A Review of the Human Health Risks from Microbial Hazards in Recreational Beach Sand
Abstract
1. Introduction
2. Scoping for Local Relevance
3. Microorganisms in the Beach Environment
4. Faecal Indicators in Beach Sands
4.1. Faecal Indicator Bacteria
4.1.1. Environmental Factors Affecting FIB Survival
4.1.2. ‘Hot Spots’ of Contamination
4.2. Genetic Indicators for Faecal Contamination
5. Microbial Hazards in Beach Sands
- Bacteria 70 days, usually <20 days (thermotolerant coliforms and Salmonella spp.)
- Viruses 70 days, usually <100 days (enterovirus)
- Protozoa 150 days, usually <75 days (Cryptosporidium spp.)
5.1. Bacterial Hazards
5.2. Viral Hazards
5.3. Protozoan Parasitic Hazards
5.4. Fungal Hazards
6. Adverse Health Events Linked to Contact with Beach Sand
6.1. Epidemiological Studies
6.2. Outbreak Reports
6.3. Quantitative Microbial Risk Assessments (QMRAs)
- 10–1000 oocysts/g sand of Cryptosporidium spp. via oral exposure.
- 5–500 MPN/g sand of enterovirus via oral exposure.
- 106–107 CFU/g sand of S. aureus via dermal exposure.
7. Integrating Beach Sand Monitoring for Improving Environmental Health
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | Colony forming units |
| CI | Confidence interval |
| FIB | Faecal indicator bacteria |
| GC | Genome copies |
| GI | Gastrointestinal illness |
| MST | Molecular source tracking |
| MPN | Most probable number |
| NGS | Next generation sequencing |
| PCR | Polymerase chain reaction |
| QMRA | Quantitative microbial risk assessment |
| STEC | Shiga toxin-forming Escherichia coli |
| WGS | Whole genome sequencing |
| WHO | World Health Organization |
References
- WHO. Guidelines on Recreational Water Quality. Volume 1: Coastal and Fresh Waters; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Devane, M.L.; Moriarty, E.; Weaver, L.; Cookson, A.; Gilpin, B. Fecal indicator bacteria from environmental sources; strategies for identification to improve water quality monitoring. Water Res. 2020, 185, 116204. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.E.; Solo-Gabriele, H.M.; Elmir, S.; Fleming, L.E. Microbial load from animal feces at a recreational beach. Mar. Pollut. Bull. 2009, 58, 1649–1656. [Google Scholar] [CrossRef]
- Ministry for the Environment. Microbiological Water Quality Guidelines for Marine and Freshwater Recreational Areas; New Zealand Ministry for the Environment: Wellington, New Zealand, 2003.
- USEPA. Recreational Water Quality Criteria; Office of Water 820-F-12-058; United States Environmental Protection Agency: Washington, DC, USA, 2012.
- Wade, T.J.; Arnold, B.F.; Schiff, K.; Colford, J.M., Jr.; Weisberg, S.B.; Griffith, J.F.; Dufour, A.P. Health risks to children from exposure to fecally-contaminated recreational water. PLoS ONE 2022, 17, e0266749. [Google Scholar] [CrossRef]
- Brandão, J.; Valério, E.; Weiskerger, C.; Veríssimo, C.; Sarioglou, K.; Novak Babič, M.; Solo-Gabriele, H.M.; Sabino, R.; Rebelo, M.T. Strategies for monitoring microbial life in beach sand for protection of public health. Int. J. Environ. Res. Public Health 2023, 20, 5710. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- ESR. Notifiable Diseases in New Zealand: Annual Report 2023; Client Report FW24038 for the Ministry of Health; Institute of Environmental Science and Research: Porirua, New Zealand, 2025. [Google Scholar]
- Lee, C.M.; Lin, T.Y.; Lin, C.C.; Kohbodi, G.A.; Bhatt, A.; Lee, R.; Jay, J.A. Persistence of fecal indicator bacteria in Santa Monica Bay beach sediments. Water Res. 2006, 40, 2593–2602. [Google Scholar] [CrossRef]
- Whitman, R.L.; Harwood, V.J.; Edge, T.A.; Nevers, M.B.; Byappanahalli, M.; Vijayavel, K.; Brandao, J.; Sadowsky, M.J.; Alm, E.W.; Crowe, A.; et al. Microbes in beach sands: Integrating environment, ecology and public health. Rev. Environ. Sci. Biotechnol. 2014, 13, 329–368. [Google Scholar] [CrossRef] [PubMed]
- Staley, C.; Sadowsky, M.J. Regional similarities and consistent patterns of local variation in beach sand bacterial communities throughout the Northern Hemisphere. Appl. Environ. Microbiol. 2016, 82, 2751–2762. [Google Scholar] [CrossRef]
- Brandão, J.; Albergaria, I.; Albuquerque, J.; José, S.; Grossinho, J.; Ferreira, F.C.; Raposo, A.; Rodrigues, R.; Silva, C.; Jordao, L.; et al. Untreated sewage contamination of beach sand from a leaking underground sewage system. Sci. Total Environ. 2020, 740, 140237. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Sarmah, A.K.; Dubey, B. Microplastics in the NZ environment: Current status and future directions. Case Stud. Chem. Environ. Eng. 2021, 3, 100076. [Google Scholar] [CrossRef]
- Hernandez, R.J.; Hernandez, Y.; Jimenez, N.H.; Piggot, A.M.; Klaus, J.S.; Feng, Z.X.; Reniers, A.; Solo-Gabriele, H.M. Effects of full-scale beach renovation on fecal indicator levels in shoreline sand and water. Water Res. 2014, 48, 579–591. [Google Scholar] [CrossRef]
- Archana, A.; Francis, C.; Boehm, A. The beach aquifer microbiome: Research gaps and data needs. Front. Environ. Sci. 2021, 9, 653568. [Google Scholar] [CrossRef]
- Brandão, J.; Gangneux, J.P.; Arikan-Akdagli, S.; Barac, A.; Bostanaru, A.C.; Brito, S.; Bull, M.; Çerikçioğlu, N.; Chapman, B.; Efstratiou, M.A.; et al. Mycosands: Fungal diversity and abundance in beach sand and recreational waters—Relevance to human health. Sci. Total Environ. 2021, 781, 146598. [Google Scholar] [CrossRef] [PubMed]
- Korajkic, A.; McMinn, B.R.; Harwood, V.J. Relationships between microbial indicators and pathogens in recreational water settings. Int. J. Environ. Res. Public Health 2018, 15, 2842. [Google Scholar] [CrossRef]
- Rodrigues, C.; Cunha, M.Â. Assessment of the microbiological quality of recreational waters: Indicators and methods. Euro-Mediterr. J. Environ. Integr. 2017, 2, 25. [Google Scholar] [CrossRef]
- Boehm, A.B.; Graham, K.E.; Jennings, W.C. Can we swim yet? Systematic review, meta-analysis, and risk assessment of aging sewage in surface waters. Environ. Sci. Technol. 2018, 52, 9634–9645. [Google Scholar] [CrossRef] [PubMed]
- Whitman, R.L.; Przybyla-Kelly, K.; Shively, D.A.; Nevers, M.B.; Byappanahalli, M.N. Hand-mouth transfer and potential for exposure to E. coli and F+ coliphage in beach sand, Chicago, Illinois. J. Water Health 2009, 7, 623–629. [Google Scholar] [CrossRef][Green Version]
- Yamahara, K.M.; Layton, B.A.; Santoro, A.E.; Boehm, A.B. Beach sands along the California coast are diffuse sources of fecal bacteria to coastal waters. Environ. Sci. Technol. 2007, 41, 4515–4521. [Google Scholar] [CrossRef]
- Rumball, N.A.; Alm, E.W.; McLellan, S.L. Genetic determinants of Escherichia coli survival in beach sand. Appl. Environ. Microbiol. 2023, 89, e0142322. [Google Scholar] [CrossRef]
- Alm, E.W.; Burke, J.; Spain, A. Fecal indicator bacteria are abundant in wet sand at freshwater beaches. Water Res. 2003, 37, 3978–3982. [Google Scholar] [CrossRef]
- Phillips, M.C.; Solo-Gabriele, H.M.; Piggot, A.M.; Klaus, J.S.; Zhang, Y.F. Relationships between sand and water quality at recreational beaches. Water Res. 2011, 45, 6763–6769. [Google Scholar] [CrossRef] [PubMed]
- Whitman, R.L.; Nevers, M.B. Foreshore sand as a source of Escherichia coli in nearshore water of a Lake Michigan beach. Appl. Environ. Microbiol. 2003, 69, 5555–5562. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, T.D.; Nowosielski, K.; Cuvelier, M.; Hartz, A.; Green, M.; Esiobu, N.; McCorquodale, D.S.; Fleisher, J.M.; Rogerson, A. Prevalence and distribution of fecal indicator organisms in South Florida beach sand and preliminary assessment of health effects associated with beach sand exposure. Mar. Pollut. Bull. 2007, 54, 1472–1482. [Google Scholar] [CrossRef]
- Zhang, Q.; He, X.; Yan, T. Differential decay of wastewater bacteria and change of microbial communities in beach sand and seawater microcosms. Environ. Sci. Technol. 2015, 49, 8531–8540. [Google Scholar] [CrossRef]
- Abdelzaher, A.M.; Wright, M.E.; Ortega, C.; Solo-Gabriele, H.M.; Miller, G.; Elmir, S.; Newman, X.; Shih, P.; Bonilla, J.A.; Bonilla, T.D.; et al. Presence of pathogens and indicator microbes at a non-point source subtropical recreational marine beach. Appl. Environ. Microbiol. 2010, 76, 724–732. [Google Scholar] [CrossRef]
- Halliday, E.; Gast, R.J. Bacteria in beach sands: An emerging challenge in protecting coastal water quality and bather health. Environ. Sci. Technol. 2011, 45, 370–379. [Google Scholar] [CrossRef]
- Sabino, R.; Rodrigues, R.; Costa, I.; Carneiro, C.; Cunha, M.; Duarte, A.; Faria, N.; Ferreira, F.C.; Gargaté, M.J.; Júlio, C.; et al. Routine screening of harmful microorganisms in beach sands: Implications to public health. Sci. Total Environ. 2014, 472, 1062–1069. [Google Scholar] [CrossRef]
- Esiobu, N.; Green, M.; Echeverry, A.; Bonilla, T.D.; Stinson, C.M.; Hartz, A.; Rogerson, A.; McCorquodale, D.S. High numbers of Staphylococcus aureus at three bathing beaches in South Florida. Int. J. Environ. Res. Public Health 2013, 23, 46–57. [Google Scholar] [CrossRef]
- Gerbersdorf, S.U.; Koca, K.; de Beer, D.; Chennu, A.; Noss, C.; Risse-Buhl, U.; Weitere, M.; Eiff, O.; Wagner, M.; Aberle, J.; et al. Exploring flow-biofilm-sediment interactions: Assessment of current status and future challenges. Water Res. 2020, 185, 116182. [Google Scholar] [CrossRef] [PubMed]
- Malham, S.K.; Rajko-Nenow, P.; Howlett, E.; Tuson, K.E.; Perkins, T.L.; Pallett, D.W.; Wang, H.; Jago, C.F.; Jones, D.L.; McDonald, J.E. The interaction of human microbial pathogens, particulate material and nutrients in estuarine environments and their impacts on recreational and shellfish waters. Environ. Sci. Process. Impacts 2014, 16, 2145–2155. [Google Scholar] [CrossRef]
- Hassard, F.; Gwyther, C.L.; Farkas, K.; Andrews, A.; Jones, V.; Cox, B.; Brett, H.; Jones, D.L.; McDonald, J.E.; Malham, S.K. Abundance and distribution of enteric bacteria and viruses in coastal and estuarine sediments—A review. Front. Microbiol. 2016, 7, 1692. [Google Scholar] [CrossRef]
- Fries, J.S.; Characklis, G.W.; Noble, R.T. Sediment-water exchange of Vibrio sp. and fecal indicator bacteria: Implications for persistence and transport in the Neuse River Estuary, North Carolina, USA. Water Res. 2008, 42, 941–950. [Google Scholar] [CrossRef]
- Piggot, A.M.; Klaus, J.S.; Johnson, S.; Phillips, M.C.; Solo-Gabriele, H.M. Relationship between enterococcal levels and sediment biofilms at recreational beaches in South Florida. Appl. Environ. Microbiol. 2012, 78, 5973–5982. [Google Scholar] [CrossRef]
- Korajkic, A.; Wanjugi, P.; Brooks, L.; Cao, Y.; Harwood, V.J. Persistence and decay of fecal microbiota in aquatic habitats. Microbiol. Mol. Biol. Rev. 2019, 83, e00005–e00019. [Google Scholar] [CrossRef]
- Yamahara, K.M.; Walters, S.P.; Boehm, A.B. Growth of enterococci in unaltered, unseeded beach sands subjected to tidal wetting. Appl. Environ. Microbiol. 2009, 75, 1517–1524. [Google Scholar] [CrossRef]
- Yamahara, K.M.; Sassoubre, L.M.; Goodwin, K.D.; Boehm, A.B. Occurrence and persistence of bacterial pathogens and indicator organisms in beach sand along the California coast. Appl. Environ. Microbiol. 2012, 78, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Mika, K.B.; Imamura, G.; Chang, C.; Conway, V.; Fernandez, G.; Griffith, J.F.; Kampalath, R.A.; Lee, C.M.; Lin, C.-C.; Moreno, R.; et al. Pilot- and bench-scale testing of faecal indicator bacteria survival in marine beach sand near point sources. J. Appl. Microbiol. 2009, 107, 72–84. [Google Scholar] [CrossRef]
- Beversdorf, L.J.; Bornstein-Forst, S.M.; McLellan, S.L. The potential for beach sand to serve as a reservoir for Escherichia coli and the physical influences on cell die-off. J. Appl. Microbiol. 2007, 102, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Abdool-Ghany, A.A.; Sahwell, P.J.; Klaus, J.; Gidley, M.L.; Sinigalliano, C.D.; Solo-Gabriele, H.M. Fecal indicator bacteria levels at a marine beach before, during, and after the COVID-19 shutdown period and associations with decomposing seaweed and human presence. Sci. Total Environ. 2022, 851, 158349. [Google Scholar] [CrossRef] [PubMed]
- Halliday, E.; Ralston, D.K.; Gast, R.J. Contribution of sand-associated enterococci to dry weather water quality. Environ. Sci. Technol. 2015, 49, 451–458. [Google Scholar] [CrossRef]
- Hartz, A.; Cuvelier, M.; Nowosielski, K.; Bonilla, T.D.; Green, M.; Esiobu, N.; McCorquodale, D.S.; Rogerson, A. Survival potential of Escherichia coli and enterococci in subtropical beach sand: Implications for water quality managers. J. Environ. Qual. 2008, 37, 898–905. [Google Scholar] [CrossRef]
- Korajkic, A.; Wanjugi, P.; Harwood, V.J. Indigenous microbiota and habitat influence Escherichia coli survival more than sunlight in simulated aquatic environments. Appl. Environ. Microbiol. 2013, 79, 5329–5337. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Goto, D.; Yan, T. Effects of autochthonous microbial community on the die-off of fecal indicators in tropical beach sand. FEMS Microbiol. Ecol. 2010, 74, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.A.; Turner, S.J.; Lewis, G.D. Enterococci in the New Zealand environment: Implications for water quality monitoring. Water Sci. Technol. 1997, 35, 325–331. [Google Scholar] [CrossRef]
- Imamura, G.J.; Thompson, R.S.; Boehm, A.B.; Jay, J.A. Wrack promotes the persistence of fecal indicator bacteria in marine sands and seawater. FEMS Microbiol. Ecol. 2011, 77, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kinzelman, J.L.; Pond, K.R.; Longmaid, K.D.; Bagley, R.C. The effect of two mechanical beach grooming strategies on Escherichia coli density in beach sand at a southwestern Lake Michigan beach. Aquat. Ecosyst. Health Manag. 2004, 7, 425–432. [Google Scholar] [CrossRef]
- Kinzelman, J.L.; Whitman, R.L.; Byappanahalli, M.; Jackson, E.; Bagley, R.C. Evaluation of beach grooming techniques on Escherichia coli density in foreshore sand at North Beach, Racine, WI. Lake Reserv. Manag. 2003, 19, 349–354. [Google Scholar] [CrossRef]
- Metcalf, R.; Fellows, R.; White, H.L.; Quilliam, R.S. Persistence of ‘wet wipes’ in beach sand: An unrecognised reservoir for localised E. coli contamination. Mar. Pollut. Bull. 2024, 201, 116175. [Google Scholar] [CrossRef]
- Cloutier, D.D.; McLellan, S.L. Distribution and differential survival of traditional and alternative indicators of fecal pollution at freshwater beaches. Appl. Environ. Microbiol. 2017, 83, e02881-16. [Google Scholar] [CrossRef]
- Cui, H.L.; Yang, K.; Pagaling, E.; Yan, T. Spatial and temporal variation in enterococcal abundance and its relationship to the microbial community in Hawaii beach sand and water. Appl. Environ. Microbiol. 2013, 79, 3601–3609. [Google Scholar] [CrossRef]
- Solo-Gabriele, H.M.; Harwood, V.J.; Kay, D.; Fujioka, R.S.; Sadowsky, M.J.; Whitman, R.L.; Wither, A.; Caniça, M.; Carvalho da Fonseca, R.; Duarte, A.; et al. Beach sand and the potential for infectious disease transmission: Observations and recommendations. J. Mar. Biol. Assoc. UK 2016, 96, 101–120. [Google Scholar] [CrossRef]
- Gast, R.J.; Elgar, S.; Raubenheimer, B. Observations of transport of bacterial-like microspheres through beach sand. Cont. Shelf Res. 2015, 97, 1–6. [Google Scholar] [CrossRef]
- Feng, Z.; Reniers, A.; Haus, B.K.; Solo-Gabriele, H.M.; Kelly, E.A. Wave energy level and geographic setting correlate with Florida beach water quality. Mar. Pollut. Bull. 2016, 104, 54–60. [Google Scholar] [CrossRef]
- Vogel, L.J.; O’Carroll, D.M.; Edge, T.A.; Robinson, C.E. Release of Escherichia coli from foreshore sand and pore water during intensified wave conditions at a recreational beach. Environ. Sci. Technol. 2016, 50, 5676–5684. [Google Scholar] [CrossRef]
- Nevers, M.B.; Byappanahalli, M.N.; Nakatsu, C.H.; Kinzelman, J.L.; Phanikumar, M.S.; Shively, D.A.; Spoljaric, A.M. Interaction of bacterial communities and indicators of water quality in shoreline sand, sediment, and water of Lake Michigan. Water Res. 2020, 178, 115671. [Google Scholar] [CrossRef]
- Staley, Z.R.; Vogel, L.; Robinson, C.; Edge, T.A. Differential occurrence of Escherichia coli and human Bacteroidales at two Great Lakes beaches. J. Great Lakes Res. 2015, 41, 530–535. [Google Scholar] [CrossRef]
- Suzuki, Y.; Teranishi, K.; Matsuwaki, T.; Nukazawa, K.; Ogura, Y. Effects of bacterial pollution caused by a strong typhoon event and the restoration of a recreational beach: Transitions of fecal bacterial counts and bacterial flora in beach sand. Sci. Total Environ. 2018, 640–641, 52–61. [Google Scholar] [CrossRef]
- Whiley, H.; Austin, J.; da Silva, G.M.; Ross, K. Faecal indicator bacteria present in sand at South Port Beach, South Australia. J. Coast. Res. 2018, 34, 215–219. [Google Scholar] [CrossRef]
- Sibanda, T.; Ramganesh, S. Taxonomic and functional analyses reveal existence of virulence and antibiotic resistance genes in beach sand bacterial populations. Arch. Microbiol. 2021, 203, 1753–1766. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.; Gilpin, B.; Horn, B.; Coxon, S.; Armstrong, B.; Scholes, P.; Haysome, I.; Priya, B.; Eaton, C.; Cornelius, A.; et al. Quantitative Microbial Risk Assessment Phase 2.1—Initial Data Collection and Recommendations; Client Report FW21019 for the Ministry for the Environment; Institute of Environmental Science and Research: Christchurch, New Zealand, 2021. [Google Scholar]
- Valério, E.; Santos, M.L.; Teixeira, P.; Matias, R.; Mendonça, J.; Ahmed, W.; Brandão, J. Microbial Source Tracking as a method of determination of beach sand contamination. Int. J. Environ. Res. Public Health 2022, 19, 7934. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Otto, M. Staphylococcus aureus colonisation and strategies for decolonisation. Lancet Microbe 2024, 5, e606–e618. [Google Scholar] [CrossRef]
- Ceylan, E.; Berktas, M.; Ağaoğlu, Z. The occurrence and antibiotic resistance of motile Aeromonas in livestock. Trop. Anim. Health Prod. 2009, 41, 199–204. [Google Scholar] [CrossRef]
- Kooh, P.; Thébault, A.; Cadavez, V.; Gonzales-Barron, U.; Villena, I. Risk factors for sporadic cryptosporidiosis: A systematic review and meta-analysis. Microb. Risk Anal. 2021, 17, 100116. [Google Scholar] [CrossRef]
- WHO. Guidelines for the Safe Use of Wastewater, Excreta and Greywater; World Health Organization: Geneva, Switzerland, 2006; Volume 2. [Google Scholar]
- Khan, I.U.; Loughborough, A.; Edge, T.A. DNA-based real-time detection and quantification of aeromonads from fresh water beaches on Lake Ontario. J. Water Health 2009, 7, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Bolton, F.J.; Surman, S.B.; Martin, K.; Wareing, D.R.; Humphrey, T.J. Presence of Campylobacter and Salmonella in sand from bathing beaches. Epidemiol. Infect. 1999, 122, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.H.; Hill, S.; Nowak, E.; Palmer, M.E.; Jarjanazi, H.; Lee, D.Y.; Mueller, M.; Schop, R.; Weir, S.; Irwin Abbey, A.M.; et al. Investigation of the prevalence of thermophilic Campylobacter species at Lake Simcoe recreational beaches. Inland Waters 2013, 3, 93–104. [Google Scholar] [CrossRef]
- Eichmiller, J.J.; Borchert, A.J.; Sadowsky, M.J.; Hicks, R.E. Decay of genetic markers for fecal bacterial indicators and pathogens in sand from Lake Superior. Water Res. 2014, 59, 99–111. [Google Scholar] [CrossRef]
- Harrison, S.; Kinra, S. Outbreak of Escherichia coli O157 associated with a busy bathing beach. Commun. Dis. Public Health 2004, 7, 47–50. [Google Scholar]
- Goodwin, K.D.; Matragrano, L.; Wanless, D.; Sinigalliano, C.D.; LaGier, M.J. A preliminary investigation of fecal indicator bacteria, human pathogens, and source tracking markers in beach water and sand. Environ. Res. J. 2009, 2, 395–417. [Google Scholar]
- Cabot, M.E.; Piccini, C.; Inchausti, P.; de la Escalera, G.M.; García-Alonso, J. Relationships between fecal indicator abundance in water and sand and the presence of pathogenic genes in sand of recreational beaches. Environ. Monit. Assess. 2024, 196, 1067. [Google Scholar] [CrossRef]
- Williams, A.P.; Avery, L.M.; Killham, K.; Jones, D.L. Persistence, dissipation, and activity of Escherichia coli O157:H7 within sand and seawater environments. FEMS Microbiol. Ecol. 2007, 60, 24–32. [Google Scholar] [CrossRef]
- Stevens, J.; Evans, G.; Aguirre, K. Human beach use affects abundance and identity of fungi present in sand. J. Coast. Res. 2012, 28, 787–792. [Google Scholar] [CrossRef]
- Sanchez, P.S.; Agudo, E.G.; Castro, F.G.; Alves, M.N.; Martins, M.T. Evaluation of the sanitary quality of marine recreational waters and sands from beaches of the São Paulo State, Brazil. Water Sci. Technol. 1986, 18, 61–72. [Google Scholar] [CrossRef]
- Thapaliya, D.; Hellwig, E.J.; Kadariya, J.; Grenier, D.; Jefferson, A.J.; Dalman, M.; Kennedy, K.; DiPerna, M.; Orihill, A.; Taha, M.; et al. Prevalence and characterization of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus on public recreational beaches in Northeast Ohio. Geohealth 2017, 1, 320–332. [Google Scholar] [CrossRef]
- Plano, L.R.; Shibata, T.; Garza, A.C.; Kish, J.; Fleisher, J.M.; Sinigalliano, C.D.; Gidley, M.L.; Withum, K.; Elmir, S.M.; Hower, S.; et al. Human-associated methicillin-resistant Staphylococcus aureus from a subtropical recreational marine beach. Microb. Ecol. 2013, 65, 1039–1051. [Google Scholar] [CrossRef]
- Mohammed, R.L.; Echeverry, A.; Stinson, C.M.; Green, M.; Bonilla, T.D.; Hartz, A.; McCorquodale, D.S.; Rogerson, A.; Esiobu, N. Survival trends of Staphylococcus aureus, Pseudomonas aeruginosa, and Clostridium perfringens in a sandy South Florida beach. Mar. Pollut. Bull. 2012, 64, 1201–1209. [Google Scholar] [CrossRef]
- Monteiro, S.; Brondani, G.; Brandão, J.; Santos, R. Viruses in beach sand. In Proceedings of the Fifth Food and Environmental Virology Congress, Kusatsu, Japan, 13–16 September 2016. [Google Scholar]
- Robalo, A.; Brandão, J.; Shibata, T.; Solo-Gabriele, H.; Santos, R.; Monteiro, S. Detection of enteric viruses and SARS-CoV-2 in beach sand. Sci. Total Environ. 2023, 901, 165836. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.H.; Abdelzaher, A.M.; Phillips, M.; Hernandez, R.; Solo-Gabriele, H.M.; Kish, J.; Scorzetti, G.; Fell, J.W.; Diaz, M.R.; Scott, T.M.; et al. Indicator microbes correlate with pathogenic bacteria, yeasts and helminthes in sand at a subtropical recreational beach site. J. Appl. Microbiol. 2011, 110, 1571–1583. [Google Scholar] [CrossRef]
- Zanoli Sato, M.; Di Bari, M.; Lamparelli, C.; Truzzi, A.; Coelho, M.; Hachich, E. Sanitary quality of sands from marine recreational beaches of São Paulo, Brazil. Braz. J. Microbiol. 2005, 36, 321–326. [Google Scholar] [CrossRef]
- Tomás, J.M. The main Aeromonas pathogenic factors. ISRN Microbiol. 2012, 2012, 256261. [Google Scholar] [CrossRef]
- Fernández-Bravo, A.; Figueras, M.J. An update on the genus Aeromonas: Taxonomy, epidemiology, and pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef]
- USFDA. Bad Bug Book. Handbook of Foodborne Pathogenic Microorganisms and Natural Toxins, 2nd ed.; United States Food and Drug Administration: Silver Spring, MD, USA, 2012.
- Chaix, G.; Roger, F.; Berthe, T.; Lamy, B.; Jumas-Bilak, E.; Lafite, R.; Forget-Leray, J.; Petit, F. Distinct Aeromonas populations in water column and associated with copepods from estuarine environment (Seine, France). Front. Microbiol. 2017, 8, 1259. [Google Scholar] [CrossRef]
- Mohan, V. Faeco-prevalence of Campylobacter jejuni in urban wild birds and pets in New Zealand. BMC Res. Notes 2015, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, E.M.; McEwan, N.; Mackenzie, M.; Karki, N.; Sinton, L.W. Incidence and prevalence of microbial indicators and pathogens in ovine faeces in New Zealand. N. Z. J. Agric. Res. 2011, 54, 71–81. [Google Scholar] [CrossRef]
- Moriarty, E.M.; Karki, N.; Mackenzie, M.; Sinton, L.W.; Wood, D.R.; Gilpin, B.J. Faecal indicators and pathogens in selected New Zealand waterfowl. N. Z. J. Mar. Freshw. Res. 2011, 45, 679–688. [Google Scholar] [CrossRef]
- Rapp, D.; Ross, C.M.; Pleydell, E.J.; Muirhead, R.W. Differences in the fecal concentrations and genetic diversities of Campylobacter jejuni populations among individual cows in two dairy herds. Appl. Environ. Microbiol. 2012, 78, 7564–7571. [Google Scholar] [CrossRef] [PubMed]
- Facciolà, A.; Riso, R.; Avventuroso, E.; Visalli, G.; Delia, S.A.; Laganà, P. Campylobacter: From microbiology to prevention. J. Prev. Med. Hyg. 2017, 58, E79–E92. [Google Scholar]
- Pakbin, B.; Brück, W.M.; Rossen, J.W.A. Virulence factors of enteric pathogenic Escherichia coli: A review. Int. J. Mol. Sci. 2021, 22, 9922. [Google Scholar] [CrossRef]
- Silby, M.W.; Winstanley, C.; Godfrey, S.A.C.; Levy, S.B.; Jackson, R.W. Pseudomonas genomes: Diverse and adaptable. FEMS Microbiol. Rev. 2011, 35, 652–680. [Google Scholar] [CrossRef]
- Wilson, M.G.; Pandey, S. Pseudomonas aeruginosa. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Esiobu, N.; Mohammed, R.; Echeverry, A.; Green, M.; Bonilla, T.; Hartz, A.; McCorquodale, D.; Rogerson, A. The application of peptide nucleic acid probes for rapid detection and enumeration of eubacteria, Staphylococcus aureus and Pseudomonas aeruginosa in recreational beaches of S. Florida. J. Microbiol. Methods 2004, 57, 157–162. [Google Scholar] [CrossRef]
- Jajere, S.M. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World 2019, 12, 504–521. [Google Scholar] [CrossRef]
- Crump, J.A. Progress in typhoid fever epidemiology. Clin. Infect. Dis. 2019, 68, S4–S9. [Google Scholar] [CrossRef]
- Kozajda, A.; Jeżak, K. Occupational exposure to Staphylococcus aureus in the wastewater treatment plants environment. Med. Pr. 2020, 71, 265–278. [Google Scholar] [CrossRef]
- Topić, N.; Cenov, A.; Jozić, S.; Glad, M.; Mance, D.; Lušić, D.; Kapetanović, D.; Mance, D.; Vukić Lušić, D. Staphylococcus aureus—An additional parameter of bathing water quality for crowded urban beaches. Int. J. Environ. Res. Public Health 2021, 18, 5234. [Google Scholar] [CrossRef]
- Steadmon, M.; Takakusagi, M.; Wiegner, T.N.; Jones, M.; Economy, L.M.; Panelo, J.; Morrison, L.A.; Medeiros, M.C.I.; Frank, K.L. Detection and modeling of Staphylococcus aureus and fecal bacteria in Hawaiian coastal waters and sands. Water Environ. Res. 2024, 96, e11037. [Google Scholar] [CrossRef]
- Goodwin, K.D.; McNay, M.; Cao, Y.P.; Ebentier, D.; Madison, M.; Griffith, J.F. A multi-beach study of Staphylococcus aureus, MRSA, and enterococci in seawater and beach sand. Water Res. 2012, 46, 4195–4207. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, J.A.; Mavridou, A.; Richardson, S.C.; Lampiri, M.; Marcelou, U. Bather-related microbial and yeast populations in sand and seawater. Water Res. 1997, 31, 799–804. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Primers 2018, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Curren, E.; Leong, S.C.Y. Profiles of bacterial assemblages from microplastics of tropical coastal environments. Sci. Total Environ. 2019, 655, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Cressey, P.; Horn, B.; Gilpin, B.; Rivas, L. The burden of yersiniosis in New Zealand, 2022. N. Z. Med. J. 2025, 138, 83–91. [Google Scholar] [CrossRef]
- Aslam, A.; Hashmi, M.; Okafor, C. Shigellosis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Elmanama, A.A.; Fahd, M.I.; Afifi, S.; Abdallah, S.; Bahr, S. Microbiological beach sand quality in Gaza Strip in comparison to seawater quality. Environ. Res. 2005, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Di Cola, G.; Fantilli, A.C.; Pisano, M.B.; Ré, V.E. Foodborne transmission of hepatitis A and hepatitis E viruses: A literature review. Int. J. Food Microbiol. 2021, 338, 108986. [Google Scholar] [CrossRef]
- Rexin, D.; Rachmadi, A.T.; Hewitt, J. Persistence of infectious human norovirus in estuarine water. Food Environ. Virol. 2024, 16, 58–64. [Google Scholar] [CrossRef]
- Teunis, P.F.M.; Le Guyader, F.S.; Liu, P.; Ollivier, J.; Moe, C.L. Noroviruses are highly infectious but there is strong variation in host susceptibility and virus pathogenicity. Epidemics 2020, 32, 100401. [Google Scholar] [CrossRef] [PubMed]
- Firquet, S.; Beaujard, S.; Lobert, P.E.; Sané, F.; Caloone, D.; Izard, D.; Hober, D. Survival of enveloped and non-enveloped viruses on inanimate surfaces. Microbes Environ. 2015, 30, 140–144. [Google Scholar] [CrossRef]
- Benkő, M.; Aoki, K.; Arnberg, N.; Davison, A.J.; Echavarría, M.; Hess, M.; Jones, M.S.; Kaján, G.L.; Kajon, A.E.; Mittal, S.K.; et al. ICTV virus taxonomy profile: Adenoviridae 2022. J. Gen. Virol. 2022, 103, 001721. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Ghimire, P.; Dhamoon, A.S. The repertoire of adenovirus in human disease: The innocuous to the deadly. Biomedicines 2018, 6, 30. [Google Scholar] [CrossRef]
- Hewitt, J.; Greening, G.E.; Leonard, M.; Lewis, G.D. Evaluation of human adenovirus and human polyomavirus as indicators of human sewage contamination in the aquatic environment. Water Res. 2013, 47, 6750–6761. [Google Scholar] [CrossRef]
- Zell, R.; Delwart, E.; Gorbalenya, A.E.; Hovi, T.; King, A.M.Q.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. ICTV virus taxonomy profile: Picornaviridae. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar] [CrossRef]
- Sayed, I.M. Dual infection of hepatitis A virus and hepatitis E virus—What Is known? Viruses 2023, 15, 298. [Google Scholar] [CrossRef]
- Vinjé, J.; Estes, M.K.; Esteves, P.; Green, K.Y.; Katayama, K.; Knowles, N.J.; L’Homme, Y.; Martella, V.; Vennema, H.; White, P.A.; et al. ICTV virus taxonomy profile: Caliciviridae. J. Gen. Virol. 2019, 100, 1469–1470. [Google Scholar] [CrossRef]
- Teunis, P.F.; Sukhrie, F.H.; Vennema, H.; Bogerman, J.; Beersma, M.F.; Koopmans, M.P. Shedding of norovirus in symptomatic and asymptomatic infections. Epidemiol. Infect. 2015, 143, 1710–1717. [Google Scholar] [CrossRef]
- Ekundayo, T.C.; Igere, B.E.; Oluwafemi, Y.D.; Iwu, C.D.; Olaniyi, O.O. Human norovirus contamination in water sources: A systematic review and meta-analysis. Environ. Pollut. 2021, 291, 118164. [Google Scholar] [CrossRef]
- Chandran, S.; Gibson, K.E. Improving the detection and understanding of infectious human norovirus in food and water matrices: A review of methods and emerging models. Viruses 2024, 16, 776. [Google Scholar] [CrossRef] [PubMed]
- Vanathy, K.; Parija, S.C.; Mandal, J.; Hamide, A.; Krishnamurthy, S. Cryptosporidiosis: A mini review. Trop. Parasitol. 2017, 7, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.D. Biology of Giardia lamblia. Clin. Microbiol. Rev. 2001, 14, 447–475. [Google Scholar] [CrossRef]
- Johnson, D.C.; Enriquez, C.E.; Pepper, I.L.; Davis, T.L.; Gerba, C.P.; Rose, J.B. Survival of Giardia, Cryptosporidium, Poliovirus and Salmonella in marine waters. Water Sci. Technol. 1997, 35, 261–268. [Google Scholar] [CrossRef]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; de Wit, L.A.; VanWormer, E.; Villena, I. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.O.; Jones, H.F.E.; Roe, W.D. The effects of Toxoplasma gondii on New Zealand wildlife: Implications for conservation and management. Pac. Conserv. Biol. 2021, 27, 208–220. [Google Scholar] [CrossRef]
- Pinto-Ferreira, F.; Caldart, E.T.; Pasquali, A.K.S.; Mitsuka-Breganó, R.; Freire, R.L.; Navarro, I.T. Patterns of transmission and sources of infection in outbreaks of human toxoplasmosis. Emerg. Infect. Dis. 2019, 25, 2177–2182. [Google Scholar] [CrossRef]
- Manjarrez, G.; Blanco, J.; Gonzalez, B.; Botero, C.M.; Diaz-Mendoza, C. Parasites in tourist beaches: Proposal for its inclusion as health quality indicators. Review for Latin America. Ecol. Apl. 2019, 18, 91–100. [Google Scholar] [CrossRef]
- Bojar, H.; Kłapeć, T. Contamination of selected recreational areas in Lublin Province, Eastern Poland, by eggs of Toxocara spp., Ancylostoma spp. and Trichuris spp. Ann. Agric. Environ. Med. 2018, 25, 460–463. [Google Scholar] [CrossRef]
- Ramos, E.L.P.; Gómez-Hernández, C.; Queiroz, L.G.; Moura, R.G.F.; Nogueira, N.P.; Ferreira, G.L.S.; Rezende-Oliveira, K. Parasite detection in sand from bays on the north coast of São Paulo state, Brazil. J. Trop. Pathol. 2020, 49, 191–205. [Google Scholar] [CrossRef]
- Vogel, C.; Rogerson, A.; Schatz, S.; Laubach, H.; Tallman, A.; Fell, J. Prevalence of yeasts in beach sand at three bathing beaches in South Florida. Water Res. 2007, 41, 1915–1920. [Google Scholar] [CrossRef]
- Deligios, M.; Mazzarello, V.; Fiamma, M.; Barac, A.; Diana, L.; Ferrari, M.; Murgia, M.; Paglietti, B.; Rubino, S. Seasonal variation in fungi in beach sand in summertime: Stintino (Italy). Int. J. Environ. Res. Public Health 2023, 20, 7134. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.; Figueira, C.; Romão, D.; Brandão, J.; Freitas, M.C.; Andrade, C.; Calado, G.; Ferreira, C.; Campos, A.; Prada, S. Sediment characteristics and microbiological contamination of beach sand—A case-study in the archipelago of Madeira. Sci. Total Environ. 2016, 573, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hu, Z.D.; Zhao, X.M.; Zhao, W.N.; Feng, Z.X.; Yurkov, A.; Alwasel, S.; Boekhout, T.; Bensch, K.; Hui, F.L.; et al. Phylogenomic analysis of the Candida auris-Candida haemuli clade and related taxa in the Metschnikowiaceae, and proposal of thirteen new genera, fifty-five new combinations and nine new species. Persoonia 2024, 52, 22–43. [Google Scholar] [CrossRef]
- Rees, J.R.; Pinner, R.W.; Hajjeh, R.A.; Brandt, M.E.; Reingold, A.L. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992-1993: Results of population-based laboratory active surveillance. Clin. Infect. Dis. 1998, 27, 1138–1147. [Google Scholar] [CrossRef]
- Weiskerger, C.J.; Brandão, J. Fungal contaminants in water and sand: A new frontier for quantitative microbial risk assessment. Curr. Opin. Environ. Sci. Health 2020, 16, 73–81. [Google Scholar] [CrossRef]
- Brandão, J.; Weiskerger, C.; Valério, E.; Pitkänen, T.; Meriläinen, P.; Avolio, L.; Heaney, C.D.; Sadowsky, M.J. Climate change impacts on microbiota in beach sand and water: Looking ahead. Int. J. Environ. Res. Public Health 2022, 19, 1444. [Google Scholar] [CrossRef]
- DeFlorio-Barker, S.; Arnold, B.F.; Sams, E.A.; Dufour, A.P.; Colford, J.M., Jr.; Weisberg, S.B.; Schiff, K.C.; Wade, T.J. Child environmental exposures to water and sand at the beach: Findings from studies of over 68,000 subjects at 12 beaches. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 93–100. [Google Scholar] [CrossRef]
- Ferguson, A.; Del Donno, C.; Obeng-Gyasi, E.; Mena, K.; Kaur Altomare, T.; Guerrero, R.; Gidley, M.; Montas, L.; Solo-Gabriele, H.M. Children exposure-related behavior patterns and risk perception associated with recreational beach use. Int. J. Environ. Res. Public Health 2019, 16, 2783. [Google Scholar] [CrossRef]
- Moran, K.; Webber, J. Surf, sand, scrapes and stings: First aid incidents involving children at New Zealand beaches, 2007–2012. J. Paediatr. Child Health 2014, 50, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Tomenchok, L.E.; Gidley, M.L.; Mena, K.D.; Ferguson, A.C.; Solo-Gabriele, H.M. Children’s abrasions in recreational beach areas and a review of possible wound infections. Int. J. Environ. Res. Public Health 2020, 17, 4060. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.S.; Eftim, S.E.; Goldstone, A.E.; Dufour, A.P.; Nappier, S.P.; Wade, T.J. Evaluating health risks associated with exposure to ambient surface waters during recreational activities: A systematic review and meta-analysis. Water Res. 2020, 176, 115729. [Google Scholar] [CrossRef]
- Collier, S.A.; Wade, T.J.; Sams, E.A.; Hlavsa, M.C.; Dufour, A.P.; Beach, M.J. Swimming in the USA: Beachgoer characteristics and health outcomes at US marine and freshwater beaches. J. Water Health 2015, 13, 531–543. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Safe Recreational Water Environments. Volume 1: Coastal and Freshwaters; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Heaney, C.D.; Sams, E.; Wing, S.; Marshall, S.; Brenner, K.; Dufour, A.P.; Wade, T.J. Contact with beach sand among beachgoers and risk of illness. Am. J. Epidemiol. 2009, 170, 164–172. [Google Scholar] [CrossRef]
- Heaney, C.D.; Sams, E.; Dufour, A.P.; Brenner, K.P.; Haugland, R.A.; Chern, E.; Wing, S.; Marshall, S.; Love, D.C.; Serre, M.; et al. Fecal indicators in sand, sand contact, and risk of enteric illness among beachgoers. Epidemiology 2012, 23, 95–106. [Google Scholar] [CrossRef]
- Wade, T.J.; Augustine, S.A.J.; Griffin, S.M.; Sams, E.A.; Oshima, K.H.; Egorov, A.I.; Simmons, K.J.; Eason, T.N.; Dufour, A.P. Asymptomatic norovirus infection associated with swimming at a tropical beach: A prospective cohort study. PLoS ONE 2018, 13, e0195056. [Google Scholar] [CrossRef]
- Leonard, A.F.C.; Garside, R.; Ukoumunne, O.C.; Gaze, W.H. A cross-sectional study on the prevalence of illness in coastal bathers compared to non-bathers in England and Wales: Findings from the Beach User Health Survey. Water Res. 2020, 176, 115700. [Google Scholar] [CrossRef]
- Gertler, M.; Dürr, M.; Renner, P.; Poppert, S.; Askar, M.; Breidenbach, J.; Frank, C.; Preußel, K.; Schielke, A.; Werber, D.; et al. Outbreak of Cryptosporidium hominis following river flooding in the city of Halle (Saale), Germany, August 2013. BMC Infect. Dis. 2015, 15, 88. [Google Scholar] [CrossRef]
- Labropoulou, S.; Charvalos, E.; Chatzipanagiotou, S.; Ioannidis, A.; Sylignakis, P.; Τaka, S.; Karageorgou, I.; Linou, M.; Mpizta, G.; Mentis, A.; et al. Sunbathing, a possible risk factor of murine typhus infection in Greece. PLoS Negl. Trop. Dis. 2021, 15, e0009186. [Google Scholar] [CrossRef]
- Kidd, S.E.; Hagen, F.; Tscharke, R.L.; Huynh, M.; Bartlett, K.H.; Fyfe, M.; Macdougall, L.; Boekhout, T.; Kwon-Chung, K.J.; Meyer, W. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc. Natl. Acad. Sci. USA 2004, 101, 17258–17263. [Google Scholar] [CrossRef]
- Tertipi, N.; Kefala, V.; Papageorgiou, E.; Rallis, E. Prevalence of common viral skin infections in beach volleyball athletes. Viruses 2021, 13, 2107. [Google Scholar] [CrossRef]
- Doorduyn, Y.; Van Den Brandhof, W.E.; Van Duynhoven, Y.T.; Wannet, W.J.; Van Pelt, W. Risk factors for Salmonella Enteritidis and Typhimurium (DT104 and non-DT104) infections in The Netherlands: Predominant roles for raw eggs in Enteritidis and sandboxes in Typhimurium infections. Epidemiol. Infect. 2006, 134, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Lucerón, C.O.; Meixeira, A.P.; Sanz, I.A.; Deleyto, V.C.; León, S.H.; Ruiz, L.G. Notes from the field: An outbreak of Salmonella Typhimurium associated with playground sand in a preschool setting—Madrid, Spain, September-October 2016. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 256–257. [Google Scholar] [CrossRef] [PubMed]
- Staff, M.; Musto, J.; Hogg, G.; Janssen, M.; Rose, K. Salmonellosis outbreak traced to playground sand, Australia, 2007–2009. Emerg. Infect. Dis. 2012, 18, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Solo-Gabriele, H.M. Quantitative microbial risk assessment of human illness from exposure to marine beach sand. Environ. Sci. Technol. 2012, 46, 2799–2805. [Google Scholar] [CrossRef]
- Casadevall, A. Immunity to invasive fungal diseases. Annu. Rev. Immunol. 2022, 40, 121–141. [Google Scholar] [CrossRef]
- Nnadi, N.E.; Carter, D.A. Climate change and the emergence of fungal pathogens. PLoS Pathog. 2021, 17, e1009503. [Google Scholar] [CrossRef]
- Weiskerger, C.J.; Brandão, J.; Ahmed, W.; Aslan, A.; Avolio, L.; Badgley, B.D.; Boehm, A.B.; Edge, T.A.; Fleisher, J.M.; Heaney, C.D.; et al. Impacts of a changing earth on microbial dynamics and human health risks in the continuum between beach water and sand. Water Res. 2019, 162, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Roca, M.A.; Brown, R.S.; Solo-Gabriele, H.M. Fecal indicator bacteria levels at beaches in the Florida Keys after Hurricane Irma. Mar. Pollut. Bull. 2019, 138, 266–273. [Google Scholar] [CrossRef] [PubMed]
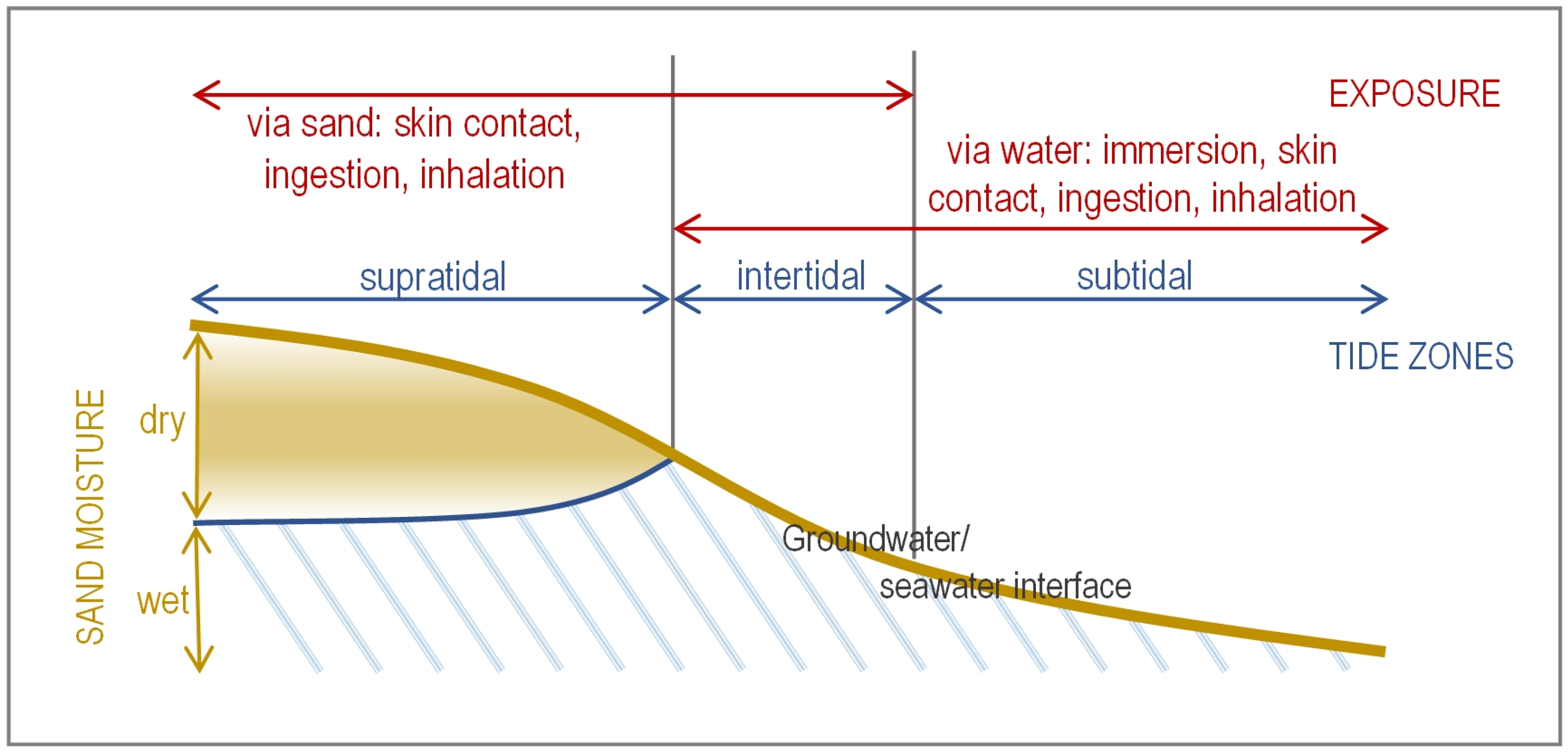
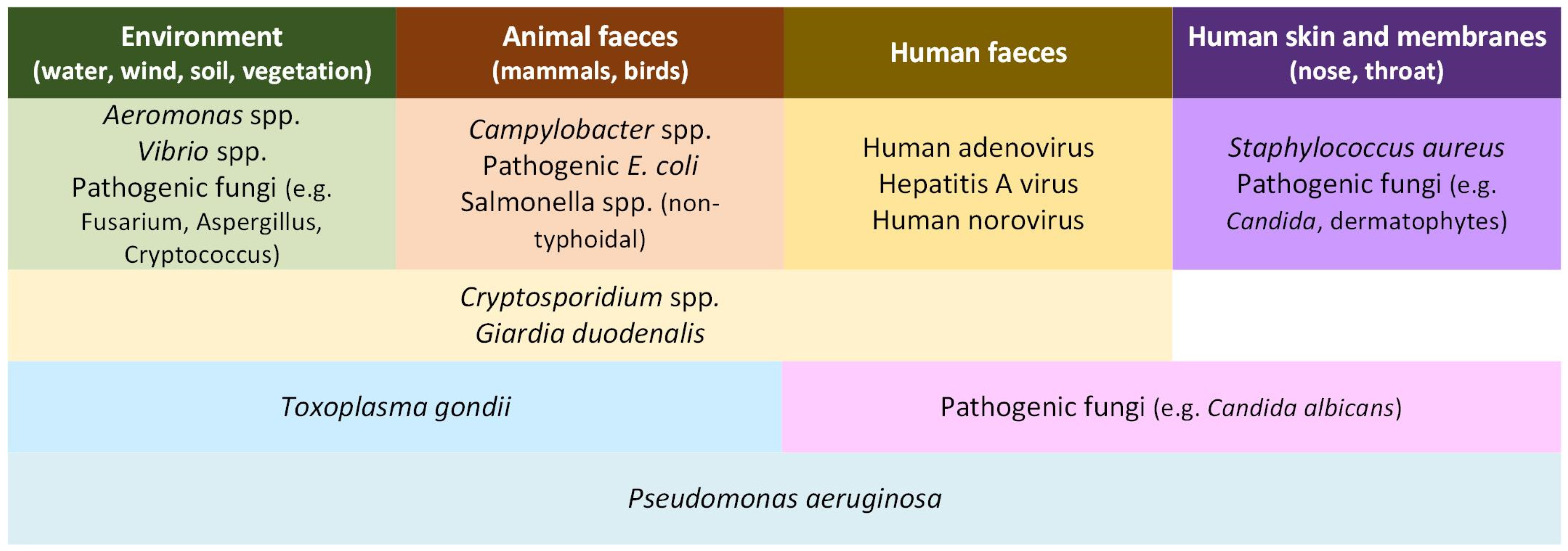
| Microbiological Agent | Most Likely Acute Conditions from Sand Exposure | Presence in Beach Sand * | Survival in Beach Sand |
|---|---|---|---|
| Bacteria | |||
| Aeromonas spp. | GI, wound infection | Canada: Detected in interstitial pore water of freshwater beach sand [70]. | No relevant data located. |
| Campylobacter spp. | GI | England: Detected in dry and wet beach sand (82/182 positive), including from beaches complying with FIB water standards [71]. USA: Detected in sand (7/53 positive) from coastal beaches [40]. Canada: Detected in pore water from freshwater sand beaches [72]. | Concentration decreased in marine beach sand or freshwater beach sand seeded with sewage [40,73]. |
| Pathogenic E. coli | GI | England: E. coli O157 not detected in 30 sand samples taken from one beach as part of an outbreak investigation [74]. USA: Viable E. coli recovered from coastal beach sand but E. coli O157:H7 were not identified through PCR-based methods [75]. Virulence genes associated with pathogenic E. coli have been detected in sand [11,76]. | E. coli O157:H7 survived five days in sand in the presence of cattle faeces, both under dry conditions and with seawater tidal simulation [77]. |
| P. aeruginosa | Soft tissue infection | USA: Detected in beach sand from temperate South Carolina [78]. Southern Brazil: Detected in marine beach sand [79]. Japan: Non-speciated Pseudomonas detected by PCR methods in sand samples taken after a typhoon event, at different depths [61]. | No relevant data located. |
| Salmonella spp. (non-typhoidal) | GI | USA: Detected in sand (6/53 positive) from coastal beaches [40]. England: Detected in dry and wet beach sand (10/182 positive), including from beaches complying with FIB water standards [71]. Southern Brazil: Not detected in marine sand [79]. | Concentration decreased in marine beach sand or freshwater beach sand seeded with sewage [40,73]. |
| S. aureus | Soft tissue infection | USA: Detected in sand (5/37 positive) from coastal beaches (one beach was MRSA positive) [40]. USA: Detected in wet sand 43/210) from freshwater beaches (MRSA detected in 15/210; methicillin-susceptible S. aureus (MSSA) in 28/210) [80]. USA: Detected in dry and swash zone marine sand but not in subtidal sand (MRSA only detected in dry sand) [81]. | Growth possible but might be limited by natural predation [82]. Concentration decreased in freshwater beach sand seeded with sewage (MRSA concentration also decreased) [73]. |
| Vibrio spp. | Wound infection | Japan (post-typhoon): Detected by PCR in sand collected from the water’s edge but not in landward sand samples [61]. Various countries: Detected in studies of beaches located in tropical and subtropical zones [11]. | No relevant data located but likely to survive well in wet sand. |
| Viruses | |||
| Human adenovirus | GI | Portugal: Detected in sand samples [83,84]. USA: Not detected in tropical/dry beaches [75]. | No relevant data located. |
| Hepatitis A virus | Hepatitis | Portugal: Detected in sand samples [83,84]. USA: Not detected in wet and dry sand from a subtropical beach [29]. | No relevant data located. |
| Human norovirus | GI | Portugal: Detected in sand samples [84]. USA: Not detected in wet and dry sand from a subtropical beach [29]. | No relevant data located. |
| Parasites | |||
| Cryptosporidium spp. | GI | USA: Detected by PCR but not microscopy in wet and dry sand from a subtropical beach in one study, detected by microscopy in another study (one intertidal sand sample only, 0.63 oocysts/100 g sand) [29,85]. | No relevant data located. |
| G. duodenalis | GI | USA: Not detected by PCR or microscopy in wet and dry sand samples from subtropical beaches [29,85]. Southern Brazil: Detected in dry sand (2/96 positive) during spring and summer months [86]. | No relevant data located. |
| T. gondii | Toxoplasmosis | No relevant data located. | No relevant data located. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, N.; Leonard, M. A Review of the Human Health Risks from Microbial Hazards in Recreational Beach Sand. Int. J. Environ. Res. Public Health 2025, 22, 1537. https://doi.org/10.3390/ijerph22101537
King N, Leonard M. A Review of the Human Health Risks from Microbial Hazards in Recreational Beach Sand. International Journal of Environmental Research and Public Health. 2025; 22(10):1537. https://doi.org/10.3390/ijerph22101537
Chicago/Turabian StyleKing, Nicola, and Margaret Leonard. 2025. "A Review of the Human Health Risks from Microbial Hazards in Recreational Beach Sand" International Journal of Environmental Research and Public Health 22, no. 10: 1537. https://doi.org/10.3390/ijerph22101537
APA StyleKing, N., & Leonard, M. (2025). A Review of the Human Health Risks from Microbial Hazards in Recreational Beach Sand. International Journal of Environmental Research and Public Health, 22(10), 1537. https://doi.org/10.3390/ijerph22101537






