Optimized Aluminum Hydroxide Adsorption–Precipitation for Improved Viral Detection in Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Concentration Methods
2.2. RNA Extraction and Analysis
2.3. Statistical Analysis
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daughton, C.G. Wastewater Surveillance for Population-Wide COVID-19: The Present and Future. Sci. Total Environ. 2020, 736, 139631. [Google Scholar] [CrossRef] [PubMed]
- Naughton, C.C.; Roman, F.A.; Alvarado, A.G.F.; Tariqi, A.Q.; Deeming, M.A.; Kadonsky, K.F.; Bibby, K.; Bivins, A.; Medema, G.; Ahmed, W.; et al. Show Us the Data: Global COVID-19 Wastewater Monitoring Efforts, Equity, and Gaps. FEMS Microbes 2023, 4, xtad003. [Google Scholar] [CrossRef] [PubMed]
- Pecson, B.; Gerrity, D.; Bibby, K.; Drewes, J.E.; Gerba, C.; Gersberg, R.; Gonzalez, R.; Haas, C.N.; Hamilton, K.A.; Nelson, K.L.; et al. Editorial Perspectives: Will SARS-CoV-2 Reset Public Health Requirements in the Water Industry? Integrating Lessons of the Past and Emerging Research. Environ. Sci. Water Res. Technol. 2020, 6, 1761–1764. [Google Scholar] [CrossRef]
- Daughton, C. The International Imperative to Rapidly and Inexpensively Monitor Community-Wide COVID-19 Infection Status and Trends. Sci. Total Environ. 2020, 726, 138149. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Lor, E.; Rousis, N.I.; Herna, F.; Zuccato, E.; Castiglioni, S. Wastewater-Based Epidemiology as a Novel Biomonitoring Tool to Evaluate Human Exposure To Pollutants. Environ. Sci. Technol. 2018, 52, 10224–10226. [Google Scholar] [CrossRef] [PubMed]
- Mousazadeh, M.; Ashoori, R.; Paital, B.; Kabdaşlı, I.; Frontistis, Z.; Hashemi, M.; Sandoval, M.A.; Sherchan, S.; Das, K.; Emamjomeh, M.M. Wastewater Based Epidemiology Perspective as a Faster Protocol for Detecting Coronavirus RNA in Human Populations: A Review with Specific Reference to SARS-CoV-2 Virus. Pathogens 2021, 10, 1008. [Google Scholar] [CrossRef]
- Hewitt, J.; Leonard, M.; Greening, G.E.; Lewis, G.D. Influence of Wastewater Treatment Process and the Population Size on Human Virus Profiles in Wastewater. Water Res. 2011, 45, 6267–6276. [Google Scholar] [CrossRef]
- Montazeri, N.; Goettert, D.; Achberger, E.C.; Johnson, C.N.; Prinyawiwatkul, W.; Janes, M.E. Pathogenic Enteric Viruses and Microbial Indicators during Secondary Treatment of Municipal Wastewater. Appl. Environ. Microbiol. 2015, 81, 6436. [Google Scholar] [CrossRef]
- Deshpande, J.M.; Shetty, S.J.; Siddiqui, Z.A. Environmental Surveillance System To Track Wild Poliovirus Transmission. Appl. Environ. Microbiol. 2003, 69, 2919–2927. [Google Scholar] [CrossRef] [PubMed]
- Hovi, T. Surveillance for Polioviruses. Biologicals 2006, 34, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Lago, P.M.; Gary, H.E., Jr.; Pérez, L.S.; Cáceres, V.; Olivera, J.B.; Puentes, R.P.; Corredor, M.B.; Jímenez, P.; Pallansch, M.A.; Cruz, R.G. Poliovirus Detection in Wastewater and Stools Following an Immunization Campaign in Havana, Cuba. Int. J. Epidemiol. 2003, 32, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Shulman, L.M.; Manor, Y.; Sofer, D.; Handsher, R.; Swartz, T.; Delpeyroux, F.; Mendelson, E. Neurovirulent Vaccine-Derived Polioviruses in Sewage from Highly Immune Populations. PLoS ONE 2006, 1, e69. [Google Scholar] [CrossRef] [PubMed]
- Vinjé, J.; Gregoricus, N.; Martin, J.; Gary, H.E., Jr.; Cáceres, V.M.; Vencze, L.; Macadam, A.; Dobbins, J.G.; Burns, C.; Wait, D.; et al. Isolation and Characterization of Circulating Type 1 Vaccine-Derived Poliovirus from Sewage and Stream Waters in Hispaniola. J. Infect. Dis. 2004, 189, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Carducci, A.; Verani, M.; Battistini, R.; Pizzi, F.; Rovini, E.; Andreoli, E.; Casini, B. Epidemiological Surveillance of Human Enteric Viruses by Monitoring of Different Environmental Matrices. Water Sci. Technol. 2006, 54, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Prevost, B.; Lucas, F.S.; Goncalves, A.; Richard, F.; Moulin, L.; Wurtzer, S. Large Scale Survey of Enteric Viruses in River and Waste Water Underlines the Health Status of the Local Population. Environ. Int. 2015, 79, 42–50. [Google Scholar] [CrossRef]
- Hellmér, M.; Paxéus, N.; Magnius, L.; Enache, L.; Arnholm, B.; Johansson, A.; Bergström, T.; Norder, H. Detection of Pathogenic Viruses in Sewage Provided Early Warnings of Hepatitis A Virus and Norovirus Outbreaks. Appl. Environ. Microbiol. 2014, 80, 6771–6781. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Della Libera, S.; Iaconelli, M.; Ciccaglione, A.R.; Bruni, R.; Taffon, S.; Equestre, M.; Alfonsi, V.; Rizzo, C.; Tosti, M.E.; et al. Surveillance of Hepatitis A Virus in Urban Sewages and Comparison with Cases Notified in the Course of an Outbreak, Italy 2013. BMC Infect. Dis. 2014, 14, 419. [Google Scholar] [CrossRef] [PubMed]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-nCoV and Naming It SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, A.; Li, H.; Zheng, K.; Zhuang, Z.; Chen, Z.; Shi, Y.; Zhang, Z.; Chen, S.; Liu, X.; et al. Isolation of Infectious SARS-CoV-2 from Urine of a COVID-19 Patient. Emerg. Microbes Infect. 2020, 9, 991–993. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged Presence of SARS-CoV-2 Viral RNA in Faecal Samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Roivainen, M.; Blomqvist, S.; al-Hello, H.; Paananen, A.; Delpeyroux, F.; Kuusi, M.; Hovi, T. Highly Divergent Neurovirulent Vaccine-Derived Polioviruses of All Three Serotypes Are Recurrently Detected in Finnish Sewage. Eurosurveillance 2010, 15, 19566. [Google Scholar] [CrossRef] [PubMed]
- Carmo dos Santos, M.; Cerqueira Silva, A.C.; dos Reis Teixeira, C.; Pinheiro Macedo Prazeres, F.; Fernandes dos Santos, R.; de Araújo Rolo, C.; de Souza Santos, E.; Santos da Fonseca, M.; Oliveira Valente, C.; Saraiva Hodel, K.V.; et al. Wastewater Surveillance for Viral Pathogens: A Tool for Public Health. Heliyon 2024, 10, e33873. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.M.; Tscharke, B.J.; Donner, E.; O’Brien, J.W.; Grant, S.C.; Kaserzon, S.L.; Mackie, R.; O’Malley, E.; Crosbie, N.D.; Thomas, K.V.; et al. Wastewater-Based Epidemiology Biomarkers: Past, Present and Future. TrAC Trends Anal. Chem. 2018, 105, 453–469. [Google Scholar] [CrossRef]
- Sharkey, M.E.; Kumar, N.; Mantero, A.M.A.; Babler, K.M.; Boone, M.M.; Cardentey, Y.; Cortizas, E.M.; Grills, G.S.; Herrin, J.; Kemper, J.M.; et al. Lessons Learned from SARS-CoV-2 Measurements in Wastewater. Sci. Total Environ. 2021, 798, 149177. [Google Scholar] [CrossRef] [PubMed]
- LaTurner, Z.W.; Zong, D.M.; Kalvapalle, P.; Gamas, K.R.; Terwilliger, A.; Crosby, T.; Ali, P.; Avadhanula, V.; Santos, H.H.; Weesner, K.; et al. Evaluating Recovery, Cost, and Throughput of Different Concentration Methods for SARS-CoV-2 Wastewater-Based Epidemiology. Water Res. 2021, 197, 117043. [Google Scholar] [CrossRef]
- Olesen, S.W.; Imakaev, M.; Duvallet, C. Making Waves: Defining the Lead Time of Wastewater-Based Epidemiology for COVID-19. Water Res. 2021, 202, 117433. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, B.W.; Innes, G.K.; Prasek, S.M.; Betancourt, W.Q.; Stark, E.R.; Foster, A.R.; Abraham, A.G.; Gerba, C.P.; Pepper, I.L. Enumerating Asymptomatic COVID-19 Cases and Estimating SARS-CoV-2 Fecal Shedding Rates via Wastewater-Based Epidemiology. Sci. Total Environ. 2021, 801, 149794. [Google Scholar] [CrossRef]
- Ahmed, W.; Bivins, A.; Simpson, S.L.; Smith, W.J.M.; Metcalfe, S.; McMinn, B.; Symonds, E.M.; Korajkic, A. Comparative Analysis of Rapid Concentration Methods for the Recovery of SARS-CoV-2 and Quantification of Human Enteric Viruses and a Sewage-Associated Marker Gene in Untreated Wastewater. Sci. Total Environ. 2021, 799, 149386. [Google Scholar] [CrossRef]
- Kitajima, M.; Ahmed, W.; Bibby, K.; Carducci, A.; Gerba, C.P.; Hamilton, K.A.; Haramoto, E.; Rose, J.B. SARS-CoV-2 in Wastewater: State of the Knowledge and Research Needs. Sci. Total Environ. 2020, 739, 139076. [Google Scholar] [CrossRef]
- Salvo, M.; Moller, A.; Alvareda, E.; Gamazo, P.; Colina, R.; Victoria, M. Evaluation of Low-Cost Viral Concentration Methods in Wastewaters: Implications for SARS-CoV-2 Pandemic Surveillances. J. Virol. Methods 2021, 297, 114249. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ellenberg, R.M.; Graham, K.E.; Wigginton, K.R. Survivability, Partitioning, and Recovery of Enveloped Viruses in Untreated Municipal Wastewater. Environ. Sci. Technol. 2016, 50, 5077–5085. [Google Scholar] [CrossRef]
- Zhang, D.; Duran, S.S.F.; Lim, W.Y.S.; Tan, C.K.I.; Cheong, W.C.D.; Suwardi, A.; Loh, X.J. SARS-CoV-2 in Wastewater: From Detection to Evaluation. Mater. Today Adv. 2022, 13, 100211. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Harwood, V.J.; Gyawali, P.; Sidhu, J.P.S.; Toze, S. Comparison of Concentration Methods for Quantitative Detection of Sewage-Associated Viral Markers in Environmental Waters. Appl. Environ. Microbiol. 2015, 81, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Carducci, A.; Federigi, I.; Dasheng, L.; Julian, R.T.; Marco, V. Making Waves: Coronavirus Detection, Presence and Persistence in the Water Environment: State of the Art and Knowledge Needs for Public Health. Water Res. 2020, 179, 115907. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Robinson, J.; Chong, M.F. A Review on Application of Flocculants in Wastewater Treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Haramoto, E.; Katayama, H.; Ohgaki, S. Detection of Noroviruses in Tap Water in Japan by Means of a New Method for Concentrating Enteric Viruses in Large Volumes of Freshwater. Appl. Environ. Microbiol. 2004, 70, 2154–2160. [Google Scholar] [CrossRef]
- John, D.E.; Rose, J.B. Review of Factors Affecting Microbial Survival in Groundwater. Environ. Sci. Technol. 2005, 39, 7345–7356. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, W.; Truchado, P.; Cuevas-Ferrando, E.; Simón, P.; Allende, A.; Sánchez, G. SARS-CoV-2 RNA in Wastewater Anticipated COVID-19 Occurrence in a Low Prevalence Area. Water Res. 2020, 181, 115942. [Google Scholar] [CrossRef]
- Ahmed, W.; Bivins, A.; Bertsch, P.M.; Bibby, K.; Choi, P.M.; Farkas, K.; Gyawali, P.; Hamilton, K.A.; Haramoto, E.; Kitajima, M.; et al. Surveillance of SARS-CoV-2 RNA in Wastewater: Methods Optimisation and Quality Control Are Crucial for Generating Reliable Public Health Information. Curr. Opin. Environ. Sci. Health 2020, 17, 100209. [Google Scholar] [CrossRef] [PubMed]
- Schrader, C.; Schielke, A.; Ellerbroek, L.; Johne, R. PCR Inhibitors—Occurrence, Properties and Removal. J. Appl. Microbiol. 2012, 113, 1014–1026. [Google Scholar] [CrossRef]
- Pepper, I.L.; Gerba, C.P. Chapter 8—Environmental Sample Collection and Processing. In Environmental Microbiology, 3rd ed.; Pepper, I.L., Gerba, C.P., Gentry, T.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 157–175. ISBN 978-0-12-394626-3. [Google Scholar]
- ASTM D449-19; Standard Practice for Recovery of Viruses From Wastewater Sludges. ASTM: West Conshohocken, PA, USA, 2019.
- Xagoraraki, I.; Yin, Z.; Svambayev, Z. Fate of Viruses in Water Systems. J. Environ. Eng. 2014, 140, 04014020. [Google Scholar] [CrossRef]
- Randazzo, W.; Piqueras, J.; Evtoski, Z.; Sastre, G.; Sancho, R.; Gonzalez, C.; Sánchez, G. Interlaboratory Comparative Study to Detect Potentially Infectious Human Enteric Viruses in Influent and Effluent Waters. Food Environ. Virol. 2019, 11, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Section 9510 D. Virus Concentration by Aluminum Hydroxide Adsorption-Precipitation, Chapter Detection of Enteric Viruses. In Standard Methods for the Examination of Water and Wastewater; American Water Works Association: Denver, CO, USA, 2018. [Google Scholar]
- Verreault, D.; Marcoux-Voiselle, M.; Turgeon, N.; Moineau, S.; Duchaine, C.; Schaffner, D.W. Resistance of Aerosolized Bacterial Viruses to Relative Humidity and Temperature. Appl. Environ. Microbiol. 2015, 81, 7305–7311. [Google Scholar] [CrossRef] [PubMed]
- Gendron, L.; Verreault, D.; Veillette, M.; Moineau, S.; Duchaine, C. Evaluation of Filters for the Sampling and Quantification of RNA Phage Aerosols. Aerosol Sci. Technol. 2010, 44, 893–901. [Google Scholar] [CrossRef]
- Haramoto, E.; Kitajima, M.; Kishida, N.; Konno, Y.; Katayama, H.; Asami, M.; Akiba, M. Occurrence of Pepper Mild Mottle Virus in Drinking Water Sources in Japan. Appl. Environ. Microbiol. 2013, 79, 7413–7418. [Google Scholar] [CrossRef] [PubMed]
- Maksimovic Carvalho Ferreira, O.; Lengar, Ž.; Kogej, Z.; Bačnik, K.; Bajde, I.; Milavec, M.; Županič, A.; Mehle, N.; Kutnjak, D.; Ravnikar, M.; et al. Evaluation of Methods and Processes for Robust Monitoring of SARS-CoV-2 in Wastewater. Food Environ. Virol. 2022, 14, 384–400. [Google Scholar] [CrossRef]
- Isaksson, F.; Lundy, L.; Hedström, A.; Székely, A.J.; Mohamed, N. Evaluating the Use of Alternative Normalization Approaches on SARS-CoV-2 Concentrations in Wastewater: Experiences from Two Catchments in Northern Sweden. Environments 2022, 9, 39. [Google Scholar] [CrossRef]
- Stahl, E.C.; Tsuchida, C.A.; Hamilton, J.R.; Lin-Shiao, E.; McDevitt, S.L.; Moehle, E.A.; Witkowsky, L.B.; Tsui, C.K.; Pestal, K.; Gildea, H.K.; et al. IGI-LuNER: Single-Well Multiplexed RT-qPCR Test for SARS-CoV-2. medRxiv 2020. [Google Scholar] [CrossRef]
- Office of Water Programs; University Enterprises, Inc.; California State University, Sacramento; California Water Pollution Control Association (Eds.) Operation of Wastewater Treatment Plants, 8th ed.; Pearson Education: New York, NY, USA, 2019; ISBN 978-0-13-568400-9. [Google Scholar]
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment. Water Intell. Online 2016, 15, 9781780407500. [Google Scholar] [CrossRef]
- Shirasaki, N.; Matsushita, T.; Matsui, Y.; Marubayashi, T. Effect of Aluminum Hydrolyte Species on Human Enterovirus Removal from Water during the Coagulation Process. Chem. Eng. J. 2016, 284, 786–793. [Google Scholar] [CrossRef]
- Baird, R.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Fumagalli, M.J.; Capato, C.F.; de Castro-Jorge, L.A.; de Souza, W.M.; Arruda, E.; Figueiredo, L.T.M. Stability of SARS-CoV-2 and Other Airborne Viruses under Different Stress Conditions. Arch. Virol. 2021, 167, 183. [Google Scholar] [CrossRef] [PubMed]
- Naceradska, J.; Pivokonska, L.; Pivokonsky, M. On the Importance of pH Value in Coagulation. J. Water Supply Res. Technol.-Aqua 2019, 68, 222–230. [Google Scholar] [CrossRef]
- Yu, L.; Tian, Z.; Joshi, D.R.; Yuan, L.; Tuladhar, R.; Zhang, Y.; Yang, M. Detection of SARS-CoV-2 and Other Viruses in Wastewater: Optimization and Automation of an Aluminum Hydroxide Adsorption–Precipitation Method for Virus Concentration. Acs EsT Water 2022, 2, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cataluña, A.; Cuevas-Ferrando, E.; Randazzo, W.; Falcó, I.; Allende, A.; Sánchez, G. Comparing Analytical Methods to Detect SARS-CoV-2 in Wastewater. Sci. Total Environ. 2021, 758, 143870. [Google Scholar] [CrossRef]
- Hamza, I.A.; Jurzik, L.; Wilhelm, M. Development of a Luminex Assay for the Simultaneous Detection of Human Enteric Viruses in Sewage and River Water. J. Virol. Methods 2014, 204, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, B.; Yue, Q.; Sun, S.; Wang, Y.; Li, Q. Determination of Active Ingredients of a New Coagulant Aid-Enteromorpha by Floc Characteristics on-Line Monitoring in Yellow River Water Treatment. Chem. Eng. J. 2013, 232, 310–318. [Google Scholar] [CrossRef]
- Pino, N.J.; Rodriguez, D.C.; Cano, L.C.; Rodriguez, A. Detection of SARS-CoV-2 in Wastewater Is Influenced by Sampling Time, Concentration Method, and Target Analyzed. J. Water Health 2021, 19, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Toribio-Avedillo, D.; Gómez-Gómez, C.; Sala-Comorera, L.; Galofré, B.; Muniesa, M. Adapted Methods for Monitoring Influenza Virus and Respiratory Syncytial Virus in Sludge and Wastewater. Sci. Total Environ. 2024, 918, 170636. [Google Scholar] [CrossRef] [PubMed]
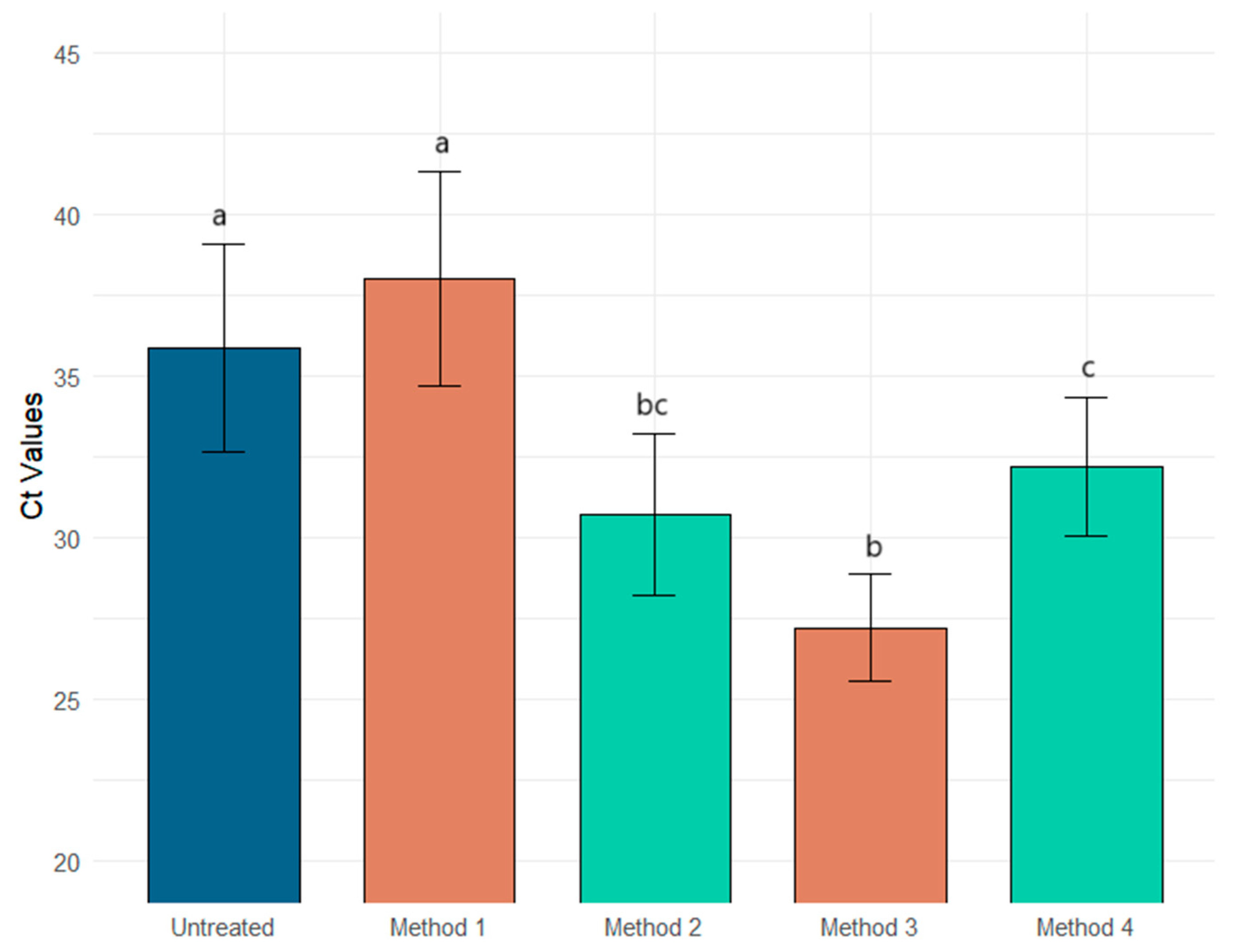
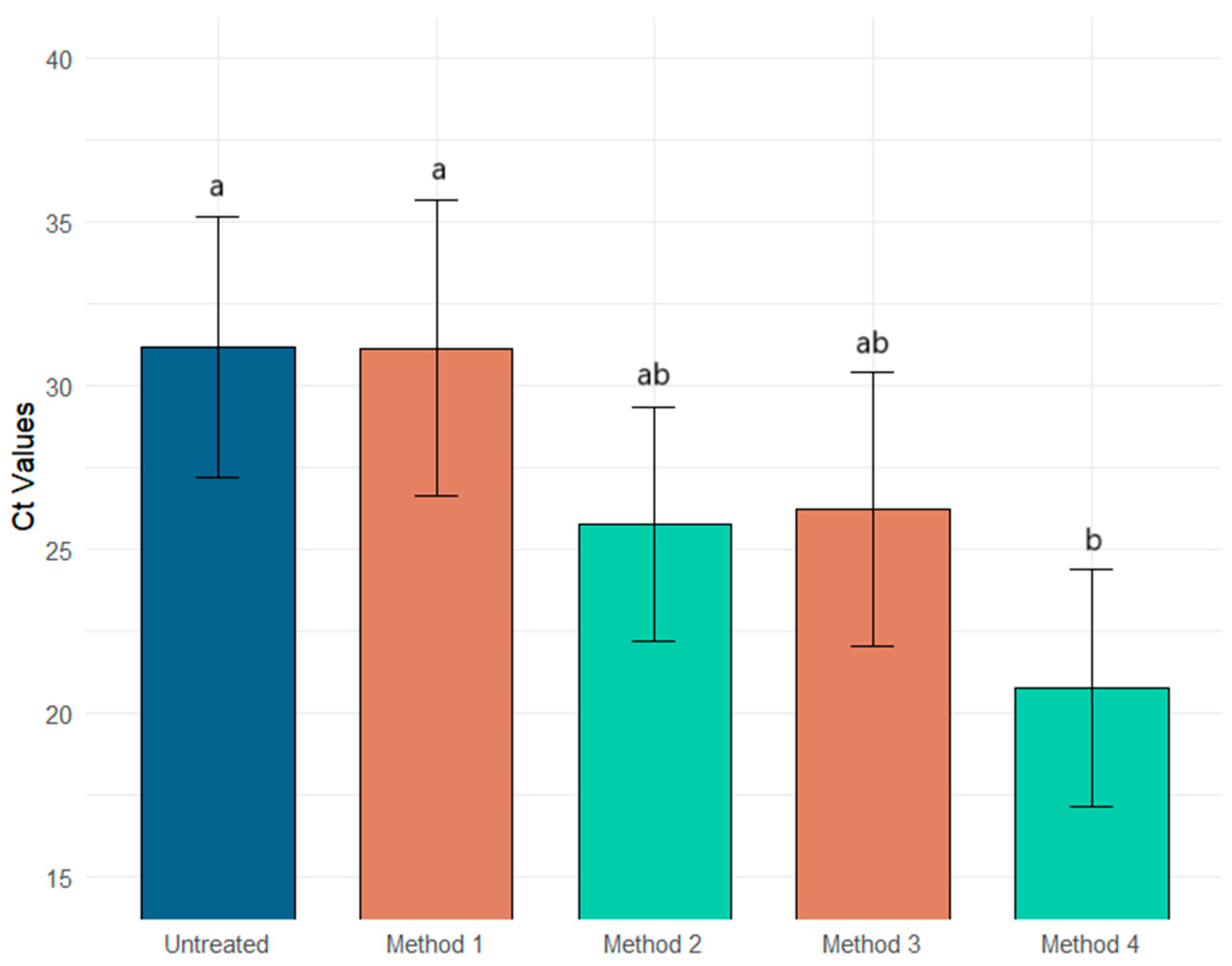
| Methods | Ct PMMOV | Intra-Replicate Standard Deviation | Ct Φ6 | Intra-Replicate Standard Deviation | Ct MS2 | Intra-Replicate Standard Deviation |
|---|---|---|---|---|---|---|
| Method 1 | 29.53 | 0.74 | 41.01 | 4.09 | 37.00 | 1.98 |
| 29.71 | 41.01 | 37.00 | ||||
| 28.23 | 33.75 | 35.41 | ||||
| 28.47 | 34.12 | 32.80 | ||||
| Method 2 | 28.50 | 0.96 | 26.74 | 3.81 | 28.48 | 3.07 |
| 28.81 | 26.94 | 32.93 | ||||
| 27.21 | 26.33 | 29.66 | ||||
| 26.83 | 34.28 | 35.20 | ||||
| Method 3 | 29.50 | 0.50 | 28.29 | 0.66 | 23.75 | 0.78 |
| 29.69 | 28.97 | 24.17 | ||||
| 30.36 | 27.80 | 22.56 | ||||
| 30.52 | 27.46 | 22.74 | ||||
| Method 4 | 27.88 | 1.62 | 30.99 | 0.24 | 15.98 | 0.68 |
| 31.81 | 31.33 | 15.60 | ||||
| 29.51 | 31.56 | 15.38 | ||||
| 29.54 | 31.37 | 16.92 |
| Methods | Target | PMMOV | Φ6 | MS2 | |
|---|---|---|---|---|---|
| Ct cut off value | >40 * | >41 | ≥37 ** | ||
| Untreated wastewater | Spiked wastewater Ct | Sample 1 | 36.90 | 39.64 | 36.84 |
| Sample 2 | 39.69 | 33.54 | 28.20 | ||
| Sample 3 | 38.13 | 34.57 | 27.04 | ||
| Sample 4 | 40.15 | 35.33 | 30.52 | ||
| Sample 5 | 39.02 | 39.97 | 35.23 | ||
| Sample 6 | 38.17 | 32.29 | 29.27 | ||
| Average Ct | 38.68 | 35.89 | 31.18 | ||
| Method 1 | Spiked wastewater AFTER flocculation Ct | Sample 1 | 28.99 | 37.47 | 35.55 |
| Sample 2 | 29.03 | 32.00 | 37.00 | ||
| Sample 3 | 37.12 | 41.01 | 25.72 | ||
| Sample 4 | 39.34 | 40.90 | 27.96 | ||
| Sample 5 | 35.08 | 38.40 | 28.41 | ||
| Sample 6 | 35.11 | 38.27 | 32.18 | ||
| Average Ct | 34.11 | 38.02 | 31.14 | ||
| Method 2 | Spiked wastewater AFTER flocculation | Sample 1 | 27.89 | 28.57 | 31.57 |
| Sample 2 | 29.91 | 29.17 | 27.74 | ||
| Sample 3 | 33.07 | 31.53 | 21.36 | ||
| Sample 4 | 35.98 | 35.44 | 23.28 | ||
| Sample 5 | 32.85 | 29.80 | 25.89 | ||
| Sample 6 | 34.67 | 29.81 | 24.80 | ||
| Average Ct | 32.38 | 30.72 | 25.77 | ||
| Method 3 | Spiked wastewater AFTER flocculation | Sample 1 | 30.02 | 28.13 | 23.31 |
| Sample 2 | 30.76 | 30.01 | 19.99 | ||
| Sample 3 | 32.14 | 25.18 | 27.30 | ||
| Sample 4 | 29.62 | 26.59 | 27.10 | ||
| Sample 5 | 34.15 | 26.58 | 32.31 | ||
| Sample 6 | 34.82 | 26.78 | 27.36 | ||
| Average Ct | 31.92 | 27.21 | 26.23 | ||
| Method 4 | Spiked wastewater AFTER flocculation | Sample 1 | 29.69 | 31.31 | 15.97 |
| Sample 2 | 34.43 | 32.79 | 16.41 | ||
| Sample 3 | 37.42 | 31.40 | 23.05 | ||
| Sample 4 | 36.97 | 29.58 | 21.81 | ||
| Sample 5 | 39.38 | 35.84 | 23.91 | ||
| Sample 6 | 40.01 | 32.40 | 23.48 | ||
| Average Ct reduction | 36.32 | 32.20 | 20.77 | ||
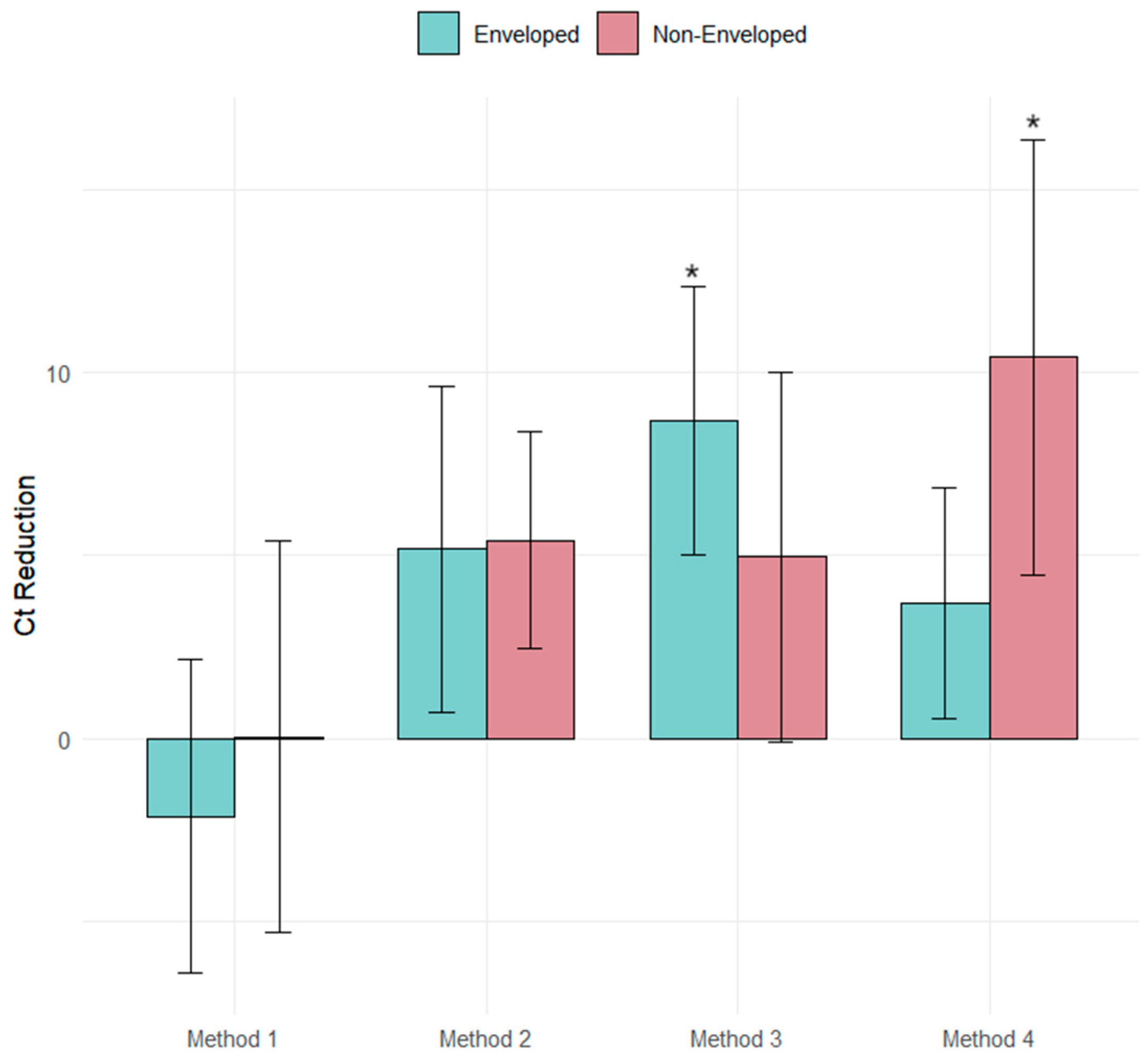
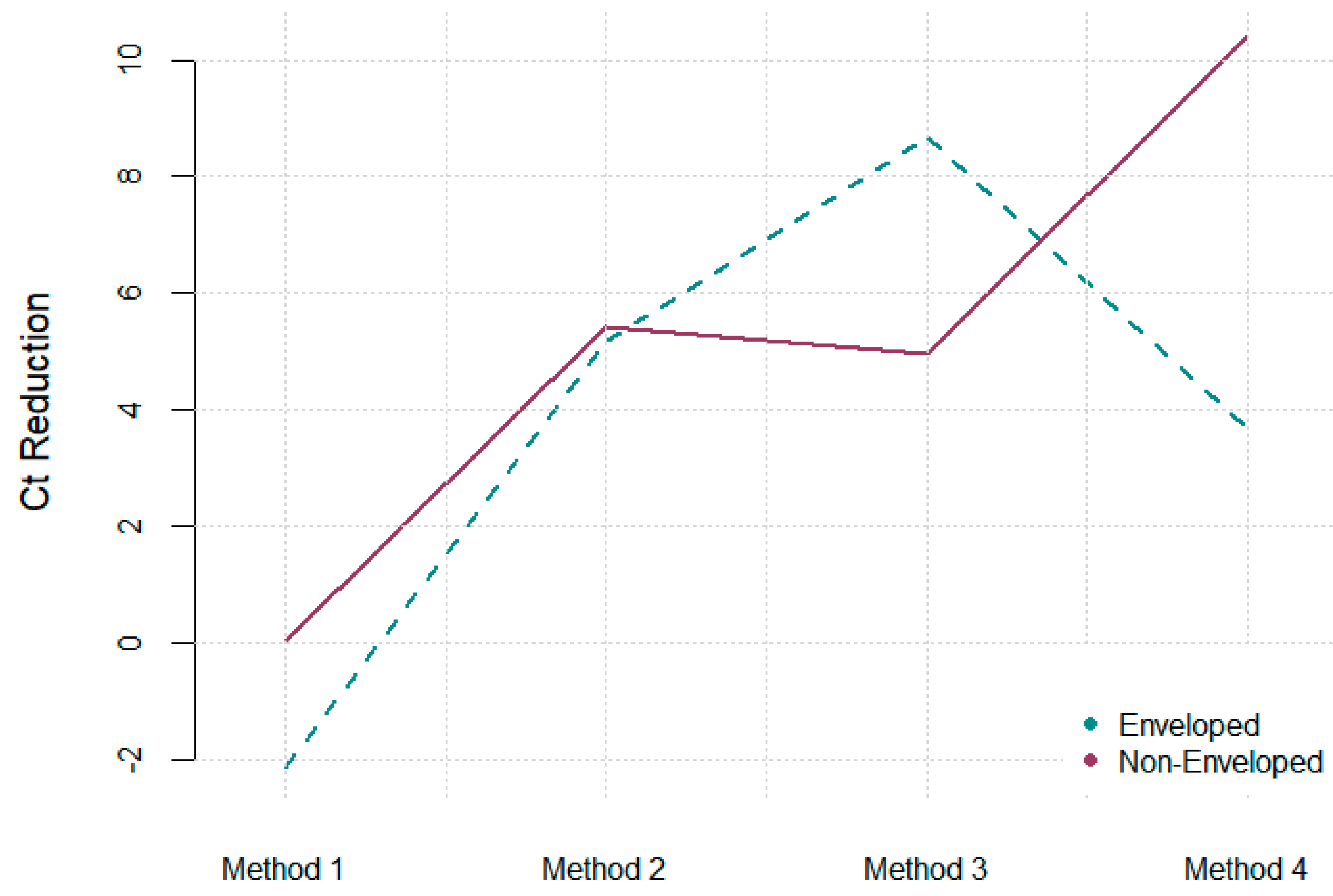
| Methods | ΔCt PMMOV | CI | ΔCt Φ6 | CI | ΔCt MS2 | CI | Time (Hours) |
|---|---|---|---|---|---|---|---|
| Method 1 | 4.53 | 1.41, 7.72 | −2.14 | −5.56, 1.28 | 0.04 | −4.22, 4.31 | 3.5 |
| Method 2 | 6.29 | 3.91, 8.32 | 5.17 | 1.59, 8.75 | 5.41 | 3.03, 7.79 | 4 |
| Method 3 | 6.76 | 4.65, 8.87 | 8.67 | 5.74, 11.61 | 3.69 | 0.92, 8.99 | 3.5 |
| Method 4 | 2.36 | −0.69, 2.88 | 4.95 | 1.16, 6.22 | 10.41 | 5.64, 15.18 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farmer-Diaz, K.; Matthew-Bernard, M.; Cheetham, S.; Mitchell, K.; Macpherson, C.N.L.; Ramos-Nino, M.E. Optimized Aluminum Hydroxide Adsorption–Precipitation for Improved Viral Detection in Wastewater. Int. J. Environ. Res. Public Health 2025, 22, 148. https://doi.org/10.3390/ijerph22020148
Farmer-Diaz K, Matthew-Bernard M, Cheetham S, Mitchell K, Macpherson CNL, Ramos-Nino ME. Optimized Aluminum Hydroxide Adsorption–Precipitation for Improved Viral Detection in Wastewater. International Journal of Environmental Research and Public Health. 2025; 22(2):148. https://doi.org/10.3390/ijerph22020148
Chicago/Turabian StyleFarmer-Diaz, Karla, Makeda Matthew-Bernard, Sonia Cheetham, Kerry Mitchell, Calum N. L. Macpherson, and Maria E. Ramos-Nino. 2025. "Optimized Aluminum Hydroxide Adsorption–Precipitation for Improved Viral Detection in Wastewater" International Journal of Environmental Research and Public Health 22, no. 2: 148. https://doi.org/10.3390/ijerph22020148
APA StyleFarmer-Diaz, K., Matthew-Bernard, M., Cheetham, S., Mitchell, K., Macpherson, C. N. L., & Ramos-Nino, M. E. (2025). Optimized Aluminum Hydroxide Adsorption–Precipitation for Improved Viral Detection in Wastewater. International Journal of Environmental Research and Public Health, 22(2), 148. https://doi.org/10.3390/ijerph22020148





