Unlocking the Potential of mHealth: Integrating Behaviour Change Techniques in Hypertension App Design
Abstract
1. Introduction
2. Materials and Methods
3. Results
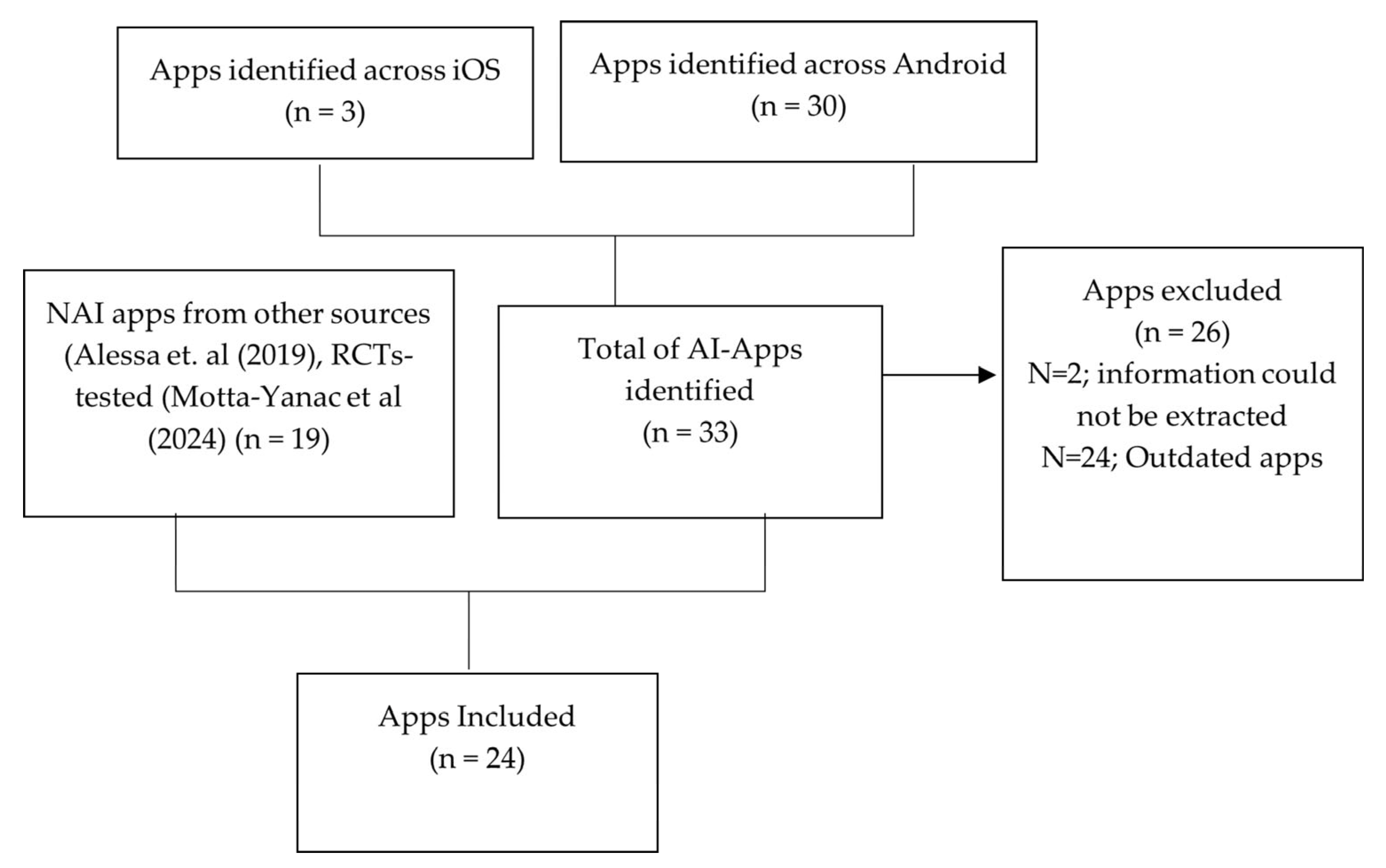
3.1. Identification of Apps
3.2. App Characteristics
3.3. Functionalities of the Apps
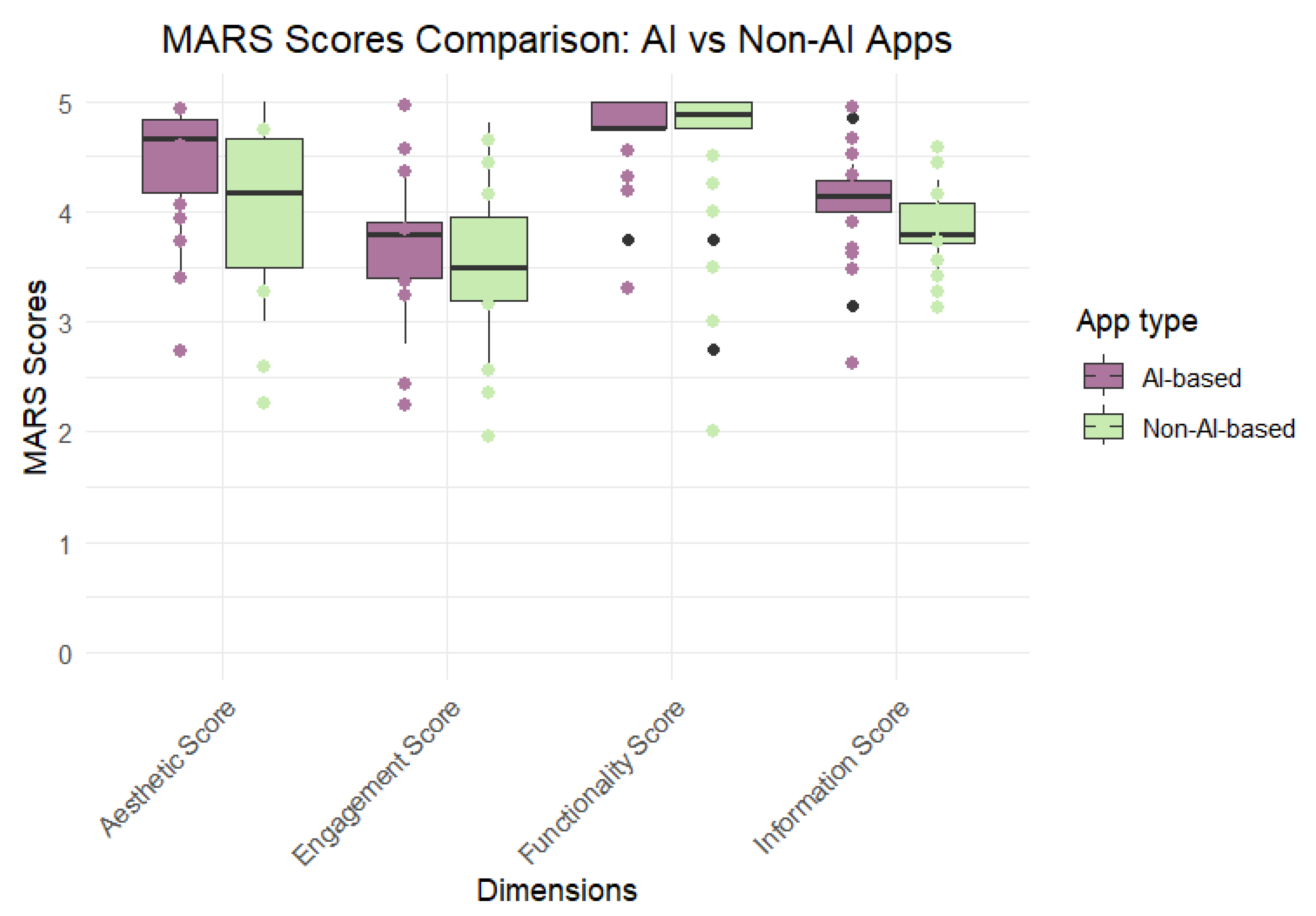
3.4. Quality Assessment Using MARS
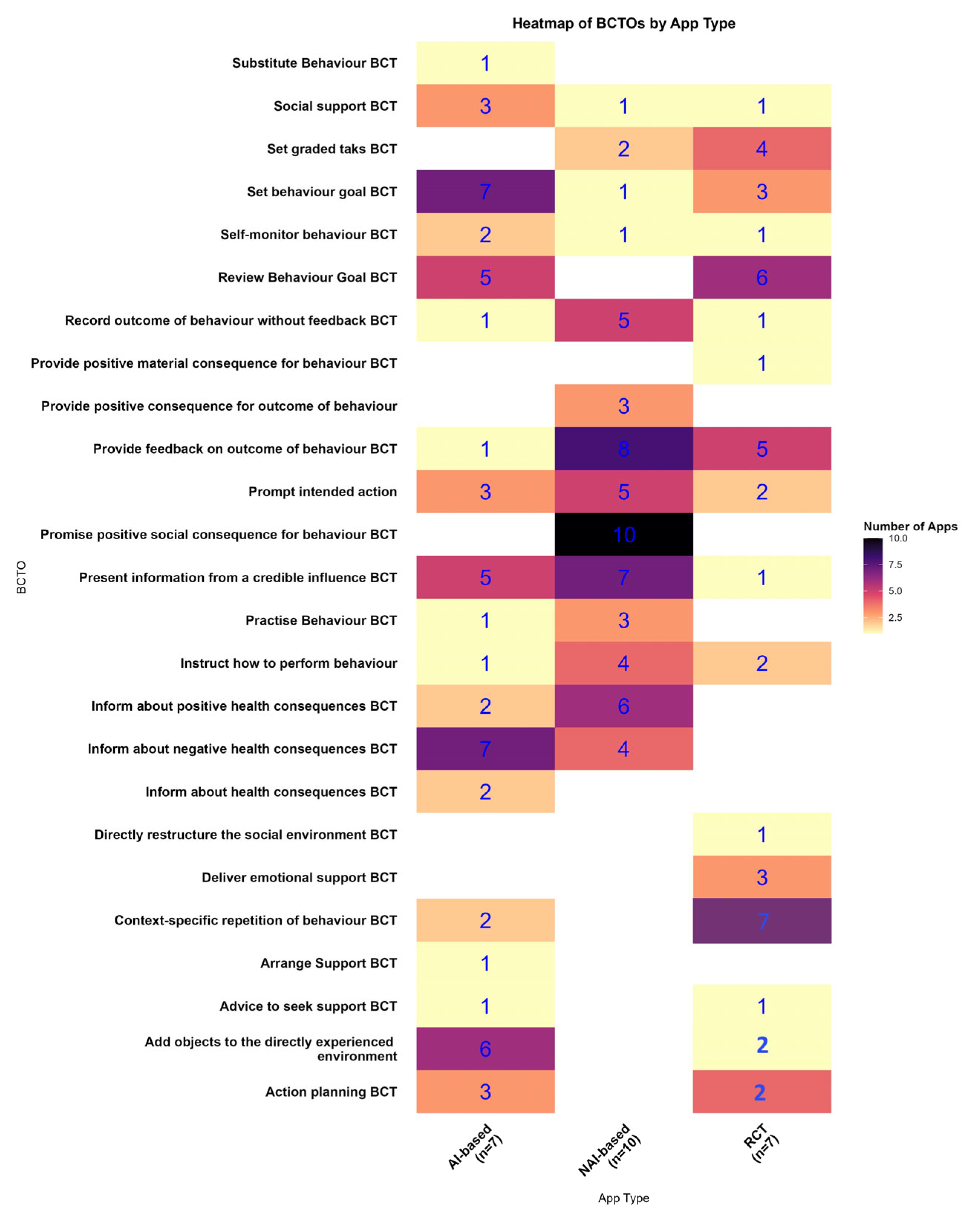
3.5. BCTOs and TDF Mapping
TDF Mapping
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCTO | Behaviour Change Technique Ontology |
| BP | Blood Pressure |
| TDF | Theoretical Domain Framework |
| MARS | Mobile Application Rating Scale |
| AI | Artificial Intelligence |
| BCT | Behaviour Change Technique |
| NAI | Non-AI |
| RCT | Randomised Controlled Trial |
References
- Patel, S.; Arya, M. The BUS Framework: A comprehensive tool in creating an mHealth App utilizing Behavior Change Theories, User-Centered Design, and Social Marketing. J. Mob. Technol. Med. 2017, 6, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Farag, N.; Noë, A.; Patrinos, D.; Zawati, M.H. Mapping the Apps: Ethical and Legal Issues with Crowdsourced Smartphone Data using mHealth Applications. Asian Bioeth. Rev. 2024, 16, 437–470. [Google Scholar] [CrossRef] [PubMed]
- Spinean, A.; Mladin, A.; Carniciu, S.; Stănescu, A.; Serafinceanu, C. Emerging Methods for Integrative Management of Chronic Diseases: Utilizing mHealth Apps for Lifestyle Interventions. Nutrients 2025, 17, 1506. [Google Scholar] [CrossRef] [PubMed]
- Alawiye, T.R. The Impact of Digital Technology on Healthcare Delivery and Patient Outcomes. E-Health Telecommun. Syst. Netw. 2024, 13, 13–22. [Google Scholar] [CrossRef]
- Mishra, S.R.; Satheesh, G.; Khanal, V.; Nguyen, T.N.; Picone, D.; Chapman, N.; Lindley, R.I. Closing the Gap in Global Disparities in Hypertension Control. Hypertension 2025, 82, 407–410. [Google Scholar] [CrossRef]
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef]
- Conn, V.S.; Ruppar, T.M.; Chase, J.A.D.; Enriquez, M.; Cooper, P.S. Interventions to Improve Medication Adherence in Hypertensive Patients: Systematic Review and Meta-analysis. Curr. Hypertens. Rep. 2015, 17, 94. [Google Scholar] [CrossRef]
- Michie, S.; Van Stralen, M.M.; West, R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement. Sci. 2011, 6, 42. [Google Scholar] [CrossRef]
- Kumar, N.; Khunger, M.; Gupta, A.; Garg, N. A content analysis of smartphone-based applications for hypertension management. J. Am. Soc. Hypertens. 2015, 9, 130–136. [Google Scholar] [CrossRef]
- Parati, G.; Torlasco, C.; Omboni, S.; Pellegrini, D. Smartphone Applications for Hypertension Management: A Potential Game-Changer That Needs More Control. Curr. Hypertens. Rep. 2017, 19, 48. [Google Scholar] [CrossRef]
- Alessa, T.; Hawley, M.S.; Hock, E.S.; De Witte, L. Smartphone Apps to Support Self-Management of Hypertension: Review and Content Analysis. JMIR Mhealth Uhealth 2019, 7, e13645. [Google Scholar] [CrossRef]
- Motta-Yanac, E.; Riley, V.; Ellis, N.J.; Mankoo, A.; Gidlow, C.J. The digital prescription: A systematic review and meta-analysis of smartphone apps for blood pressure control. Int. J. Med. Inform. 2025, 195, 105755. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; West, R.; Finnerty, A.N.; Norris, E.; Wright, A.J.; Marques, M.M.; Johnston, M.; Kelly, M.P.; Thomas, J.; Hastings, J. Representation of behaviour change interventions and their evaluation: Development of the Upper Level of the Behaviour Change Intervention Ontology. Wellcome Open Res. 2021, 5, 123. [Google Scholar] [CrossRef] [PubMed]
- Atkins, L.; Francis, J.; Islam, R.; O’Connor, D.; Patey, A.; Ivers, N.; Foy, R.; Duncan, E.M.; Colquhoun, H.; Grimshaw, J.M.; et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement. Sci. 2017, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Staniford, L.J.; Schmidtke, K.A. A systematic review of hand-hygiene and environmental-disinfection interventions in settings with children. BMC Public Health 2020, 20, 195. [Google Scholar] [CrossRef]
- Stoyanov, S.R.; Hides, L.; Kavanagh, D.J.; Zelenko, O.; Tjondronegoro, D.; Mani, M. Mobile App Rating Scale: A New Tool for Assessing the Quality of Health Mobile Apps. JMIR Mhealth Uhealth 2015, 3, e27. [Google Scholar] [CrossRef]
- Wang, S.; Leung, M.; Leung, S.Y.; Han, J.; Leung, W.; Hui, E.; Mihailidou, A.S.; Tsoi, K.K.-F.; Wong, M.C.-S.; Wong, S.Y.-S.; et al. Safety, Feasibility, and Acceptability of Telemedicine for Hypertension in Primary Care: A Proof-of-concept and Pilot Randomized Controlled Trial (SATE-HT). J. Med. Syst. 2023, 47, 34. [Google Scholar] [CrossRef]
- Susanto, Y.; Pristianty, L.; Hermansyah, A. The impact of mHealth application on improving medication adherence and hypertension management: A systematic review of randomised trials. Pharm. Educ. 2023, 23, 208–218. [Google Scholar] [CrossRef]
- Alzahrani, S.A.; Bin Muammar, M.F.; Bin Muammar, A.F.; Alolah, A.; Almutawa, M. The Adoption and Acceptance of mHealth Interventions for Self-Management of Hypertension Among Adult Patients: A Systematic Review. Cureus 2022, 14, e31584. [Google Scholar] [CrossRef]
- Almasi, S.; Hosseini, A.; Emami, H.; Sabahi, A. Mobile Health Technology for Hypertension Management: A Systematic Review. Acta Med. Iran. 2020, 58, 249–259. [Google Scholar] [CrossRef]
- Guo, C.; Ashrafian, H.; Ghafur, S.; Fontana, G.; Gardner, C.; Prime, M. Challenges for the evaluation of digital health solutions—A call for innovative evidence generation approaches. NPJ Digit. Med. 2020, 3, 110. [Google Scholar] [CrossRef] [PubMed]
- Monachelli, R.; Davis, S.W.; Barnard, A.; Longmire, M.; Docherty, J.P.; Oakley-Girvan, I. Designing mHealth Apps to Incorporate Evidence-Based Techniques for Prolonging User Engagement. Interact. J. Med. Res. 2024, 13, e51974. [Google Scholar] [CrossRef] [PubMed]
- Alessa, T.; Abdi, S.; Hawley, M.S.; De Witte, L. Mobile Apps to Support the Self-Management of Hypertension: Systematic Review of Effectiveness, Usability, and User Satisfaction. JMIR Mhealth Uhealth 2018, 6, e10723. [Google Scholar] [CrossRef]
- Schoeppe, S.; Alley, S.; Van Lippevelde, W.; Bray, N.A.; Williams, S.L.; Duncan, M.J.; Vandelanotte, C. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 127. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.J.; Huang, R.Q.; Ma, H.M.; Wang, A.Q.; Tang, X.Y.; Pei, R.Y.; Piao, M.H. Behavior Change Techniques Used in Self-Management Interventions Based on mHealth Apps for Adults With Hypertension: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Med. Internet Res. 2024, 26, e54978. [Google Scholar] [CrossRef]
- Aguiar, M.; Trujillo, M.; Chaves, D.; Álvarez, R.; Epelde, G. mHealth Apps Using Behavior Change Techniques to Self-report Data: Systematic Review. JMIR Mhealth Uhealth 2022, 10, e33247. [Google Scholar] [CrossRef]
- Natale, P.; Ni, J.Y.; Martinez-Martin, D.; Kelly, A.; Chow, C.K.; Thiagalingam, A.; Caillaud, C.; Eggleton, B.; Scholes-Robertson, N.; Craig, J.C.; et al. Perspectives and Experiences of Self-monitoring of Blood Pressure Among Patients with Hypertension: A Systematic Review of Qualitative Studies. Am. J. Hypertens. 2023, 36, 372–384. [Google Scholar] [CrossRef]
- Yang, L.; Hao, Y.; Zhong, L.; Xu, S.; Zhong, S.; Lu, Y.; Liu, H. Conceptual Analysis of Self-Management in Patients with Hypertension. J. Clin. Nurs. Res. 2024, 8, 356–368. [Google Scholar] [CrossRef]
- Bhimanapati, V.B.R.; Pandian, P.K.G.; Goel, P.P. UI/UX Design Principles for Mobile Health Applications. Int. J. Res. Publ. Semin. 2024, 15, 216–231. [Google Scholar] [CrossRef]
- McLean, G.; Band, R.; Saunderson, K.; Hanlon, P.; Murray, E.; Little, P.; McManus, R.J.; Yardley, L.; Mair, F.S. Digital interventions to promote self-management in adults with hypertension systematic review and meta-analysis. J. Hypertens. 2016, 34, 600–612. [Google Scholar] [CrossRef]
- Tornero-Costa, R.; Martinez-Millana, A.; Azzopardi-Muscat, N.; Lazeri, L.; Traver, V.; Novillo-Ortiz, D. Methodological and Quality Flaws in the Use of Artificial Intelligence in Mental Health Research: Systematic Review. JMIR Ment. Health 2023, 10, e42045. Available online: https://mental.jmir.org/2023/1/e42045 (accessed on 23 June 2025). [CrossRef]
- Alzahrani, A.; Gay, V.; Alturki, R. Enabled Artificial Intelligence (AI) to Develop Sehhaty Wa Daghty App of Self-Management for Saudi Patients with Hypertension: A Qualitative Study. Information 2023, 14, 334. [Google Scholar] [CrossRef]
- Cao, W.; Milks, M.W.; Liu, X.; Gregory, M.E.; Addison, D.; Zhang, P.; Li, L. mHealth Interventions for Self-management of Hypertension: Framework and Systematic Review on Engagement, Interactivity, and Tailoring. JMIR Mhealth Uhealth 2022, 10, e29415. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, M.; Venerito, V.; Ming Azevedo, P.; Hügle, T. Generative AI-based knowledge graphs for the illustration and development of mHealth self-management content. Front. Digit. Health 2024, 6, 1466211. [Google Scholar] [CrossRef]
- Khan, B.; Fatima, H.; Qureshi, A.; Kumar, S.; Hanan, A.; Hussain, J.; Abdullah, S. Drawbacks of Artificial Intelligence and Their Potential Solutions in the Healthcare Sector. Biomed. Mater. Devices 2023, 1, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.D.; Biller-Andorno, N. The regulatory gap in digital health and alternative pathways to bridge it. Health Policy Technol. 2022, 11, 100663. [Google Scholar] [CrossRef]
- Svempe, L. Exploring Impediments Imposed by the Medical Device Regulation EU 2017/745 on Software as a Medical Device. JMIR Med. Inform. 2024, 12, e58080. [Google Scholar] [CrossRef]
- Alon, N.; Stern, A.D.; Torous, J. Assessing the Food and Drug Administration’s Risk-Based Framework for Software Precertification with Top Health Apps in the United States: Quality Improvement Study. JMIR Mhealth Uhealth 2020, 8, e20482. [Google Scholar] [CrossRef]
- Lobach, D.F.; Boxwala, A.; Kashyap, N.; Heaney-Huls, K.; Chiao, A.B.; Rafter, T.; Lomotan, E.A.; Harrison, M.I.; Dymek, C.; Swiger, J.; et al. Integrating a Patient Engagement App into an Electronic Health Record-Enabled Workflow Using Interoperability Standards. Appl. Clin. Inform. 2022, 13, 1163–1171. [Google Scholar] [CrossRef]
- Alzghaibi, H. Healthcare Practitioners’ Perceptions of mHealth Application Barriers: Challenges to Adoption and Strategies for Enhancing Digital Health Integration. Healthcare 2025, 13, 494. [Google Scholar] [CrossRef]
- Zakerabasali, S.; Ayyoubzadeh, S.M.; Baniasadi, T.; Yazdani, A.; Abhari, S. Mobile Health Technology and Healthcare Providers: Systemic Barriers to Adoption. Healthc. Inform. Res. 2021, 27, 267–278. [Google Scholar] [CrossRef]

| Functionality | AI-Empowered (N = 7), n (%) | Non-AI (NAI) (N = 10), n(%) | RCT-Tested (N = 7), n (%) | Total (N = 24), n (%) |
|---|---|---|---|---|
| Self-Monitoring | 7 (100) | 10 (100) | 7 (100) | 24 (100) |
| Goal Setting | 3 (42.8) | 2 (20) | 3 (42.8) | 8 (33.3) |
| Reminders | 5 (71.4) | 9 (90) | 4 (57.1) | 18 (75) |
| Educational Information | 3 (42.8) | 2 (20) | 4 (57.1) | 9 (37.5) |
| Feedback | 7 (100) | 7 (70) | 5 (71.4) | 19 (79.2) |
| Stress management | 2 (28.6) | 0 | 0 | 2 (8.3) |
| Communication with HCPs and other | 4 (57.1) | 3 (30) | 4 (57.1) | 11 (45.8) |
| Export of user’s data to others via email | 2 (28.6) | 10 (100) | 2 (28.6) | 14 (58.3) |
| Prevention | 6 (85.7) | 0 | 0 | 6 (25) |
| Total BCTOs | AI-Based (N = 7) | NAI-Based (N = 10) | RCTs (N = 7) | Total (N = 24) | |
|---|---|---|---|---|---|
| 25 | Mean ± SD | 7.57 ± 2.44 | 4.29 ± 2.66 | 2.45 ± 1.80 | 0.0152 |
| Median | 7 | 4 | 2 | ||
| Min, Max | 4, 12 | 1, 10 | 1, 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motta-Yanac, E.; Victoria, R.; Ellis, N.J.; Gidlow, C.J. Unlocking the Potential of mHealth: Integrating Behaviour Change Techniques in Hypertension App Design. Int. J. Environ. Res. Public Health 2025, 22, 1487. https://doi.org/10.3390/ijerph22101487
Motta-Yanac E, Victoria R, Ellis NJ, Gidlow CJ. Unlocking the Potential of mHealth: Integrating Behaviour Change Techniques in Hypertension App Design. International Journal of Environmental Research and Public Health. 2025; 22(10):1487. https://doi.org/10.3390/ijerph22101487
Chicago/Turabian StyleMotta-Yanac, Emily, Riley Victoria, Naomi J. Ellis, and Christopher James Gidlow. 2025. "Unlocking the Potential of mHealth: Integrating Behaviour Change Techniques in Hypertension App Design" International Journal of Environmental Research and Public Health 22, no. 10: 1487. https://doi.org/10.3390/ijerph22101487
APA StyleMotta-Yanac, E., Victoria, R., Ellis, N. J., & Gidlow, C. J. (2025). Unlocking the Potential of mHealth: Integrating Behaviour Change Techniques in Hypertension App Design. International Journal of Environmental Research and Public Health, 22(10), 1487. https://doi.org/10.3390/ijerph22101487







