polypharmacy and Multimorbidity. Drugs Aging 2022, 39, 59–73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.; Lee, E.; Jang, I.Y. Frailty and Comprehensive Geriatric Assessment. J. Korean Med. Sci. 2020, 35, e16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, J.; Zhou, Y.; Cai, Z. Outcome of novel oral anticoagulant versus warfarin in frail elderly patients with atrial fibrillation: A systematic review and meta-analysis of retrospective studies. Acta Clin. Belg. 2023, 78, 367–377. [Google Scholar] [CrossRef]
- Mitchell, A.; Watson, M.C.; Welsh, T.; McGrogan, A. Effectiveness and Safety of Direct Oral Anticoagulants versus Vitamin K Antagonists for People Aged 75 Years and over with Atrial Fibrillation: A Systematic Review and Meta-Analyses of Observational Studies. J. Clin. Med. 2019, 8, 554. [Google Scholar] [CrossRef]
- Pilotto, A.; Veronese, N.; Polidori, M.C.; Strandberg, T.; Topinkova, E.; Cruz-Jentoft, A.J.; Custodero, C.; Barbagallo, M.; Maggi, S.; EUROSAF Study Investigators. Frailty and anticoagulants in older subjects with atrial fibrillation: The EUROSAF study. Age Ageing 2023, 52, afad216. [Google Scholar] [CrossRef]
- Joosten, L.P.T.; van Doorn, S.; van de Ven, P.M.; Köhlen, B.T.G.; Nierman, M.C.; Koek, H.L.; Hemels, M.E.W.; Huisman, M.V.; Kruip, M.; Faber, L.M.; et al. Safety of Switching From a Vitamin K Antagonist to a Non-Vitamin K Antagonist Oral Anticoagulant in Frail Older Patients With Atrial Fibrillation: Results of the FRAIL-AF Randomized Controlled Trial. Circulation 2024, 149, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Sato, N. Global frailty screening tools: Review and application of frailty screening tools from 2001 to 2023. Intractable Rare Dis. Res. 2024, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mandt, S.R.; Thadathil, N.; Klem, C.; Russ, C.; McNamee, P.L.; Stigge, K.; Cheng, D. Apixaban Use in Patients with Kidney Impairment: A Review of Pharmacokinetic, Interventional, and Observational Study Data. Am. J. Cardiovasc. Drugs 2024, 24, 603–624. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.T.; Giugliano, R.P.; Ruff, C.T.; Koretsune, Y.; Yamashita, T.; Kiss, R.G.; Nordio, F.; Murphy, S.A.; Kimura, T.; Jin, J.; et al. Efficacy and Safety of Edoxaban in Elderly Patients with Atrial Fibrillation in the ENGAGE AF-TIMI 48 Trial. J. Am. Heart Assoc. 2016, 5, e003432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schäfer, A.; Flierl, U.; Berliner, D.; Bauersachs, J. Anticoagulants for Stroke Prevention in Atrial Fibrillation in Elderly Patients. Cardiovasc. Drugs Ther. 2020, 34, 555–568. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okumura, K.; Akao, M.; Yoshida, T.; Kawata, M.; Okazaki, O.; Akashi, S.; Eshima, K.; Tanizawa, K.; Fukuzawa, M.; Hayashi, T.; et al. Low-Dose Edoxaban in Very Elderly Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.I.; Benyaminov, R.; Rahman, M.; Ngu, D.; Reinhardt, M. Frailty: A Multidimensional Biopsychosocial Syndrome. Med. Clin. N. Am. 2023, 107, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Alobaida, M.; Alrumayh, A. Rate control strategies for atrial fibrillation. Ann. Med. 2021, 53, 682–692. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mariani, M.V.; Pierucci, N.; Piro, A.; Trivigno, S.; Chimenti, C.; Galardo, G.; Miraldi, F.; Vizza, C.D. Incidence and Determinants of Spontaneous Cardioversion of Early Onset Symptomatic Atrial Fibrillation. Medicina 2022, 58, 1513. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Şaylık, F.; Çınar, T.; Akbulut, T.; Hayıroğlu, M.İ. Comparison of catheter ablation and medical therapy for atrial fibrillation in heart failure patients: A meta-analysis of randomized controlled trials. Heart Lung 2023, 57, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Nesapiragasan, V.; Hayıroğlu, M.İ.; Sciacca, V.; Sommer, P.; Sohns, C.; Fink, T. Catheter Ablation Approaches for the Treatment of Arrhythmia Recurrence in Patients with a Durable Pulmonary Vein Isolation. Balkan Med. J. 2023, 40, 386–394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Woodford, H.J.; McKenzie, D.; Pollock, L.M. Appropriate management of heart failure in older people with frailty. BMJ 2024, 387, e078188. [Google Scholar] [CrossRef] [PubMed]
- Rosas Diaz, A.N.; Troy, A.L.; Kaplinskiy, V.; Pritchard, A.; Vani, R.; Ko, D.; Orkaby, A.R. Assessment and Management of Atrial Fibrillation in Older Adults with Frailty. Geriatrics 2024, 9, 50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masoudi, F.A.; Havranek, E.P.; Wolfe, P.; Gross, C.P.; Rathore, S.S.; Steiner, J.F.; Ordin, D.L.; Krumholz, H.M. Most hospitalized older persons do not meet the enrollment criteria for clinical trials in heart failure. Am. Heart J. 2003, 146, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, A.; Oristrell, J.; Pla, X.; Ruggiero, C.; Ferretti, R.; Diestre, G.; Clarfield, A.M.; Crome, P.; Hertogh, C.; Lesauskaite, V.; et al. The persistent exclusion of older patients from ongoing clinical trials regarding heart failure. Arch. Intern. Med. 2011, 171, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Ofori-Asenso, R.; Chin, K.L.; Mazidi, M.; Zomer, E.; Ilomaki, J.; Zullo, A.R.; Gasevic, D.; Ademi, Z.; Korhonen, M.J.; LoGiudice, D.; et al. Global Incidence of Frailty and Prefrailty Among Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e198398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giallauria, F.; Di Lorenzo, A.; Venturini, E.; Pacileo, M.; D’Andrea, A.; Garofalo, U.; De Lucia, F.; Testa, C.; Cuomo, G.; Iannuzzo, G.; et al. Frailty in Acute and Chronic Coronary Syndrome Patients Entering Cardiac Rehabilitation. J. Clin. Med. 2021, 10, 1696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walston, J.; Buta, B.; Xue, Q.L. Frailty Screening and Interventions: Considerations for Clinical Practice. Clin. Geriatr. Med. 2018, 34, 25–38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pandey, A.; Kitzman, D.W.; Nelson, M.B.; Pastva, A.M.; Duncan, P.; Whellan, D.J.; Mentz, R.J.; Chen, H.; Upadhya, B.; Reeves, G.R. Frailty and Effects of a Multidomain Physical Rehabilitation Intervention Among Older Patients Hospitalized for Acute Heart Failure: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 167–176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lauretani, F.; Testa, C.; Salvi, M.; Zucchini, I.; Lorenzi, B.; Tagliaferri, S.; Cattabiani, C.; Maggio, M. Reward System Dysfunction and the Motoric-Cognitive Risk Syndrome in Older Persons. Biomedicines 2022, 10, 808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cuomo, G.; Di Lorenzo, A.; Tramontano, A.; Iannone, F.P.; D’Angelo, A.; Pezzella, R.; Testa, C.; Parlato, A.; Merone, P.; Pacileo, M.; et al. Exercise Training in Patients with Heart Failure: From Pathophysiology to Exercise Prescription. Rev. Cardiovasc. Med. 2022, 23, 144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zanforlini, B.M.; Sambo, S.; Devita, M.; Cignarella, A.; Vezzali, F.; Sturani, S.; Grandieri, A.; Noale, M.; Siviero, P.; Limongi, F.; et al. A multidisciplinary approach to improve adherence to medical recommendations in older adults at hospital discharge: The APPROACH study protocol. PLoS ONE 2024, 19, e0297238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gleason, K.T.; Wu, M.M.J.; Wec, A.; Powell, D.S.; Zhang, T.; Gamper, M.J.; Green, A.R.; Nothelle, S.; Amjad, H.; Wolff, J.L. Use of the patient portal among older adults with diagnosed dementia and their care partners. Alzheimer’s Dement. 2023, 19, 5663–5671. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giallauria, F.; Testa, C.; Cuomo, G.; Di Lorenzo, A.; Venturini, E.; Lauretani, F.; Maggio, M.G.; Iannuzzo, G.; Vigorito, C. Exercise Training in Elderly Cancer Patients: A Systematic Review. Cancers 2023, 15, 1671. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, Y.; Romiti, G.F.; Corica, B.; Proietti, M.; Bonini, N.; Zhang, H.; Lip, G.Y.; mAF-App II trial Investigators. Mobile health-technology integrated care in atrial fibrillation patients with heart failure: A report from the mAFA-II randomized clinical trial. Eur. J. Intern. Med. 2023, 107, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chu, M.; Shen, Y.; Zhang, S.; Yin, X.; Yang, S.; Lip, G.Y.H.; Chen, M.; MIRACLE-AF Trial Investigators. A Novel Model of Integrated Care of Older Patients with Atrial Fibrillation in Rural China. JACC Asia 2024, 4, 764–773. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Becker, C.; Zumbrunn, S.; Beck, K.; Vincent, A.; Loretz, N.; Müller, J.; Amacher, S.A.; Schaefert, R.; Hunziker, S. Interventions to Improve Communication at Hospital Discharge and Rates of Readmission: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2119346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testa, C.; Salvi, M.; Zucchini, I.; Cattabiani, C.; Giallauria, F.; Petraglia, L.; Leosco, D.; Lauretani, F.; Maggio, M. Atrial Fibrillation as a Geriatric Syndrome: Why Are Frailty and Disability Often Confused? A Geriatric Perspective from the New Guidelines. Int. J. Environ. Res. Public Health 2025, 22, 179. https://doi.org/10.3390/ijerph22020179
Testa C, Salvi M, Zucchini I, Cattabiani C, Giallauria F, Petraglia L, Leosco D, Lauretani F, Maggio M. Atrial Fibrillation as a Geriatric Syndrome: Why Are Frailty and Disability Often Confused? A Geriatric Perspective from the New Guidelines. International Journal of Environmental Research and Public Health. 2025; 22(2):179. https://doi.org/10.3390/ijerph22020179
Chicago/Turabian StyleTesta, Crescenzo, Marco Salvi, Irene Zucchini, Chiara Cattabiani, Francesco Giallauria, Laura Petraglia, Dario Leosco, Fulvio Lauretani, and Marcello Maggio. 2025. "Atrial Fibrillation as a Geriatric Syndrome: Why Are Frailty and Disability Often Confused? A Geriatric Perspective from the New Guidelines" International Journal of Environmental Research and Public Health 22, no. 2: 179. https://doi.org/10.3390/ijerph22020179
APA StyleTesta, C., Salvi, M., Zucchini, I., Cattabiani, C., Giallauria, F., Petraglia, L., Leosco, D., Lauretani, F., & Maggio, M. (2025). Atrial Fibrillation as a Geriatric Syndrome: Why Are Frailty and Disability Often Confused? A Geriatric Perspective from the New Guidelines. International Journal of Environmental Research and Public Health, 22(2), 179. https://doi.org/10.3390/ijerph22020179







