The Influence of Parental Behaviours and Socioeconomic Factors on Offspring’s Precursors of Atherosclerosis: A Systematic Review and Meta-Analysis of Observational Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Inclusion and Exclusion Criteria
2.3. Search Strategy
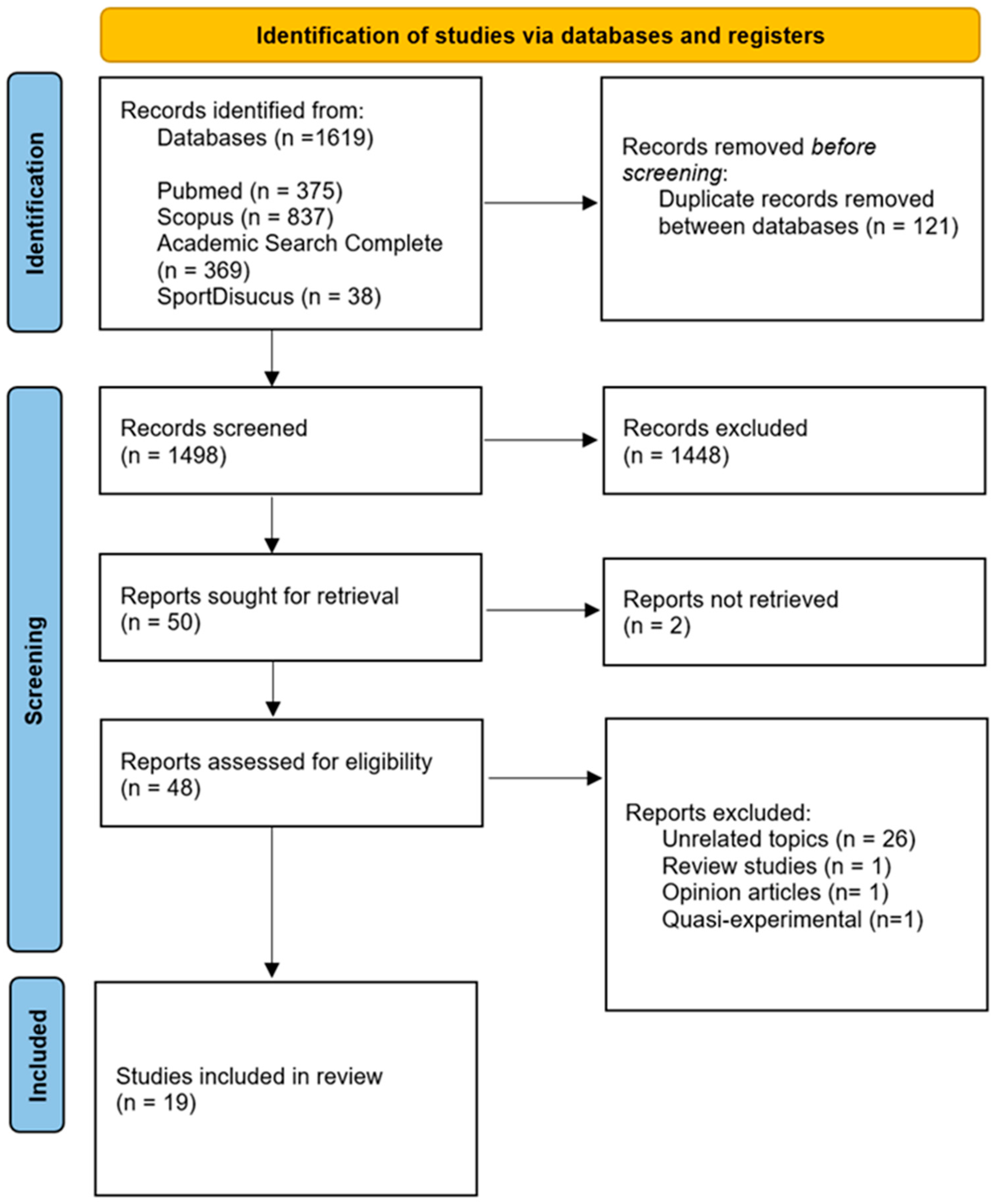
2.4. Study Selection, Data Extraction
2.5. Quality Assessment of the Included Studies
2.6. Data Analysis
3. Results
3.1. Systematic Review
3.1.1. Arterial Stiffness
3.1.2. Blood Pressure
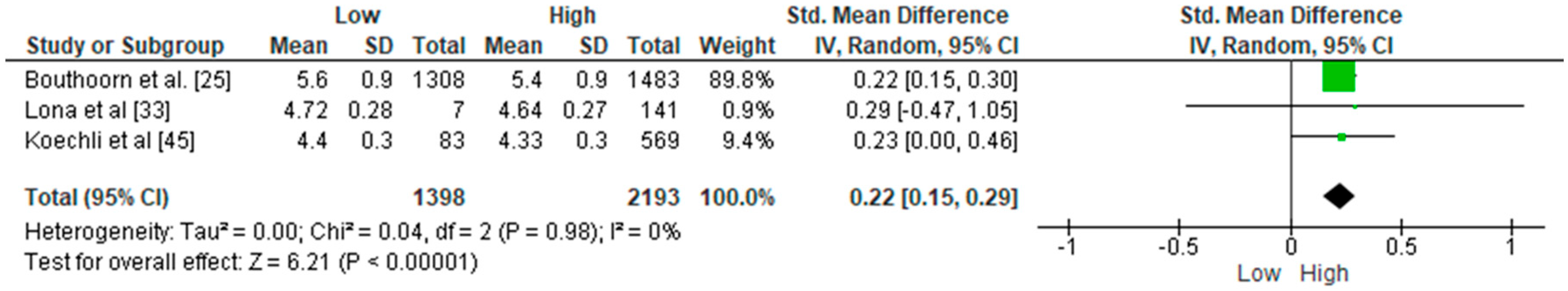
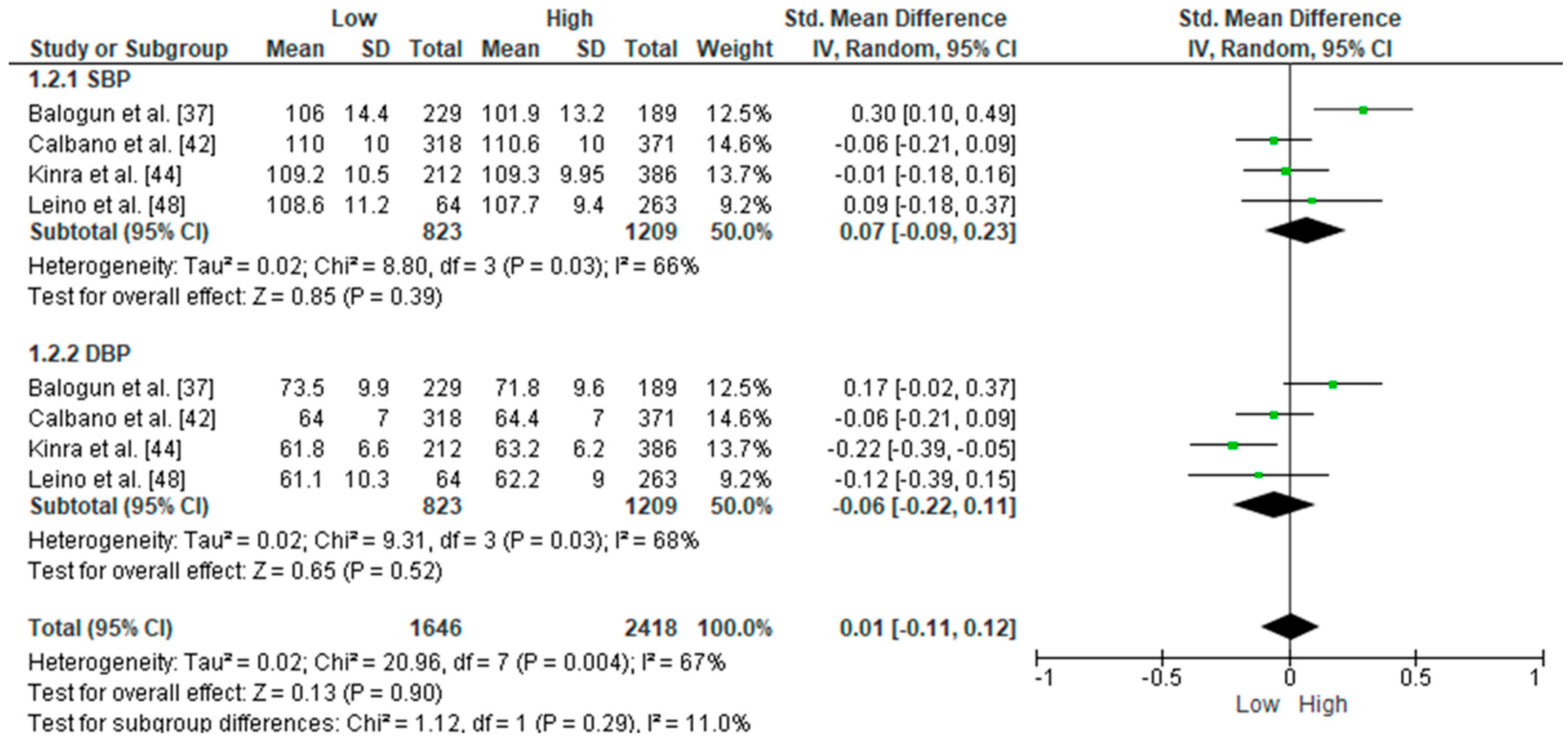
3.2. Meta-Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIx | Augmentation Index |
| AS | Arterial Stiffness |
| BP | Blood Pressure |
| CVD | Cardiovascular Disease |
| DBP | Diastolic Blood Pressure |
| EDL | Educational Level |
| FMD | Flow-mediated Dilation |
| IMT | Intima Media Thickness |
| PA | Physical Activity |
| PP | Pulse Pressure |
| PWV | Pulse Wave Velocity |
| SBP | Systolic Blood Pressure |
| SES | Socioeconomic Status |
| SMD | Standardised Mean Difference |
Appendix A
| Search | Query |
|---|---|
| Pubmed | 375 |
| (“parental lifestyle” [Text Word] OR “parents lifestyle” [Text Word] OR “parents physical activity” [Text Word] OR “parental physical activity” [Text Word] OR “parental exercise” [Text Word] OR “parental sleep” [Text Word] OR “parents exercise” [Text Word] OR “parents sleep” [Text Word] OR “mother-child relations” [MeSH Terms] OR “mother child relationship *” [Text Word] OR “parent-child relations” [Text Word] OR “parents” [MeSH Terms]) AND (“children” [Text Word] OR “young age” [Text Word] OR “child” [Text Word] OR “adolescents” [Text Word] OR “young people” [Text Word] OR “teenage” [Text Word] OR “youth” [Text Word] OR “youth *” [Text Word] OR “child” [MeSH Terms] OR “child *” [MeSH Terms] OR “youth *” [MeSH Terms] OR “adolescent” [MeSH Terms]) AND (((“arterial stiffness” [Text Word] OR “vascular stiffness” [Text Word] OR “aortic stiffness” [Text Word] OR “pulse wave velocity” [Text Word] OR “augmentation index” [Text Word] OR “central aortic pressure” [Text Word] OR “central blood pressure” [Text Word] OR “peripheral blood pressure” [Text Word] OR “pulse pressure” [Text Word] OR “atherosclerosis” [Text Word] OR “atherogenesis” [Text Word] OR “carotid intima-media thickness” [All Fields]) AND “vascular stiffness” [MeSH Terms]) OR “carotid artery diseases” [MeSH Terms] OR “blood pressure” [MeSH Terms] OR “pulse wave analysis” [MeSH Terms] OR “arterial pressure” [MeSH Terms] OR “atherosclerosis” [MeSH Terms]) | |
| Scopus | 837 |
| (“parental lifestyle” OR “parental exercise” OR “parental physical activity” OR “parents physical activity” OR “parents exercise” OR “parental behavior” OR “parental behavior” OR “parental sleep” OR “parents sleep” OR “parental sedentary behavior” OR “child parent relation” OR “child parent relation” OR “correlation, parent child” OR “correlation, parent child”) AND (children OR “young age” OR child OR adolescents OR “young people” OR young OR teenage OR youth * OR child * OR adolescent *) AND (“arterial stiffness” OR “vascular stiffness” OR “aortic stiffness” OR “aorta stiffness” OR “carotid intima-media thickness” OR “pulse pressure” OR “pulse wave velocity” OR “augmentation index” OR “central aortic pressure” OR “aortic pressure” OR “central blood pressure” OR “peripheral blood pressure” OR “carotid artery disease” OR atherosclerosis OR “atherosclerotic disease” OR “carotid artery diseases”) | |
| Academic Search Complete | 369 |
| (“parental lifestyle” OR “parental exercise” OR “parents exercise” OR “parental physical activity” OR “parents physical activity” OR “parents exercise” OR “parental behavior” OR “parental behavior” OR “parental sleep” OR “parents sleep” OR “parental sedentary behavior” OR mother OR father OR parents) AND (children OR “young age” OR child OR adolescents OR infant OR “young people” OR young OR teenage OR youth * OR child * OR adolescent *) AND (“arterial stiffness” OR “vascular stiffness” OR “aortic stiffness” OR “pulse pressure” OR “pulse wave velocity” OR “augmentation index” OR “central aortic pressure” OR “central blood pressure” OR “peripheral blood pressure” OR “carotid artery diseases” OR “carotid artery diseases” OR “carotid intima-media thickness”) | |
| SportDiscus | 38 |
| (“parental lifestyle” OR “parental exercise” OR “parents exercise” OR “parental physical activity” OR “parents physical activity” OR “parents exercise” OR “parental behavior” OR “parental behavior” OR “parental sleep” OR “parents sleep” OR “parental sedentary behavior” OR mother OR father OR parents) AND (children OR “young age” OR child OR adolescents OR infant OR “young people” OR young OR teenage OR youth * OR child * OR adolescent *) AND (“arterial stiffness” OR “vascular stiffness” OR “aortic stiffness” OR “pulse pressure” OR “pulse wave velocity” OR “augmentation index” OR “central aortic pressure” OR “central blood pressure” OR “peripheral blood pressure” OR “carotid artery diseases” OR “carotid artery diseases” OR “carotid intima-media thickness”) | |
References
- McGill, H.C., Jr.; McMahan, C.A.; Herderick, E.E.; Malcom, G.T.; Tracy, R.E.; Strong, J.P.; Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Origin of atherosclerosis in childhood and adolescence. Am. J. Clin. Nutr. 2000, 72, 1307s–1315s. [Google Scholar] [CrossRef]
- Kaminsky, L.A.; German, C.; Imboden, M.; Ozemek, C.; Peterman, J.E.; Brubaker, P.H. The importance of healthy lifestyle behaviors in the prevention of cardiovascular disease. Prog. Cardiovasc. Dis. 2022, 70, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Watanabe, T. Atherosclerosis: Known and unknown. Pathol. Int. 2022, 72, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-artery stiffness in health and disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Trucksäss, A.; Lichtenstein, A.H.; von Känel, R. Lifestyle factors as determinants of atherosclerotic cardiovascular health. Atherosclerosis 2024, 395, 117577. [Google Scholar] [CrossRef]
- Gundeti, V.; Aroor, S.; Handattu, K.; Lewis, L.E.; Mundkur, S.C.; Ramesh Bhat, Y.; Kini, P. Alarming rise in prevalence of obesity among children with essential hypertension: Reflection of larger global epidemiological change of adolescent nutritional status. Clin. Epidemiol. Glob. Health 2025, 32, 101948. [Google Scholar] [CrossRef]
- Karatzi, K.; Protogerou, A.D.; Moschonis, G.; Tsirimiagou, C.; Androutsos, O.; Chrousos, G.P.; Lionis, C.; Manios, Y. Prevalence of hypertension and hypertension phenotypes by age and gender among schoolchildren in Greece: The Healthy Growth Study. Atherosclerosis 2017, 259, 128–133. [Google Scholar] [CrossRef]
- Taşdemir, M.; Erginöz, E.; Gayret, Ö.B.; Bilge, I. Ambulatory arterial stiffness index is increased in obese children. Turk. J. Pediatr. 2020, 62, 259–266. [Google Scholar] [CrossRef]
- Schnermann, M.E.; Schulz, C.-A.; Herder, C.; Alexy, U.; Nöthlings, U. A lifestyle pattern during adolescence is associated with cardiovascular risk markers in young adults: Results from the DONALD cohort study. J. Nutr. Sci. 2021, 10, e92. [Google Scholar] [CrossRef]
- Chen, T.; Lin, J.; Lin, Y.; Xu, L.; Lu, D.; Li, F.; Hou, L.; Yu, C.C.W. Effects of aerobic exercise and resistance exercise on physical indexes and cardiovascular risk factors in obese and overweight school-age children: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0257150. [Google Scholar] [CrossRef]
- Diaz-Castro, J.; Garcia-Vega, J.E.; Ochoa, J.J.; Puche-Juarez, M.; Toledano, J.M.; Moreno-Fernandez, J. Implementation of a Physical Activity Program Protocol in Schoolchildren: Effects on the Endocrine Adipose Tissue and Cognitive Functions. Front. Nutr. 2021, 8, 761213. [Google Scholar] [CrossRef]
- German, C.; Makarem, N.; Fanning, J.; Redline, S.; Elfassy, T.; McClain, A.; Abdalla, M.; Aggarwal, B.; Allen, N.; Carnethon, M. Sleep, sedentary behavior, physical activity, and cardiovascular health: MESA. Med. Sci. Sports Exerc. 2021, 53, 724. [Google Scholar] [CrossRef]
- Sequi-Dominguez, I.; Mavridis, D.; Cavero-Redondo, I.; Saz-Lara, A.; Martinez-Vizcaino, V.; Núñez de Arenas-Arroyo, S. Comparative effectiveness of different types of exercise in reducing arterial stiffness in children and adolescents: A systematic review and network meta-analysis. Br. J. Sports Med. 2023, 57, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.B.; Riddoch, C.; Kriemler, S.; Hills, A.P. Physical activity and cardiovascular risk factors in children. Br. J. Sports Med. 2011, 45, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.; de Azevedo, C.V.M.; Santos, R.M.R. Sleep and health-related physical fitness in children and adolescents: A systematic review. Sleep. Sci. 2021, 14, 357–365. [Google Scholar] [CrossRef]
- Horta, B.L.; Schaan, B.D.; Bielemann, R.M.; Vianna, C.Á.; Gigante, D.P.; Barros, F.C.; Ekelund, U.; Hallal, P.C. Objectively measured physical activity and sedentary-time are associated with arterial stiffness in Brazilian young adults. Atherosclerosis 2015, 243, 148–154. [Google Scholar] [CrossRef]
- Lisboa, T.; Silva, W.R.d.; Silva, D.A.S.; Felden, É.P.G.; Pelegrini, A.; Lopes, J.d.J.D.; Beltrame, T.S. Social support from family and friends for physical activity in adolescence: Analysis with structural equation modeling. Cad. Saude Publica 2021, 37, e00196819. [Google Scholar] [CrossRef]
- Xu, H.; Wen, L.M.; Rissel, C. Associations of parental influences with physical activity and screen time among young children: A systematic review. J. Obes. 2015, 2015, 546925. [Google Scholar] [CrossRef]
- Sun, D.; Ding, Y.; Yu, C.; Sun, D.; Pang, Y.; Pei, P.; Yang, L.; Millwood, I.Y.; Walters, R.G.; Du, H.; et al. Joint impact of polygenic risk score and lifestyles on early- and late-onset cardiovascular diseases. Nat. Hum. Behav. 2024, 8, 1810–1818. [Google Scholar] [CrossRef]
- Said, M.A.; Verweij, N.; van der Harst, P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK Biobank Study. JAMA Cardiol. 2018, 3, 693–702. [Google Scholar] [CrossRef]
- Reimers, A.K.; Boxberger, K.; Schmidt, S.C.; Niessner, C.; Demetriou, Y.; Marzi, I.; Woll, A. Social support and modelling in relation to physical activity participation and outdoor play in preschool children. Children 2019, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Carayanni, V.; Vlachopadopoulou, E.; Koutsouki, D.; Bogdanis, G.C.; Psaltopoulou, T.; Manios, Y.; Karachaliou, F.; Hatzakis, A.; Michalacos, S. Effects of Body Mass Index (BMI), demographic and socioeconomic factors on organized physical activity (OPA) participation in children aged 6–15 years: A cross-sectional study comparing primary and secondary school children in Greece. BMC Pediatr. 2020, 20, 491. [Google Scholar] [CrossRef] [PubMed]
- Su, D.L.; Tang, T.C.; Chung, J.S.; Lee, A.S.; Capio, C.M.; Chan, D.K. Parental influence on child and adolescent physical activity level: A meta-analysis. Int. J. Environ. Res. Public. Health 2022, 19, 16861. [Google Scholar] [CrossRef] [PubMed]
- Schreier, H.M.; Chen, E. Socioeconomic status in one’s childhood predicts offspring cardiovascular risk. Brain Behav. Immun. 2010, 24, 1324–1331. [Google Scholar] [CrossRef]
- Bouthoorn, S.H.; Van Lenthe, F.J.; De Jonge, L.L.; Hofman, A.; Van Osch-Gevers, L.; Jaddoe, V.W.; Raat, H. Maternal educational level and blood pressure, aortic stiffness, cardiovascular structure and functioning in childhood: The generation R study. Am. J. Hypertens. 2014, 27, 89–98. [Google Scholar] [CrossRef]
- Muñoz-Galiano, I.M.; Connor, J.D.; Gómez-Ruano, M.A.; Torres-Luque, G. Students’ Physical Activity Profiles According to Children’s Age and Parental Educational Level. Children 2021, 8, 516. [Google Scholar] [CrossRef]
- Ruedl, G.; Niedermeier, M.; Wimmer, L.; Ploner, V.; Pocecco, E.; Cocca, A.; Greier, K. Impact of Parental Education and Physical Activity on the Long-Term Development of the Physical Fitness of Primary School Children: An Observational Study. Int. J. Environ. Res. Public Health 2021, 18, 8736. [Google Scholar] [CrossRef]
- Komulainen, K.; Mittleman, M.A.; Jokela, M.; Laitinen, T.T.; Pahkala, K.; Elovainio, M.; Juonala, M.; Tammelin, T.; Kähönen, M.; Raitakari, O. Socioeconomic position and intergenerational associations of ideal health behaviors. Eur. J. Prev. Cardiol. 2019, 26, 1605–1612. [Google Scholar] [CrossRef]
- Muñoz-Galiano, I.M.; Connor, J.D.; Gómez-Ruano, M.A.; Torres-Luque, G. Influence of the parental educational level on physical activity in schoolchildren. Sustainability 2020, 12, 3920. [Google Scholar] [CrossRef]
- Kwok, M.K.; Schooling, C.M.; Subramanian, S.V.; Leung, G.M.; Kawachi, I. Pathways from parental educational attainment to adolescent blood pressure. J. Hypertens. 2016, 34, 1787–1795. [Google Scholar] [CrossRef]
- Mannoh, I.; Hussien, M.; Commodore-Mensah, Y.; Michos, E.D. Impact of social determinants of health on cardiovascular disease prevention. Curr. Opin. Cardiol. 2021, 36, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Lona, G.; Hauser, C.; Bade, S.; Köchli, S.; Infanger, D.; Endes, K.; Faude, O.; Hanssen, H. Association of Parental Socioeconomic Status and Physical Activity with Development of Arterial Stiffness in Prepubertal Children. Int. J. Environ. Res. Public Health 2021, 18, 8227. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, G.D.; Schwertz, R.; Klett, M.; Hoffmann, G.F.; Schaefer, F.; Wühl, E. Determinants of blood pressure in preschool children: The role of parental smoking. Circulation 2011, 123, 292–298. [Google Scholar] [CrossRef]
- Hacke, C.; Weisser, B. Effects of parental smoking on exercise systolic blood pressure in adolescents. J. Am. Heart Assoc. 2015, 4, e001936. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Wang, Y.; Zhao, Y.; Han, L.; Zhong, L.; Zhang, Q.; Speakman, J.R.; Li, M.; Gao, S. Impact of parental smoking on adipokine profiles and cardiometabolic risk factors in Chinese children. Atherosclerosis 2020, 301, 23–29. [Google Scholar] [CrossRef]
- Balogun, J.A.; Obajuluwa, V.A.; Olaogun, M.O.; Abereoje, O.K.; Oyeyemi, A.Y.; Adeodu, O.O.; Balogun, M.O. Influence of parental socioeconomic status on casual blood pressures of Nigerian school children. Int. J. Cardiol. 1990, 29, 63–69. [Google Scholar] [CrossRef]
- Kvaavik, E.; Glymour, M.; Klepp, K.-I.; Tell, G.S.; Batty, G.D. Parental education as a predictor of offspring behavioural and physiological cardiovascular disease risk factors. Eur. J. Public. Health 2012, 22, 544–550. [Google Scholar] [CrossRef]
- Khanolkar, A.R.; Byberg, L.; Koupil, I. Parental influences on cardiovascular risk factors in Swedish children aged 5–14 years. Eur. J. Public. Health 2012, 22, 840–847. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, J.; Wang, Z.; Dong, Y.; Yang, Z.; Dong, B.; Ma, Y. Parental smoking and blood pressure in children and adolescents: A national cross-sectional study in China. BMC Pediatr. 2019, 19, 116. [Google Scholar] [CrossRef]
- Kwok, M.; Schooling, C.; Leung, G.; Subramanian, S. Grandparental education, parental education and adolescent blood pressure. Prev. Med. 2016, 90, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Calbano, A.G.; Moyano, M.Á.; Mamondi, V.; Berra, S. Prevalence of high blood pressure among schoolchildren from Córdoba, Argentina, and its relation to socioeconomic status. Arch. Argent. Pediatr. 2018, 116, 340–344. [Google Scholar] [CrossRef]
- Ansa, V.; Anah, M.; Odey, F.; Mbu, P.; Agbor, E. Relationship between parental socio-economic status and casual blood pressure in coastal Nigerian adolescents. West Afr. J. Med. 2011, 29, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Kinra, S.; Johnson, M.; Kulkarni, B.; Sarma, K.R.; Ben-Shlomo, Y.; Smith, G.D. Socio-economic position and cardiovascular risk in rural indian adolescents: Evidence from the Andhra Pradesh children and parents study (APCAPS). Public Health 2014, 128, 852–859. [Google Scholar] [CrossRef]
- Koechli, S.; Endes, K.; Grenacher, J.; Streese, L.; Lona, G.; Hauser, C.; Deiseroth, A.; Zahner, L.; Hanssen, H. Socioeconomic status and parental lifestyle are associated with vascular phenotype in children. Front. Public. Health 2021, 9, 610268. [Google Scholar] [CrossRef]
- Kallio, K.; Jokinen, E.; Raitakari, O.T.; Hämäläinen, M.; Siltala, M.; Volanen, I.; Kaitosaari, T.; Viikari, J.; Rönnemaa, T.; Simell, O. Tobacco smoke exposure is associated with attenuated endothelial function in 11-year-old healthy children. Circulation 2007, 115, 3205–3212. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Stawińska-Witoszyńska, B.; Krzyżaniak, A.; Krzywińska-Wiewiorowska, M.; Siwińska, A. Who is at higher risk of hypertension? Socioeconomic status differences in blood pressure among Polish adolescents: A population-based ADOPOLNOR study. Eur. J. Pediatr. 2015, 174, 1461–1473. [Google Scholar] [CrossRef]
- Leino, M.; Porkka, K.V.; Raitakari, O.T.; Laitinen, S.; Taimela, S.; ViikariIIK, J.S. Influence of parental occupation on coronary heart disease risk factors in children. The Cardiovascular Risk in Young Finns Study. Int. J. Epidemiol. 1996, 25, 1189–1195. [Google Scholar] [CrossRef]
- Lona, G.; Hauser, C.; Köchli, S.; Infanger, D.; Endes, K.; Schmidt-Trucksäss, A.; Hanssen, H. Association of blood pressure, obesity and physical activity with arterial stiffness in children: A systematic review and meta-analysis. Pediatr. Res. 2022, 91, 502–512. [Google Scholar] [CrossRef]
- Lona, G.; Hauser, C.; Köchli, S.; Infanger, D.; Endes, K.; Faude, O.; Hanssen, H. Blood pressure increase and microvascular dysfunction accelerate arterial stiffening in children: Modulation by physical activity. Front. Physiol. 2020, 11, 613003. [Google Scholar] [CrossRef]
- Heil, L.; Oberhoffer, R.; Böhm, B. Association between physical activity intensity levels and arterial stiffness in healthy children. J. Phys. Act. Health 2020, 17, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Sanchez-Gonzalez, M.A.; Son, W.-M.; Kwak, Y.-S.; Park, S.-Y. The Effects of a 12-Week Combined Exercise Training Program on Arterial Stiffness, Vasoactive Substances, Inflammatory Markers, Metabolic Profile, and Body Composition in Obese Adolescent Girls. Pediatr. Exerc. Sci. 2018, 30, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Kolanowski, W.; Ługowska, K.; Trafialek, J. Increased physical activity at school benefits arterial blood pressure in children—A prospective follow-up cohort study. Int. J. Environ. Res. Public. Health 2022, 19, 4662. [Google Scholar] [CrossRef] [PubMed]
- Jago, R.; Solomon-Moore, E.; Macdonald-Wallis, C.; Thompson, J.L.; Lawlor, D.A.; Sebire, S.J. Association of parents’ and children’s physical activity and sedentary time in Year 4 (8–9) and change between Year 1 (5–6) and Year 4: A longitudinal study. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 110. [Google Scholar] [CrossRef]
- Blanco, M.; Veiga, O.L.; Sepúlveda, A.R.; Izquierdo-Gomez, R.; Román, F.J.; López, S.; Rojo, M. Family environment, physical activity and sedentarism in preadolescents with childhood obesity: ANOBAS case-control study. Aten. Primaria 2020, 52, 250–257. [Google Scholar] [CrossRef]
- Bronikowski, M.; Bronikowska, M.; Pluta, B.; Maciaszek, J.; Tomczak, M.; Glapa, A. Positive Impact on Physical Activity and Health Behaviour Changes of a 15-Week Family Focused Intervention Program: “Juniors for Seniors”. BioMed Res. Int. 2016, 2016, 5489348. [Google Scholar] [CrossRef]
- Aguilar-Cordero, M.J.; León Ríos, X.A.; Rojas-Carvajal, A.M.; Latorre-García, J.; Expósito-Ruiz, M.; Sánchez-López, A.M. Effects of physical activity on quality of life in overweight and obese children. Nutr. Hosp. 2021, 38, 736–741. [Google Scholar] [CrossRef]
- Yun, M.; Li, S.; Sun, D.; Ge, S.; Lai, C.C.; Fernandez, C.; Chen, W.; Srinivasan, S.R.; Berenson, G.S. Tobacco smoking strengthens the association of elevated blood pressure with arterial stiffness: The Bogalusa Heart Study. J. Hypertens. 2015, 33, 266–274. [Google Scholar] [CrossRef]
- Raghuveer, G.; White, D.A.; Hayman, L.L.; Woo, J.G.; Villafane, J.; Celermajer, D.; Ward, K.D.; de Ferranti, S.D.; Zachariah, J. Cardiovascular Consequences of Childhood Secondhand Tobacco Smoke Exposure: Prevailing Evidence, Burden, and Racial and Socioeconomic Disparities: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e336–e359. [Google Scholar] [CrossRef]
- Yaylaoglu, S.; Dundar, C. Assessing parental awareness and concerns about children’s tobacco smoke exposure: A community-based analysis. Arch. Public. Health 2025, 83, 39. [Google Scholar] [CrossRef]
- Peralta, L.R.; Mihrshahi, S.; Bellew, B.; Reece, L.J.; Hardy, L.L. Influence of school-level socioeconomic status on children’s physical activity, fitness, and fundamental movement skill levels. J. Sch. Health 2019, 89, 460–467. [Google Scholar] [CrossRef]
- Mesquita, E.D.d.L.; Tebar, W.R.; Correia, D.C.Q.; Guica, J.T.; Torres, W.; Fernandes, R.A.; Agostinete, R.R.; Christofaro, D.G.D. Physical activity and sedentary behaviour of adolescents and their parents: A specific analysis by sex and socioeconomic status. Arch. Public. Health 2023, 81, 189. [Google Scholar] [CrossRef]
- Amrhein, V.; Greenland, S.; McShane, B. Scientists rise up against statistical significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P. Why most published research findings are false. PLoS Med. 2005, 2, e124. [Google Scholar] [CrossRef] [PubMed]
- Aatola, H.; Koivistoinen, T.; Tuominen, H.; Juonala, M.; Lehtimäki, T.; Viikari, J.S.; Raitakari, O.T.; Kähönen, M.; Hutri-Kähönen, N. Influence of child and adult elevated blood pressure on adult arterial stiffness: The Cardiovascular Risk in Young Finns Study. Hypertension 2017, 70, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Mynard, J.P.; Smith, K.J.; Juonala, M.; Urbina, E.M.; Niiranen, T.; Daniels, S.R.; Xi, B.; Magnussen, C.G. Pediatric blood pressure and cardiovascular health in adulthood. Curr. Hypertens. Rep. 2024, 26, 431–450. [Google Scholar] [CrossRef]
- Aatola, H.; Hutri-Kähönen, N.; Juonala, M.; Viikari, J.S.; Hulkkonen, J.; Laitinen, T.; Taittonen, L.; Lehtimäki, T.; Raitakari, O.T.; Kahonen, M. Lifetime risk factors and arterial pulse wave velocity in adulthood: The cardiovascular risk in young Finns study. Hypertension 2010, 55, 806–811. [Google Scholar] [CrossRef]
- Bronfenbrenner, U.; Morris, P.A. The bioecological model of human development. In Handbook of Child Psychology; Damon, W., Lerner, R.M., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Frosch, C.A.; Schoppe-Sullivan, S.J.; O’Banion, D.D. Parenting and child development: A relational health perspective. Am. J. Lifestyle Med. 2021, 15, 45–59. [Google Scholar] [CrossRef]
- Lambrinou, C.P.; Androutsos, O.; Karaglani, E.; Cardon, G.; Huys, N.; Wikström, K.; Kivelä, J.; Ko, W.; Karuranga, E.; Tsochev, K.; et al. Effective strategies for childhood obesity prevention via school based, family involved interventions: A critical review for the development of the Feel4Diabetes-study school based component. BMC Endocr. Disord. 2020, 20, 52. [Google Scholar] [CrossRef]
- Michaelson, V.; Pilato, K.A.; Davison, C.M. Family as a health promotion setting: A scoping review of conceptual models of the health-promoting family. PLoS ONE 2021, 16, e0249707. [Google Scholar] [CrossRef]
| Patient/Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Children and Adolescents | Parental Lifestyle Behaviours | Parental Lifestyle Behaviours Sleep Physical Activity Sedentary Behaviours Smoking SES EDL | Arterial stiffness: PWV, AIx, IMT, and PP Blood Pressure |
| T/A | I | M | R | D | O | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 * | 9 | 10 | 11 | 12 | 13 * | 14 * | 15 * | 16 | 17 | 18 | 19 | 20 | 21 | 22 | |||||||||||||
| (a) | (b) | (a) | (b) | (a) | (b) | (c) | (d) | (e) | (a) | (b) | (c) | (a) | (b) | (c) | (a) | (b) | (c) | |||||||||||||||||
| Lona et al. [33] | + | + | + | + | + | + | + | NA | + | + | + | + | + | + | + | − | + | − | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + |
| Simonetti et al. [34] | − | + | + | + | − | + | + | NA | + | + | + | − | + | + | + | − | + | − | + | + | − | + | − | NA | + | + | + | − | + | + | + | + | + | + |
| Hacke and Weisser [35] | + | + | + | + | + | + | + | NA | + | + | + | − | + | + | + | + | + | − | + | + | − | + | − | NA | + | + | + | − | + | + | + | + | + | + |
| Kwok et al. [30] | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | − | + | + | + | + | + | + |
| Li et al. [36] | + | + | + | + | + | + | + | NA | + | + | + | − | + | + | + | + | + | + | + | − | − | + | − | NA | + | + | + | − | + | + | + | + | + | + |
| Balogun et al. [37] | + | + | + | + | + | + | + | NA | + | + | + | − | + | + | + | − | + | − | − | − | − | + | − | NA | + | + | + | − | + | + | + | + | + | + |
| Bouthoorn et al. [25] | + | + | + | + | + | + | + | NA | + | + | + | − | + | + | + | + | + | − | − | − | − | + | + | NA | + | + | + | − | + | + | + | + | + | + |
| Kvaavik et al. [38] | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | − | + | − | − | + | + | + | + | + | + | − | + | + | + | + | + | + |
| Khanolkar et al. [39] | + | + | + | + | + | + | + | NA | + | + | + | − | + | + | + | − | + | − | + | − | − | + | − | NA | + | + | + | − | + | + | + | + | + | + |
| Zhang et al. [40] | + | + | + | + | + | + | + | NA | + | + | + | − | + | + | + | + | + | + | + | − | − | + | + | NA | + | + | + | − | + | + | + | + | + | + |
| Kwok et al. [41] | + | + | + | + | + | + | + | NA | + | + | + | − | + | + | + | + | + | − | + | + | − | + | + | NA | + | + | + | − | + | + | + | + | + | + |
| Calbano et al. [42] | + | + | + | + | + | + | + | NA | + | + | + | − | + | − | + | − | + | − | + | + | − | + | + | NA | + | + | + | − | − | + | + | + | + | + |
| Ansa et al. [43] | + | + | + | + | + | + | + | NA | + | + | + | − | + | + | − | − | + | − | + | − | − | + | + | NA | + | + | + | − | + | + | + | + | + | − |
| Kinra et al. [44] | + | + | + | + | + | + | + | NA | + | + | + | + | + | + | + | − | + | − | + | + | − | + | + | NA | + | + | + | − | − | + | + | + | + | + |
| Koechli et al. [45] | + | + | + | + | + | + | + | NA | + | + | + | + | + | + | + | − | − | + | + | + | + | + | + | NA | + | + | + | − | − | + | + | + | + | − |
| Schreier and Chen [24] | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | − | − | − | + | − | − | + | − | + | + | + | + | − | + | + | + | + | + | − |
| Kallio et al. [46] | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | − | − | + | + | − | − | + | − | + | + | + | + | − | + | + | + | + | + | + |
| Kaczmarek et al. [47] | + | + | + | + | + | + | + | NA | + | + | + | − | + | + | + | − | + | − | + | − | − | + | − | NA | + | + | + | − | + | + | + | + | + | + |
| Leino et al. [48] | + | + | + | + | + | + | + | NA | + | + | + | − | + | − | − | − | − | − | + | − | − | + | − | NA | + | + | + | − | − | + | − | + | + | − |
| Authors (Year) | Sample Size | Age | Parents | Country | Method (Device) | Outcome | Model Adjustment | Main Results |
|---|---|---|---|---|---|---|---|---|
| Parental Physical Activity | ||||||||
| Koechli et al. [45] | 833 | 6–8 | Both | Switzerland | Oscillometric Mobil-OGraph | PWV | M1: age and sex M2: age, sex and smoke | Both Mean (95%CI): M1: L = 4.39 (4.35; 4.44); M = 4.32 (4.27; 4.37); H = 4.33 (4.30; 4.36); p = 0.035; M2: L = 4.38 (4.34; 4.43); M = 4.32 (4.28; 4.37); H = 4.34 (4.31; 4.36); p = 0.098 Mother Mean (95%CI) M1: L = 4.37 (4.34; 4.41); M = 4.31 (4.27; 4.35), H = 4.33 (4.30; 4.37); p = 0.040; Mother M2: L = 4.37 (4.33; 4.40); M = 4.31 (4.27; 4.35); H = 4.34 (4.30; 4.38); p = 0.085 Father M1 Mean (95%CI): L = 4.37 (4.34; 4.41); M = 4.32 (4.27; 4.36), H = 4.33 (4.30; 4.36); p = 0.068; Father M2: L = 4.37 (4.34; 4.40); M = 4.32 (4.27; 4.36), H = 4.33 (4.30; 4.36); p = 0.124 |
| Lona et al. [33] | 223 | 6–8 | Both | Switzerland | Oscillometric Mobil-OGraph | PWV | Age and sex | Both Mean (95%CI): L = 4.73 (4.65; 4.82); M = 4.62 (4.57; 4.68); H = 4.66 (4.6; 4.72), p = 0.108 Mother Mean (95%CI): L = 4.7 (4.64; 4.77); M = 4.69 (4.62; 4.75); H = 4.6 (4.54; 4.66), p = 0.049 Father Mean (95%CI): L = 4.69 (4.62; 4.76); M = 4.64 (4.56; 4.71); H = 4.65 (4.6; 4.71), p = 0.483 |
| Parental Smoking Habits | ||||||||
| Koechli et al. [45] | 833 | 6–8 | Both | Switzerland | Oscillometric Mobil-OGraph | PWV | M1: age and sex M2: age, sex, and parental PA | Both Mean (95%CI) M1: No = 4.32 (4.29; 4.34); Yes = 4.39 (4.35; 4.42), p < 0.001; M2: No = 4.32 (4.29; 4.34); Yes = 4.39 (4.35; 4.42), p = 0.001 Mother Mean (95%CI): M1: No = 4.33 (4.31; 4.36); Yes = 4.37 (4.32; 4.41); p = 0.219; M2: No = 4.33 (4.31; 4.36); Yes = 4.37 (4.32; 4.41); p = 0.247 Father M1: No = 4.33 (4.31; 4.36); Yes = 4.37 (4.32; 4.41), p < 0.001; M2: No = 4.32 (4.30; 4.34); Yes = 4.39 (4.36; 4.43), p = 0.001 |
| Kallio et al. [46] | 402 | 8–11 | Both | Finland | Vascular Sonographer | FMD | Covariates with p < 0.10 in preliminary analyses were included in multivariable models using backwards stepwise selection. | A single measurement of cotinine effects over FMD responses in offspring at a single time point showed no statistical significance; p = 0.054. Offspring longitudinal exposure to high cotinine levels leads to significant changes in the FMD pattern’s response, time-by-group interactions (p < 0.001), and the magnitude of response (p = 0.002) |
| Authors (Year) | Sample Size | Age | Parents | Country | Method (Device) | Outcome | Model Adjustment | Main Results |
|---|---|---|---|---|---|---|---|---|
| Parental Educational Level | ||||||||
| Koechli et al. [45] | 833 | 6–8 | Both | Switzerland | Oscillometric Mobil-OGraph | PWV | M1: age and sex M2: age, sex, and HI | Both Mean (95%CI): M1 L = 4.40 (4.34; 4.47); M = 4.37 (4.33; 4.42); M2: H = 4.33 (4.30; 4.35), p = 0.041; L = 4.39 (4.31; 4.46); M = 4.33 (4.28; 4,38); H = 4.34 (4.31; 4.37), p = 0.420 Mother Mean (95%CI): M1: L = 4.39 (4.34; 4.44); M = 4.35 (4.31; 4.39); H = 4.33 (4.30; 4.35), p = 0.051; M2: L = 4.37 (4.32; 4.43); M = 4.33 (4.29; 4.37); H = 4.34 (4.31; 4.37), p = 0.386 Father Mean (95%CI): M1: L = 4.37 (4.32; 4.42); M = 4.37 (4.33; 4.41), H = 4.33 (4.30; 4.35); p = 0.118; M2: L = 4.35 (4.29; 4.41), M = 4.34 (4.29; 4.38), H = 4.34 (4.31; 4.37), p = 0.918 |
| Lona et al. [33] | 223 | 6–8 | Both | Switzerland | Oscillometric Mobil-OGraph | PWV | M3: age, sex, and EDL M4: age, sex, and HI | M3: L = 4.72 (4.52; 4.93); M = 4.68 (4.62; 4.75); H = 4.64 (4.6; 4.69), p = 0.544 M4: L = 4.77 (4.54; 5); M = 4.68 (4.61; 4.75); H = 4.64 (4.59; 4.69), p = 0.466 |
| Bouthoorn et al. [25] | 6 | Mother | Netherlands | Automatic Complior Device | PWV | Sex, age, and ethnicity | L = 0.06 (−0.02; 0.14); ML = 0.02 (−0.05; 0.09); MH = 0.03 (−0.05; 0.11), H = Reference; p = 0.22 | |
| Parental Socioeconomic Status | ||||||||
| Koechli et al. [45] | 833 | 6–8 | Both | Switzerland | Oscillometric Mobil-OGraph | PWV | M1: age and sex M2: age, sex, and EDL | Mean (95%CI) M1: L = 4.37 (4.33; 4.41); M = 4.37 (4.33; 4.41); H = 4.29 (4.26; 4.33), p = 0.002; M2: L = 4.36 (4.32; 4.41); M = 4.37 (4.34; 4.41); H = 4.30 (4.26; 4.34); p = 0.033 |
| Lona et al. [33] | 223 | 6–8 | Both | Switzerland | Oscillometric Mobil-OGraph | PWV | M2: age and sex M1: age, sex, and EDL | Mean (95%CI) M1: L = 4.56 (4.43; 4.68), M = 4.66 (4.57; 4.75), H = 4.69 (4.58; 4.79), p = 0.273; Both M2: L = 4.6 (4.51; 4.69); M = 4.67 (4.6; 4.73); H = 4.68 (4.62; 4.74), p = 0.382 |
| Balogun et al. [37] | 229 | 8–20 | Both | Nigeria | Computation PP = SBP-DBP | PP | Age, height, and weight | Mean ± SD: L = 31 ± 12.5; M = 32.2 ± 12.7, H = 30.3 ± 12.1; p = 0.2141 (Fratio 1.54) |
| Kinra et al. [44] | 862 | 13–18 | Both | India | Sphygmocor Apparatus | AIx | M1: age, sex, NS, AR and HR M2: M1 + Height M3: M2 + PS, FMI and cPSR | Mean ± SD Girls: L = 6.8 (12.3); M = 3.9 (11.1); H = 4.8 (9.9), p = 0.365; Mean ± SD Boys: L = 5.1 (10.4); M = 3.8 (9.8); H = 1.8 (10.9), p = 0.035 B (95%CI) M1: −1.21 (−2.36; −0.06), p = 0.04; B (95%CI) M2: −0.72 (−1.84; 0.40), p = 0.20; B (95%CI) M3: −0.63 (−1.72; 0.45), p = 0.24 |
| Authors (Year) | Sample Size | Age | Parents | Country | Method (Device) | Outcome | Model Adjustment | Main Results |
|---|---|---|---|---|---|---|---|---|
| Hacke and Weisser [35] | 532 | 12–17 | Both | Germany | Standard Stethoscope and Sphygmomanometer | SBP (rest) SBP (exercise) | Age, sex, height, BMI percentile, and physical fitness | Physical Inactivity Mean ± SB Rest:1.0 (0.1; 1.9); p = 0.467 Mean ± SB Exercise = 4.99 (3.5; 6.4), p = 0.013 |
| Khanolkar et al. [39] | 1204 | 5–14 | Both | Swedish | Dinamap Compact T’Monitor | SBP | Age; gender, PS, family clustering, and parental EDL, occupation, and height | Total MET (hours/week) B (95%CI) Mother: ≤20.25 = Reference Group; Group 20.26–33.75 = −0.91 (−2.68; 0.85); Group ≥33.76 = −0.52 (−2.36; 1.30) Father: ≤7.5 = Reference Group; Group 7.6–25.5 = −1.26 (−3.15; 0.62); Group ≥25.6 = −0.69 (−2,48; 1.10) Total hours of vigorous PA/week (min/week) B (95%CI) Mother: None = Reference Group; Group ≤75 = 0.06 (−3.64; 3.78); Group 76–150 = −1.18 (−4.93; 2.56); Group >150 = −0.21 (−4.13; 3.70) Father: None or ≤30 = Reference Group; Group 60 = −0.74 (−3.92; 2.44); Group 90–120 = −0.76 (−4.00; 2.45); Group 150–360 = −0.06 (−3.09; 2.96); Group >360 = −3.72 (−7.94; 0.51) |
| Khanolkar et al. [39] | 1171 | 5–14 | Both | Swedish | Dinamap Compact T’Monitor | DBP | Age; gender, PS, family clustering, and parental EDL, occupation, and height | Total MET (hours/week) B (95%CI) Mother: ≤20.25 = Reference Group; Group 20.26–33.75 = 0.15 (−0.87; 1.18); Group ≥33.76 = −0.40 (−1.42; 0.63) Father: ≤7.5 = Reference Group; Group 7.6–25.5 = −0.48 (−1.61; 0.65); Group ≥25.6 = −0.17 (−1.28; 0.93) Total hours of vigorous PA/week (min/week) Mother: None = Reference Group; Group ≤75 = −0.82 (−2.97; 1.32); Group 76–150 = −0.96 (−3.13; 1.20); Group >150 = −1.36 (−3.60; 0.88) Father: None or 30 = Reference Group; Group 60 = 0.77 (−0.92; 2.46); Group 90–120 = 1.47 (−0.25; 3.21) |
| Authors (Year) | Sample Size | Age | Parents | Country | Method (Device) | Outcome | Model Adjustment | Main Results |
|---|---|---|---|---|---|---|---|---|
| Hacke and Weisser [35] | 532 | 12–17 | Both | Germany | Standard Stethoscope and Sphygmomanometer | SBP (rest) SBP (exercise) | Age, sex, height, BMI percentile, and physical fitness | Mean ± SB Rest = 0.1 (−0.7; 0.5), p = 0.945 Mean ± SB Exercise = 4.0 (3.1; 4.9), p = 0.030 |
| Khanolkar et al. [39] | 1171 | 5–14 | Both | Swedish | Dinamap Compact T’monitor | SBP | Age; gender, PS, family clustering, and parental EDL, occupation, and height | Mother: N = Reference; F = −0.44 (−2.21; 1.32); C = −0.04 (−2.34; 2.33). Father N = Reference; F = 0.70 (−1.06; 2.50); C = −0.40 (−3.22; 2.40) |
| Simonetti et al. [34] | 4236 | 5.7 ± 0.4 | Both | Germany | Auscultatory Aneroid Sphygmomanometry | SBP | Full adjustment for potential confounders by multivariable Regression analysis identified | Univariate Regression B (SE): 0.0044 (0.0010); p < 0.0001, R2 = 0.0049 |
| Kallio et al. [46] | 402 | 8–11 | Both | Finland | Standard Sphygmomanometer | SBP | Values are mean ± SD unless | Mean ± SB N = 104 ± 8; L = 104 ± 9; H = 104 ± 7; p = 0.99 |
| Zhang et al. [40] | 42.745 | 7–18 | Both | China | Auscultation Mercury Sphygmomanometer | SBP Hypertension | Crude M1: Age, height, and BMI M2: several life factors; maternal EDL; parental hypertension | Girls B (95%CI): Crude = 1.03 (0.72; 1.34); p < 0.001; M1 = 0.6 (0.41; 0.96), p < 0.001; M2 = 0.44 (0.16; 0.72), p = 0.0002 Boys B (95%CI): Crude = 0.30 (−0.03; 0.64), p = 0.01 M1 = 0.24 (−0.04; 0.52), p = 0.09; M2 = 0.06 (−0.22; 0.33), p = 0.69 Hypertension OR (95%CI) Girls: Crude = 1.19 (1.10; 1.29), p < 0.001; M1 = 1.15 (1.06; 1.25), p < 0.001; M2 = 1.11 (1.02; 1.20, p = 0.01 Boys: Crude = 1.01 (0.93,1.09), p = 0.90; M1 = 0.96 (0.89; 1.04), p = 0.32; M2 = 0.93 (0.86; 1.01), p = 0.09 |
| Li et al. [36] | 3150 | 6–18 | Both | China | standardised protocol | SBP BP | M1: age, sex, Tanner stage M2: M1 + residence, diet scores, parents’ EDL, parents’ BMI, and family history (diabetes) | Mean ± SB No-exposed = 106 ± 14; Parental smoking = 109 ± 14 M1 OR (95% CI) = 1.33 (1.13–1.58), p = 0.001 M2 OR (95% CI) = 1.22 (1.02–1.46), p = 0.028 |
| Khanolkar et al. [39] | 1171 | 5–14 | Both | Swedish | Dinamap Compact T’Monitor | DBP | Age; height, gender, PS, family clustering, parental EDL, and occupation | Mother B (95%CI): Never = Reference; Former = 0.72 (−1.71; 0.28); Current = −0.46 (−1.75; 0.84). Father B (95%CI): Never = Reference; Former = 0.24 (−0.77; 1.26); Current = −0.62 (−1.92; 0.68) |
| Simonetti et al. [34] | 4236 | 5.7 ± 0.4 | Both | Germany | Auscultatory Aneroid Sphygmomanometer | DBP | Full adjustment for potential confounders by multivariable Regression analysis identified | Univariate Regression B (SE): 0.0030 (0.0012); p = 0.01, R2 = 0.0013 Full Model B (SE): 0.1608 (0.2686), p = 0.5. |
| Kallio et al. [46] | 402 | 8–11 | Both | Finland | Standard Sphygmomanometer | DBP | Values are mean_SD unless otherwise stated | Serum Cotinine Concentration Mean ± SB Nondetectable = 63 ± 5; Low = 64 ± 6; High = 63 ± 5; p = 0.98 |
| Zhang et al. [40] | 42.745 | 7–18 | Both | China | Auscultation Mercury Sphygmomanometer | DBP Hypertension | Crude M1: Age, height, BMI M2: Several life factors; maternal EDL and parental hypertension | Girls B (95%CI): Crude = 0.60 (038; 0.83); p < 0.001; M1: 0.40 (0.19; 0.62); p < 0.001; M2: 0.26 (0.04; 0.47); p = 0.02 Boys B (95%CI): Crude = 0.18 (−0.06; 0.41), p = 0.14; M1: 0.13 (−0.08; 0.35); p = 0.23; M2: 0.0001 (−0.21; 0.21); p = 0.99 |
| Li et al. [36] | 3150 | 6–18 | Both | China | standardised protocol | DBP | M1: Age, sex, Tanner stage M2: M1 + residence, diet scores, parents’ EDL, parents’ BMI, and family history (diabetes) | Mean ± SB No-exposed = 66 ± 10; Parental smoking = 69 ± 10, p < 0.001 |
| Authors (Year) | Sample Size | Age | Parents | Country | Method (Device) | Outcome | Model Adjustment | Main Results |
|---|---|---|---|---|---|---|---|---|
| Schreier and Chen [24] | 88 | 9–18 | Both | Canada | BPM−100 automated BP monitor | SBP | Unadjusted Associations | 12-month SBP for: Aggregate SBP B (SE) = −0.010 (0.243), p = 0.97 Trajectories of SBP B (SE) = 0.185 (0.187) |
| Hacke and Weisser [35] | 532 | 12–17 | Both | Germany | Standard Stethoscope And Sphygmomanometer | SBP (rest) SBP (exercise) | Age, sex, height, BMI percentile, and physical fitness | Rest MD (95%CI): 1.3 (0.6; 1.9), p = 0.315 Exercise MD (95%C): 5.2 (4.3; 6.2), p = 0.006 |
| Khanolkar et al. [39] | 1171 | 5–14 | Both | Swedish | Dinamap Compact T’Monitor | SBP | Child’s age; gender, PS, family clustering, and parental EDL, occupation, and height | Mother B (95%CI): Other = 2.18 (−0.05; 4.42); Secondary = 2.17 * (0.63; 3.70); University = Reference; p < 0.05 * Father B (95%CI): Other = 2.72 * (0.11; 5.33); Secondary = 1.11 (−0.42; 2.65); University = Reference; p = p < 0.05 * |
| Calbano et al. [42] | 1486 | 8–14 | Mother | Argentina | Oscillometric Sphygmomanometer | SBP | Values are mean ± SD unless | Average SBP: Low = 110.24; Middle = 111.35; High = 110.68; p = 0.16 |
| Bouthoorn et al. [25] | 5843 | 6 (0.5) | Mother | Netherlands | Automatic Complior | SBP | Sex, age, and ethnicity | B (95%CI) Low = 2.28 * (1.62; 2.94); Mid-Low = 1.29 * (0.71; 1,87); Mid-High = 0.62 (−0.001; 1.24); High = Reference group; p < 0.001 * |
| Kvaavik et al. [38] | 325 | 11–16 | Both | Norway | 1979 and 1981: a random-zero sphygmomanometer; 1991: dinamap 2006: instrument not recorded | SBP | Sex; intervention and maturation | Mother B (95%CI) = −0.10 (−0.21; 0.002) Father B (95%CI) = −0.14 (−0.24; −0.03) |
| Simonetti et al. [34] | 4236 | 5.7 ± 0.4 | Both | Germany | Auscultatory Aneroid Sphygmomanometry | SBP | Full adjustment for potential confounders by multivariable Regression analysis identified | Univariate regression B (SE) = −0.0053 (0.0009); p < 0.0010; R2 = 0.0068. Multiple Regression (Full Model) B (SE) = −0.5756 (0.3008), p = 0.06 |
| Kwok et al. [41] | 5604 | ~13 | Both | Hong Kong | Oscillometric Device | SBP Prehypertension Hypertension | Unadjusted associations | Both SBP Mean (95%CI): Grade 9 or below = Reference, Grade 10–11 = −0.04 (−0.11; 0.02) Grade 12 or above = −0.11 * (−0.19; −0.04); Prehypertension in OR Grade 9 or below = 1.00; Grade 10–11 = 0.97 (0.84, 1.12); Grade 12 or above = 0.86 (0.73, 1.02); Hypertension in OR grade 9 or below = 1.00; Grade 10–11 = 0.84 (0.67; 1.05); Grade 12 or above = 0.78 (0.60; 1.02) Mother SBP Mean (95%CI): Grade 9 or below = Reference; Grade 10–11 = −0.08 * (−0.13; −0.02); Grade 12 or above = −0.11 * (−0.19; −0.03); Prehypertension in OR Grade 9 or below = 1.00; Grade 10–11 = 0.91 (0.79,1.04); Grade 12 or above = 0.92 (0.76,1.11); Hypertension in OR Grade 9 or below = 1.00; Grade 10–11 = 0.90 (0.73; 1.12); Grade 12 or above = 0.89 (0.66; 1.21) Father: SBP in mean difference Grade 9 or below = Reference; Grade 10–11 = −0.03 (−0.09; 0.04); Grade 12 or above = −0.10 * (−0.17; −0.03); Prehypertension in OR Below = 1.00; Grade 10–11 = 0.99 (0.86; 1.14); Grade 12 or above = 0.90 (0.76; 1.05); hypertension in OR Grade 9 or below = 1.00; Grade 10–11 = 1.00 (0.81; 1.25); Grade 12 or above = 0.82 (0.63, 1.07) |
| Kaczmarek et al. [47] | 4941 | 10–18 | Both | Poland | TECH MED TM-Z Mercury Gauge Sphygmomanometer | Prehypertension SBP Hypertension SBP | Multivariate analyses: FHH, sex, age, and weight status | Parents’ years of study Univariate analysis: Mother (<12 yrs) = 7.2%; (12 yrs) = 5.3%; (>12 yrs) = 3.5%; p = 0.002; Father: (<12 yrs) = 7.0%; (12 yrs) = 4.5%; (>12 yrs) = 2.7, p < 0.001 Results for hypertensive Maternal EDL Multivariate level (stepwise with backward elimination) in OR: Prehypertension (<12 yrs) = 1; (12 yrs) = 0.75 (0.69; 0.92); (>12 yrs) = 0.60 (0.48; 0.91); p = 0.002; hypertension (<12 yrs) = 1; (12 yrs) = 0.73 (0.62; 0.86); (>12 yrs) = 0.54 (0.39; 0.72), p = 0.0002 |
| Khanolkar et al. [39] | 1171 | 5–14 | Both | Swedish | Dinamap Compact T’Monitor | DBP | Child’s age, gender, pubertal stage, family clustering, and parental EDL, occupation, and height | Mother B (95%CI): Other = 0.88 (−0.42; 2.20); Secondary = 1.37 * (0.47 2.27); p < 0.05 *; University = Reference Father B (95%CI): Other = 0.46 (−0.92; 1.42); Secondary = 0.51 (−0.40; 1.42); University = Reference |
| Calbano et al. [42] | 1486 | 8–14 | Mother | Argentina | Oscillometric Sphygmomanometer | DBP | Values are mean ± SD unless otherwise stated | Average DBP Low = 63.78; Middle = 65.66; High = 64.29; p = 0.04. |
| Bouthoorn et al. [25] | 5843 | 6 (0.5) | Mother | Netherlands | Automatic Complior | DBP | Sex, age, and ethnicity | B (95%CI) Low = 1.80 (1.25; 2.35); p < 0.001; Mid-Low:1.27 (0.79; 1.76); p < 0.001; Mid-High = 0.69 (0.18; 1.21); p < 0.01; High = Reference |
| Kvaavik et al. [38] | 325 | 11–16 | Both | Norway | 1979 and 1981: a random-zero sphygmomanometer; 1991: dinamap 2006: instrument not recorded | DP | Sex, intervention, and maturation | Mother: B (95%CI) = −0.03 (−0.14; 0.08) Father: B (95%CI) = −0.03 (−0.13; 0.08) |
| Simonetti et al. [34] | 4236 | 5.7 ± 0.4 | Both | Germany | Auscultatory Aneroid Sphygmomanometry | DBP | Full adjustment for potential confounders by multivariable Regression analysis identified | Univariate Regression B (SD) = 0.0048 (0.0011), p < 0.000; R2 = 0.0036 Multiple Regression (Full Model) B (SD) = −0.1851 (0.2619), p = 0.5 |
| Kwok et al. [41] | 5604 | ~13 | Both | Hong Kong | Oscillometric Device | DBP | Unadjusted associations | Both B (95% CI): Low = Reference; Middle = −0.05 * (−0.08; 0.01); High = −0.07 * (−0.11; −0.04); Mother B (95% CI): Low = Reference; Mid = −0.06 * (−0.009; −0.02); High = −0.07 * (−0.11; −0.03) Father B (95% CI): Low = Reference; Middle = −0.04 * (−0.07; −0.003); High = −0.06 (−0.09; −0.02) |
| Kaczmarek et al. [47] | 4941 | 10–18 | Both | Poland | TECH MED TM-Z Mercury Gauge Sphygmomanometer | DBP | FHH, sex, age, and weight status | Univariate analysis: Mother Low = 6.1%; Middle = 3.6%; High = 2.5%; p < 0.001; Father: Low = 5.8%; Middle = 2.7%; High = 1.7, p < 0.001 Maternal EDL Multivariate level (stepwise with backward elimination) in OR: Prehypertension Low = 1; Middle = 0.66 (0.54; 0.81); High = 0.44 (0.29; 0.66); p < 0.0001; hypertension Low = 1; Middle = 0.60 (0.49; 0.74); High = 0.36 (0.24; 0.55), p < 0.0001 |
| Authors (Year) | Sample Size | Age | Parents | Country | Method (Device) | Outcome | Model Adjustment | Main Results |
|---|---|---|---|---|---|---|---|---|
| Khanolkar et al. [39] | 1171 | 5–14 | Both | Swedish | Dinamap Compact T’Monitor | SBP | Child’s age, gender, PS, family clustering, and parental EDL, occupation, and height | Occupation Mother B (95%CI): Higher = Reference; Lower = 0.43 (−1.78; 2.65); Skilled = 1.68 (−0.62; 3.98); Unskilled = 0.50 (−1.30; 2.27); Farmer = 2.28 * (−0.53; 5.10), p < 0.05 * Father B (95%CI): Higher = Reference; Lower = 1.68 (−0.61; 3.98); Skilled = 3.04 * (1.05; 5.03); Unskilled = 1.96 (−0.38; 4.30); Farmer = 0.92 (−1.85; 3.70), p < 0.05 *; |
| Calbano et al. [42] | 1486 | 8–14 | Mother | Argentina | Oscillometric Sphygmomanometer | SBP | Values are mean ± SD unless otherwise stated | Average SBP: Low = 110.06; Middle = 110.74; High = 110.66, p = 0. 074. |
| Kwok et al. [30] | 4989 | ~13 | Both | Hong Kong | Oscillometric Device | SBP Prehypertension Hypertension | Total effect, direct effect of parental education (effect not via mediators was adjusted for maternal and paternal ages and birthplace). | Absolute Income, p = 0.35 Indirect Effect (IE) B (95%CI) = 0.005 (−0.01; 0.020), Direct Effect € B (95%CI) = 0.09 (0.02; 0.16), Total Effect (TE) B (95%CI) = 0.10 (0.03; 0.16); Proportion Mediator (PM) B (95%CI) = 0.05 (−0.17; 0.35) Relative Income, p = 0.99 IE B (95%CI) = 0.01 (−0.03; 0.05); DE B (95%CI) = 0.08 (0.002; 0.16); TE B (95%CI) = (0.02; 0.16); PM B (95%CI) = (−0.41; 0.86) |
| Kaczmarek et al. [47] | 4941 | 10–18 | Both | Poland | TECH MED TM-Z Mercury Gauge Sphygmomanometer | Prehypertension SBP Hypertension SBP | FHH, sex, age, and weight status | Place of residence: A higher prevalence tendency of SBP hypertension in the rural areas group (9.0%, p < 0.001). Urban ≥ 100.000 inhabitants’ group is less likely to develop prehypertension OR = 0.56 (0.37; 0.82); p = 0.004 and hypertension OR = 0.40 (0.29; 0.55), p < 0.0001 than rural areas group Occupation: A higher prevalence tendency of SBP hypertension in the Economically inactive (EI) group (5.7%, p = 0.005 for fathers and 5.9%, p = 0.004 for mothers). This group is more likely to develop. Prehypertension OR = 1.53 (1.04; 2.25); p = 0.029 p < 0.0001 than those group of economically active Income Adequacy: higher prevalence of SBP hypertensive income inadequacy group (5.3%, p = 0.047. This is more likely to develop prehypertension OR = 1.40 (1.17; 1.94); p = 0.019 than those from income adequacy group |
| Balogun et al. [37] | 229 | 8–20 | Both | Nigeria | Electronic BP Monitoring Kit | SBP | Age, height, and weight | Income and Occupation Mean ± SD: Low = 106.0 ± 14.4; Middle = 05.0 ± 13.9; Upper = 101.9 ± 13.2; p = 0.0268, F ratio = 3.6 |
| Ansa et al. [43] | 964 | 10–17 | Both | Nigeria | Accuson® Mercury Sphygmomanometer | SBP | Values are mean ± SD unless otherwise stated | School’s Average Fees Girls Mean ± SD: Low5 = 106.8 ± 11.8; Low4 = 103.9 ± 11.3; Middle Class3 = 104.4 ± 12.3; Middle class2 = 105.7 ± 13.2; Upper = 100.8 ± 12.3; p = 0.009 Boys Mean ± SD: Low 5 = 07.4 ± 12.8; Low 4 = 108.5 ± 14.5; Middle Class 3 = 106.2 ± 12.4; Middle class 2 = 105.4 ± 11.4; Upper = 102.9 ± 12.3; p = 0.143 |
| Kinra et al. [44] | 862 | 13–18 | Both | India | Oscillometric Device | SBP | M1: Age, sex, nutritional supplementation, AR, and HR M2: M1 + height M3: M2 + Pubertal stage, FTM, and cPSR | Standard of Living Index Mean ± SD Girls: Low = 107.5 (8.3); Middle = 107.5 (9.3); High = 107.2 (9.2); p = 0.917 Mean ± SD Boys: Low = 109.7 (11.1); Middle = 110.2 (10.7); High = 112.2 (10.9); p = 0.080 B (95%CI) M1: 0.55 (−0.23; 1.33); p = 0.16 B (95%CI) M2: 0.09 (−0.61; 0.78); p = 0.80 B (95%CI) M3: 0.15 (−0.58; 0.87); p = 0.68 |
| Leino et al. [48] | 1211 | 9, 12 and 15 | Father | Finns | Zero Sphygmomanometer | SBP | Values are mean ± SD unless otherwise stated | Occupation Mean ± SD Girls: Upper level = 109.1 (9.1); F = 108.2 (10.5), p = 0.59 Mean ± SD Boys: Upper level = 106.3 (9.7); Boys F = 111.2 (12.3), p = 0.02 |
| Khanolkar et al. [39] | 1171 | 5–14 | Both | Swedish | Dinamap Compact T’Monitor | DBP | Child’s age; gender, PS, family clustering, and parental EDL, occupation, and height | Mother B (95%CI): H = Reference; L = −0.27 (−1.64; 1.08); S = 0.45 (−0.75; 1.65); U = 0.20 (−0.88; 1.30); F = 1.24 (−0.73; 3.21) Father B (95%CI): H = Reference; L = 0.06 (−1.50; 1.63); S = 0.97 (−0.10; 2.05); U = 0.06 (−1.15; 1.30); F = 0.45 (−1.70; 2.60) |
| Calbano et al. [42] | 1486 | 8–14 | Mother | Argentina | Oscillometric Sphygmomanometer | DBP | Values are mean ± SD unless otherwise stated | Average DBP: Low = 64.03; Middle = 64.21; High = 64.44; p = 0.089 |
| Kwok et al. [30] | 4989 | ~13 | Both | Hong Kong | Oscillometric Device | DBP Prehypertension Hypertension | Total effect, direct effect of parental education (effect not via mediators was adjusted for parents’ ages and birthplace). | Absolute Income, p = 0.10 Indirect Effect (IE) B (95%CI) = −0.001 (−0.01; 0.01); Direct Effect (DE) B (95%CI) = 0.04 (0.002; 0.08); Total Effect (TE) B (95%CI) = 0.04 (0.01; 0.08); Proportion Mediated (PM) B (95%CI) = −0.02 (−0.52; 0.42) Relative Income, p = 0.20 IE B (95%CI) = 0.02 (−0.005; 0.05); DE B (95%CI) = 0.02 (−0.02; 0.07); TE B (95%CI) = 0.04 (−0.005; 0.09); PM B (95%CI) = 0.38 (−1.07; 2.23) |
| Kaczmarek et al. [47] | 4941 | 10–18 | Both | Poland | TECH MED TM-Z Mercury Gauge Sphygmomanometer | Prehypertension DBP Hypertension DBP | FHH, sex, age, and weight | Place of Residence: A higher prevalence of DBP hypertension in the rural areas group (7.2%, p < 0.001). UH group is less likely to develop DBP prehypertension OR = 0.26 (0.17; 0.39); p < 0.0001 and hypertension OR = 0.17 (0.11; 0.27), p < 0.0001 than RA group Occupation: A higher prevalence tendency of DBP hypertension in fathers’ EI group (5.3%, p = 0.003) and mothers’ other group (7.0%, p < 0.001). Income Adequacy: A higher prevalence of DBP hypertensive in enough adequacy (5.5%, p = 0.041). |
| Balogun et al. [37] | 229 | 8–20 | Both | Nigeria | Electronic BP Monitoring Kit | DBP | Age, height, and weight | Income and Occupation Mean ± SD: Low = 73.5 ± 9.9; Middle = 72.9 ± 9.3; Upper = 71.8 ± 9.6; p = 0.1918, F ratio = 1.7 |
| Ansa et al. [43] | 964 | 10–17 | Both | Nigeria | Accuson® Mercury Sphygmomanometer | DBP | Values are mean ± SD unless otherwise stated | School’s Average Fees Girls Mean ± SD: Low5 = 68.5 ± 8.0; Low4 = 66.5 ± 9.1; Middle class3 = 64.6 ± 9.8; Middle class2 = 65.5 ± 9.8; Upper = 62.8 ± 9.8; p = 0.002 Boys Mean ± SD: Low5 = 18.1 ± 10.4; Low4 = 66.2 ± 9.7; Middle class3 = 65.4 ± 8.8; Middle class2 = 64.9 ± 9.5; Upper = 64.3 ± 9.3, p = 0.125 In girls, DBP was significantly associated with SES. The lower the SES, the lower the DBP |
| Kinra et al. [44] | 862 | 13–18 | Both | India | Oscillometric Device | DBP | M1: Age, sex, nutritional supplementation, AR, and HR M2: M1 + height M3: M2 + Pubertal stage, FMI, and cPSR | Standard of Living Index Girls: Low = 63.0 (5.7); Middle = 62.6 (7.0); High = 62.7 (6.0); p = 0.924 Boys: Low = 61.4 (6.8); Middle = 61.7 (6.5); High = 63.8 (6.4); p = 0.001 B (95%CI) M1: 0.54 (0.007; 1.00), p = 00.03; B (95%CI) M2: 0.40 (−0.07; 0.87); p = 0.09; B (95%CI) M3: 0.38 (−0.08; 0.84); p = 0.10 |
| Leino et al. [48] | 1211 | 9, 12 and 15 | Father | Finns | Zero Sphygmomanometer | DBP | Values are mean ± SD unless otherwise stated | Mean ± SD Girls: I = 63.2 (9.6); F = 61.9 (10.0); p = 0.78 Mean ± SD boys: I = 61.1 (8.3); F = 58 (9.2); p = 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.C.; Maia, F.; Estima, F.; Gaya, A.; Christofaro, D.; Mota, J.; Aires, L.; Silva, G. The Influence of Parental Behaviours and Socioeconomic Factors on Offspring’s Precursors of Atherosclerosis: A Systematic Review and Meta-Analysis of Observational Studies. Int. J. Environ. Res. Public Health 2025, 22, 1791. https://doi.org/10.3390/ijerph22121791
Silva AC, Maia F, Estima F, Gaya A, Christofaro D, Mota J, Aires L, Silva G. The Influence of Parental Behaviours and Socioeconomic Factors on Offspring’s Precursors of Atherosclerosis: A Systematic Review and Meta-Analysis of Observational Studies. International Journal of Environmental Research and Public Health. 2025; 22(12):1791. https://doi.org/10.3390/ijerph22121791
Chicago/Turabian StyleSilva, Ana Carvalhinho, Filipe Maia, Francisco Estima, Anelise Gaya, Diego Christofaro, Jorge Mota, Luísa Aires, and Gustavo Silva. 2025. "The Influence of Parental Behaviours and Socioeconomic Factors on Offspring’s Precursors of Atherosclerosis: A Systematic Review and Meta-Analysis of Observational Studies" International Journal of Environmental Research and Public Health 22, no. 12: 1791. https://doi.org/10.3390/ijerph22121791
APA StyleSilva, A. C., Maia, F., Estima, F., Gaya, A., Christofaro, D., Mota, J., Aires, L., & Silva, G. (2025). The Influence of Parental Behaviours and Socioeconomic Factors on Offspring’s Precursors of Atherosclerosis: A Systematic Review and Meta-Analysis of Observational Studies. International Journal of Environmental Research and Public Health, 22(12), 1791. https://doi.org/10.3390/ijerph22121791









