Capacity Planning (Capital, Staff and Costs) of Inpatient Maternity Services: Pitfalls for the Unwary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Hospital Episode Statistics
2.3. Additional Bespoke Data
2.4. Estimating Real-Time Length of Stay (LOS)
2.5. Variability in the Gender Ratio
3. Results
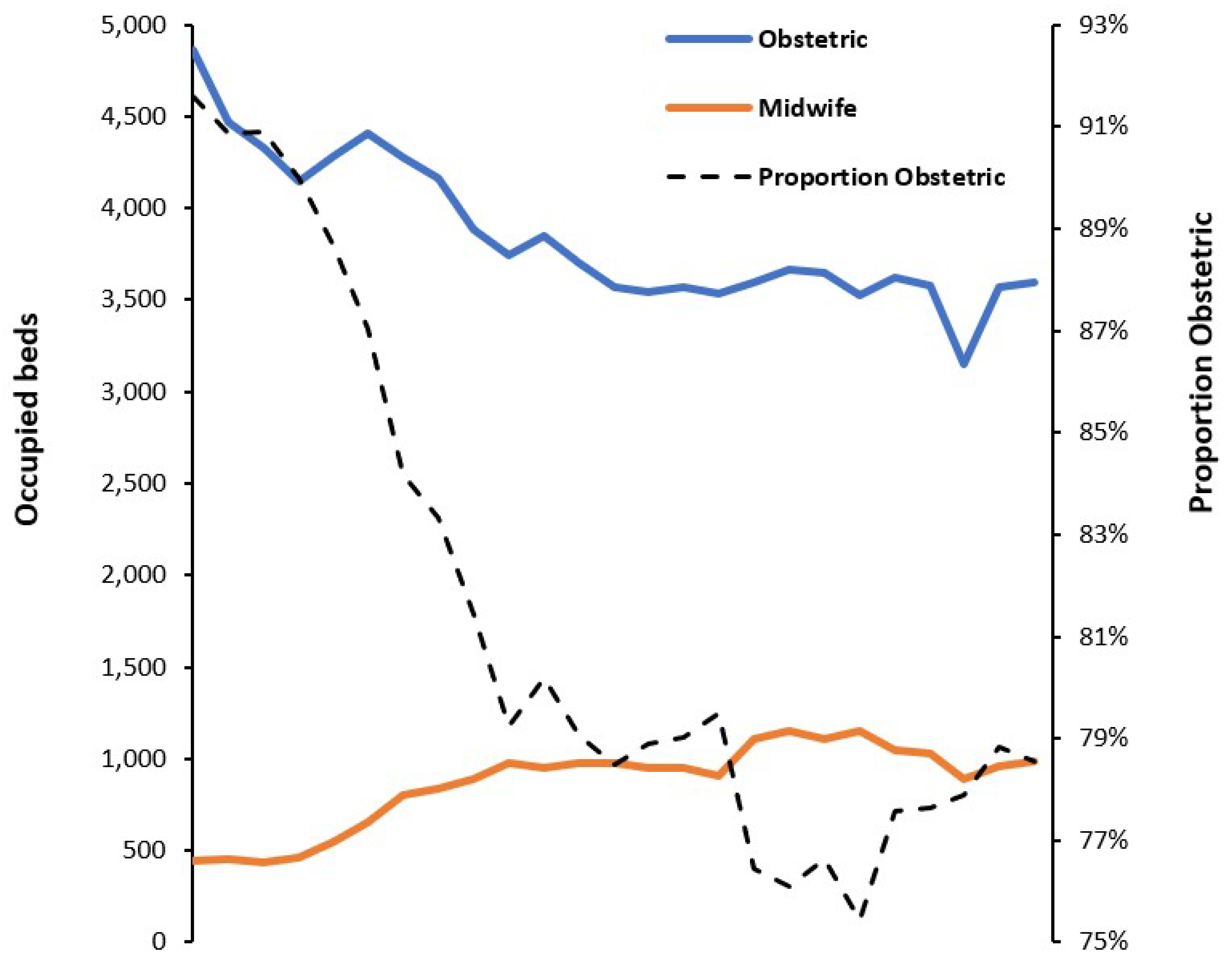
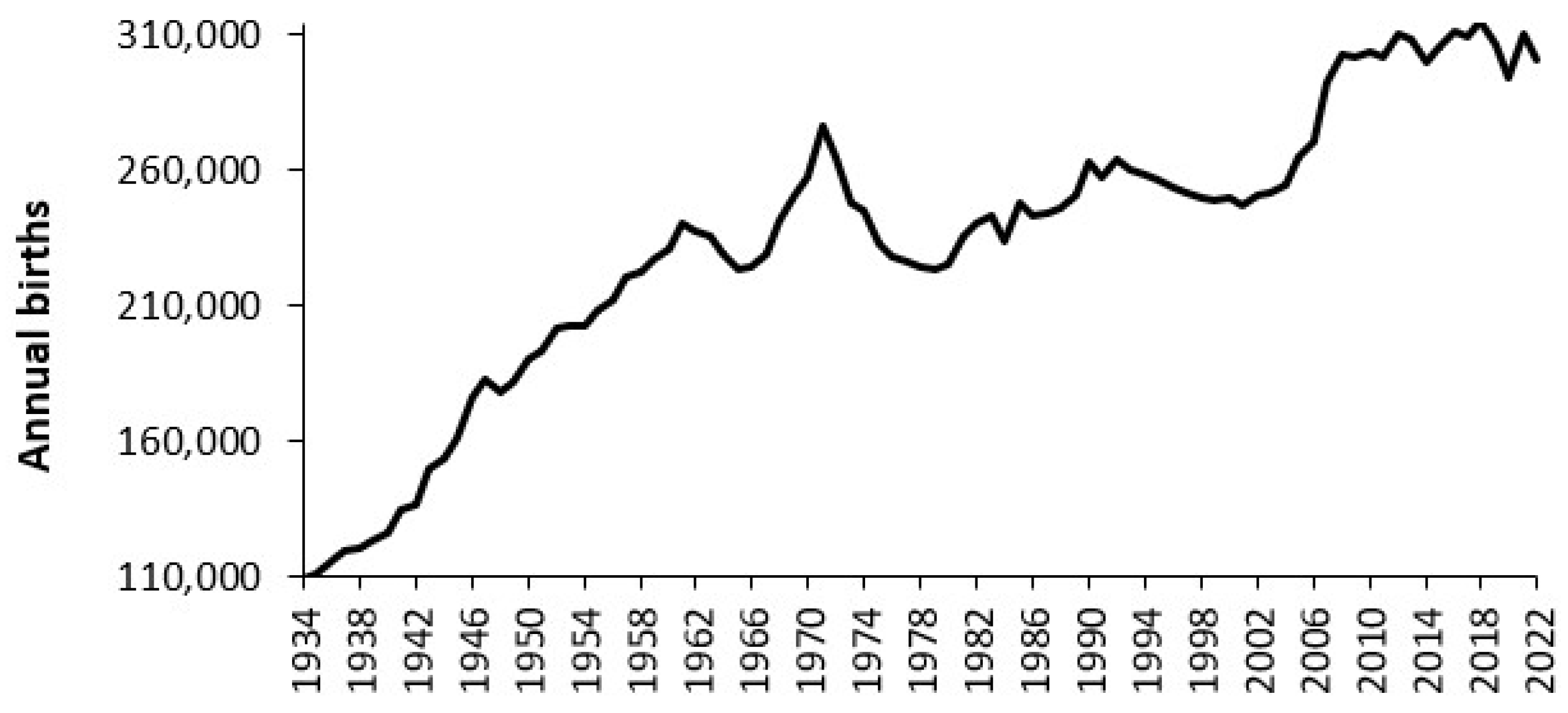
3.1. Trends in Available Beds in England
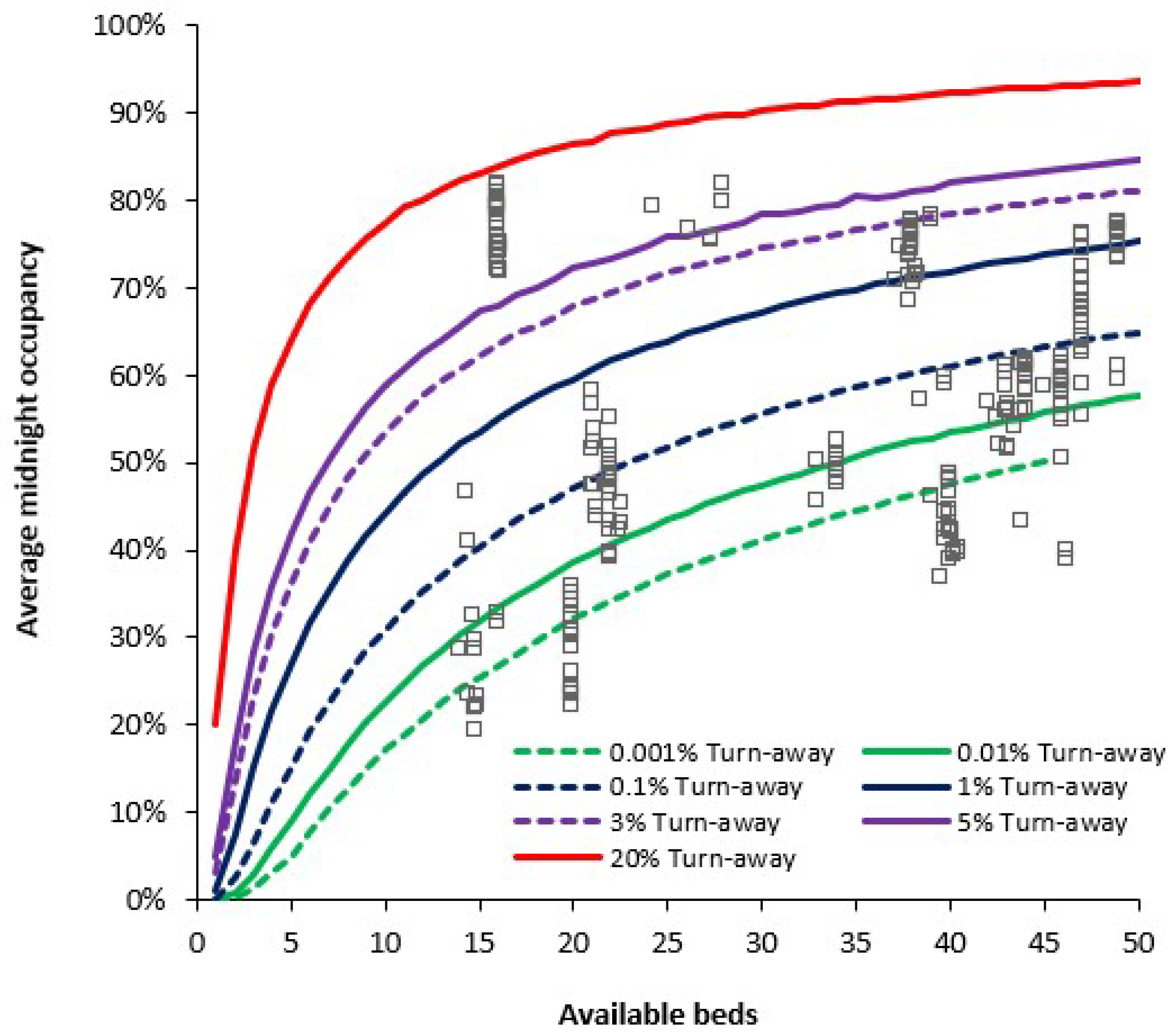
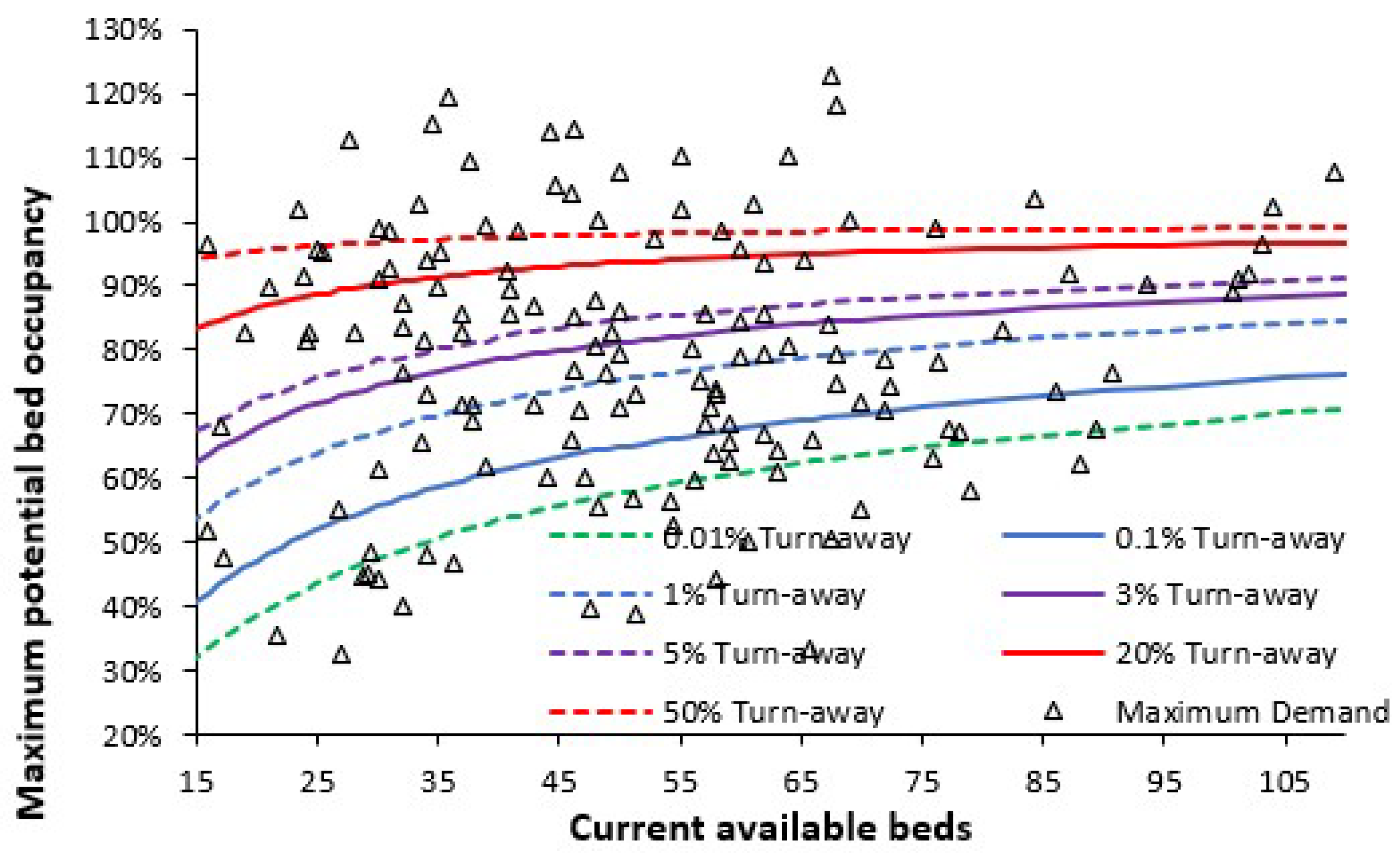
3.2. Assessing Current Bed Sufficiency Using Average Bed Occupancy
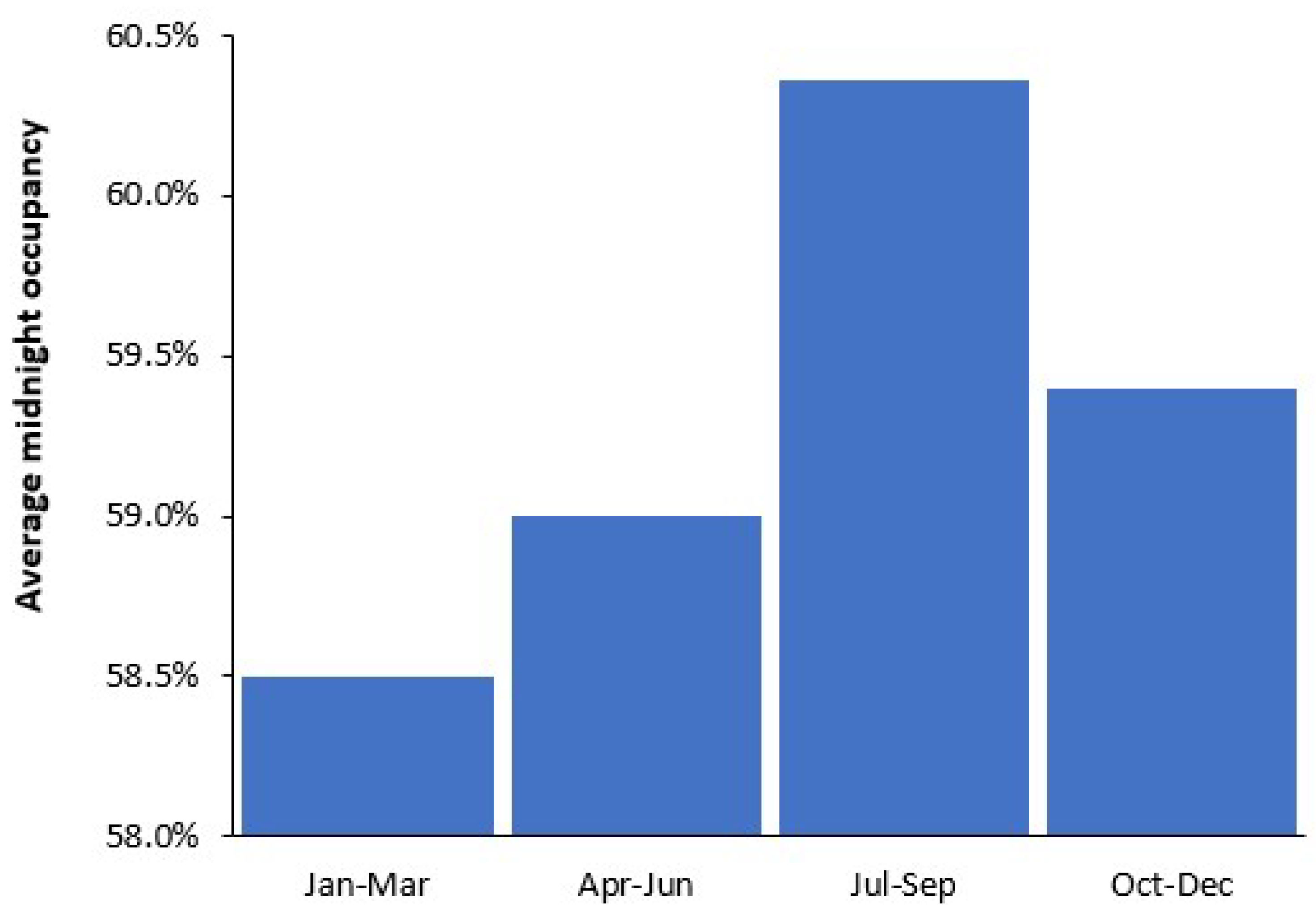
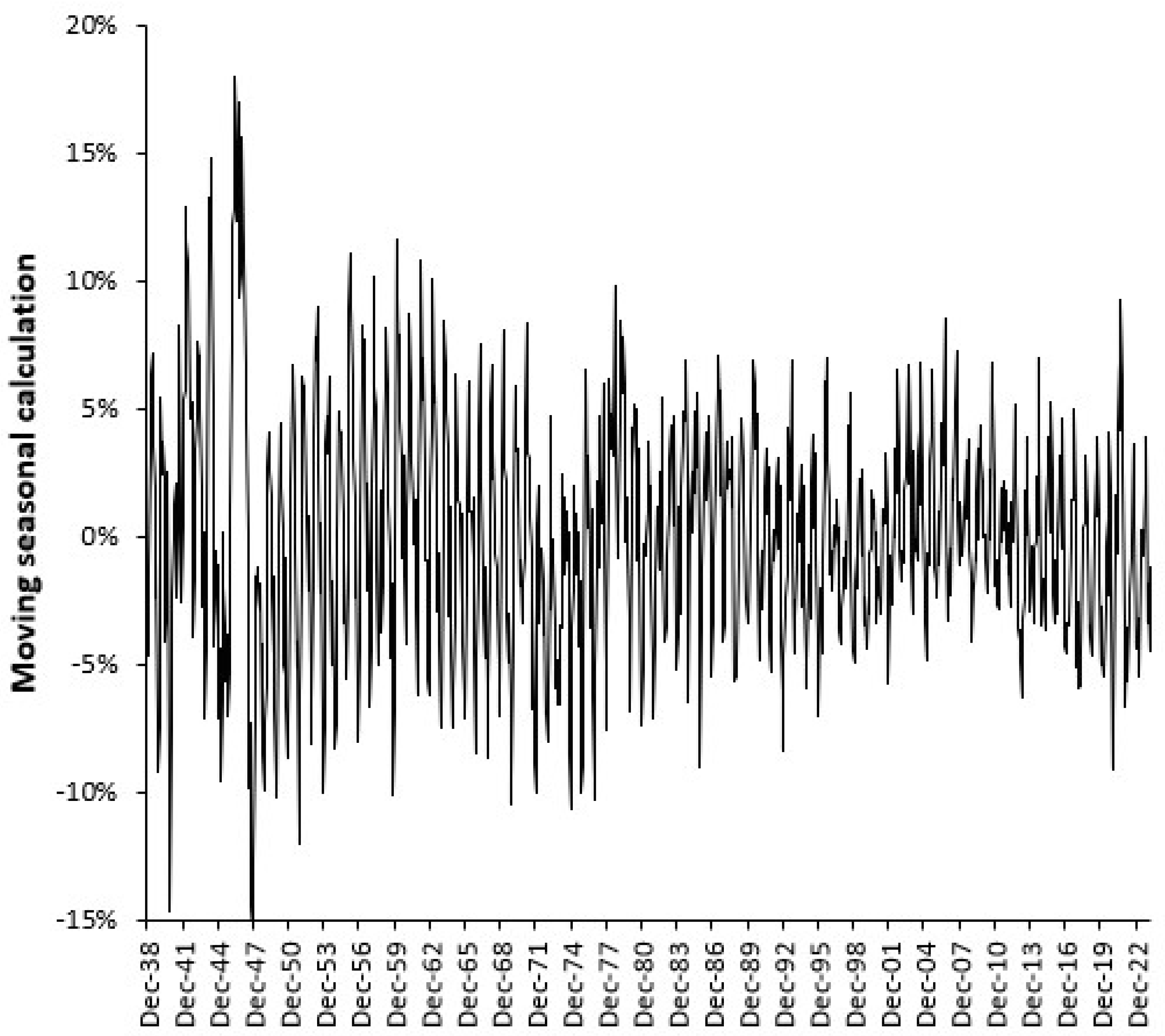
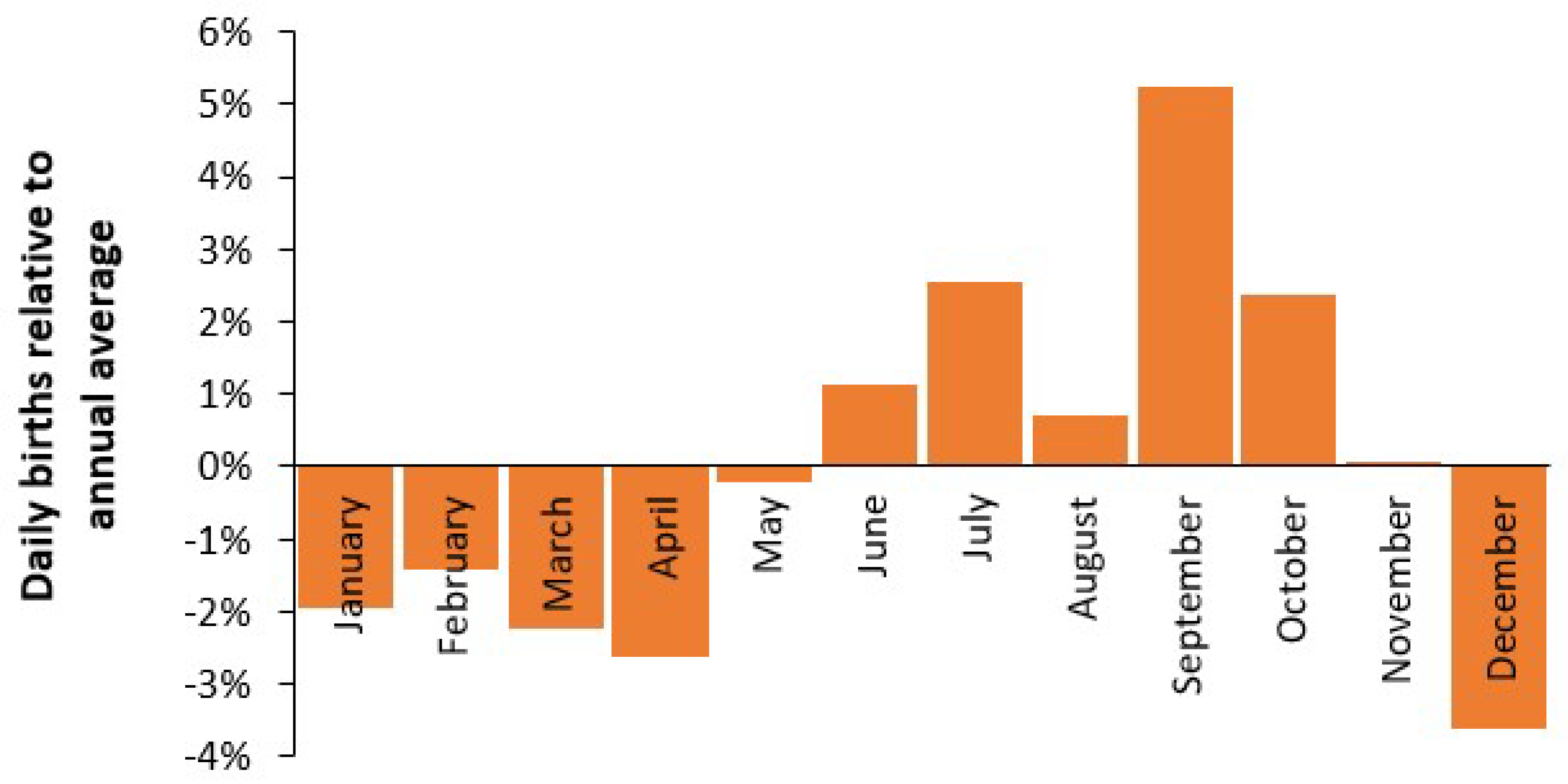
3.3. Seasonality in Births and Bed Demand
3.4. Circadian Profiles
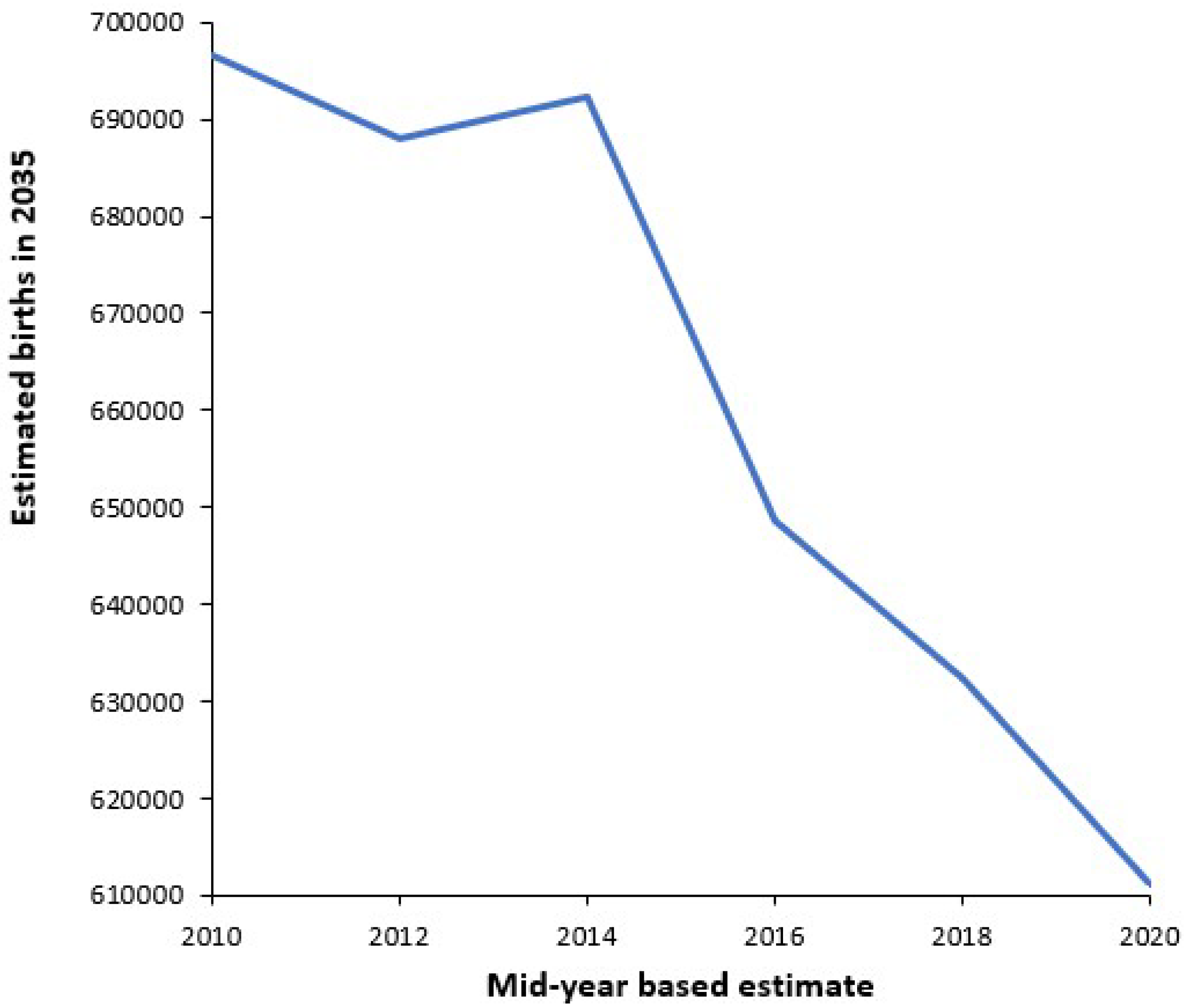
3.5. Forecasting Births
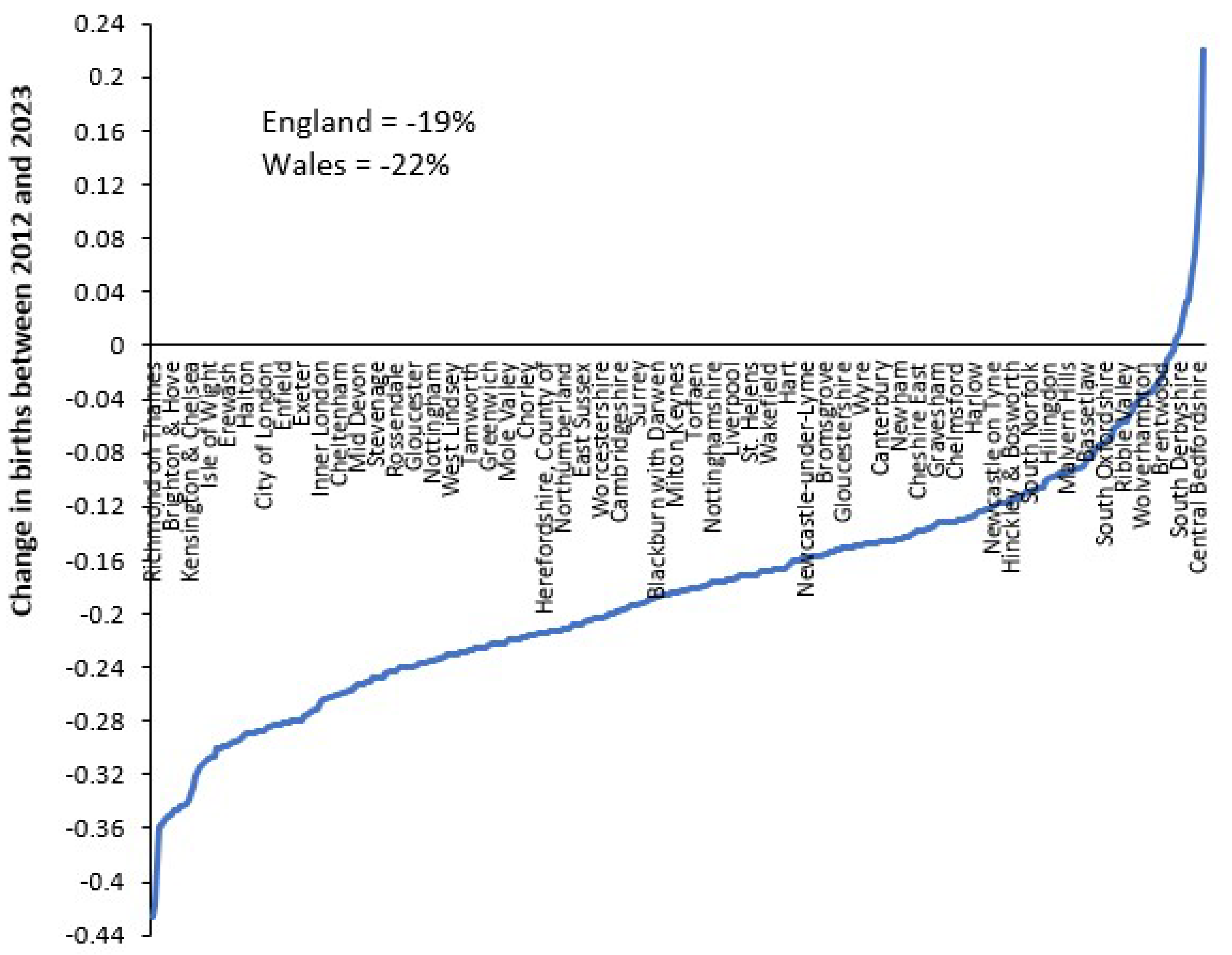
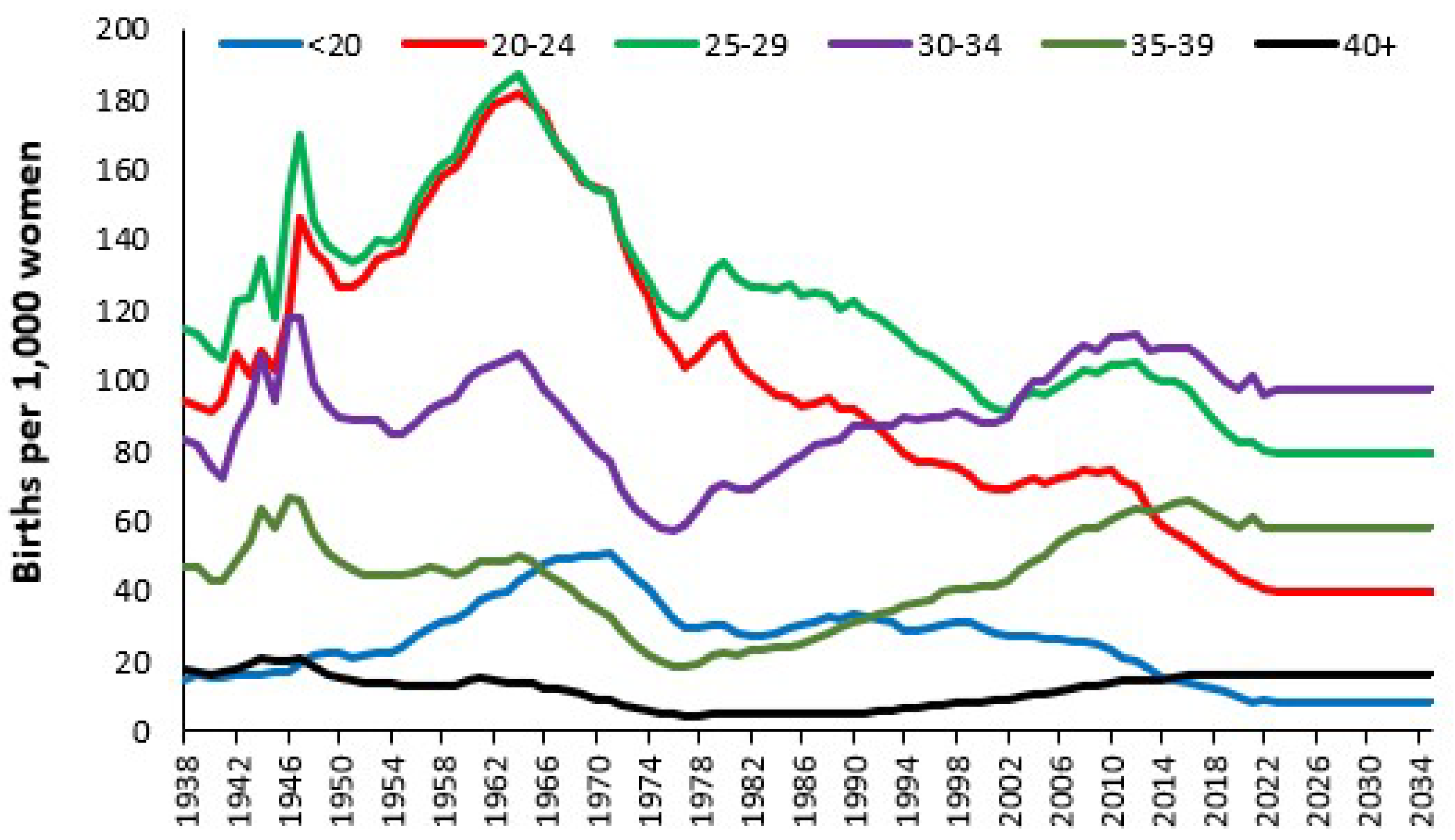
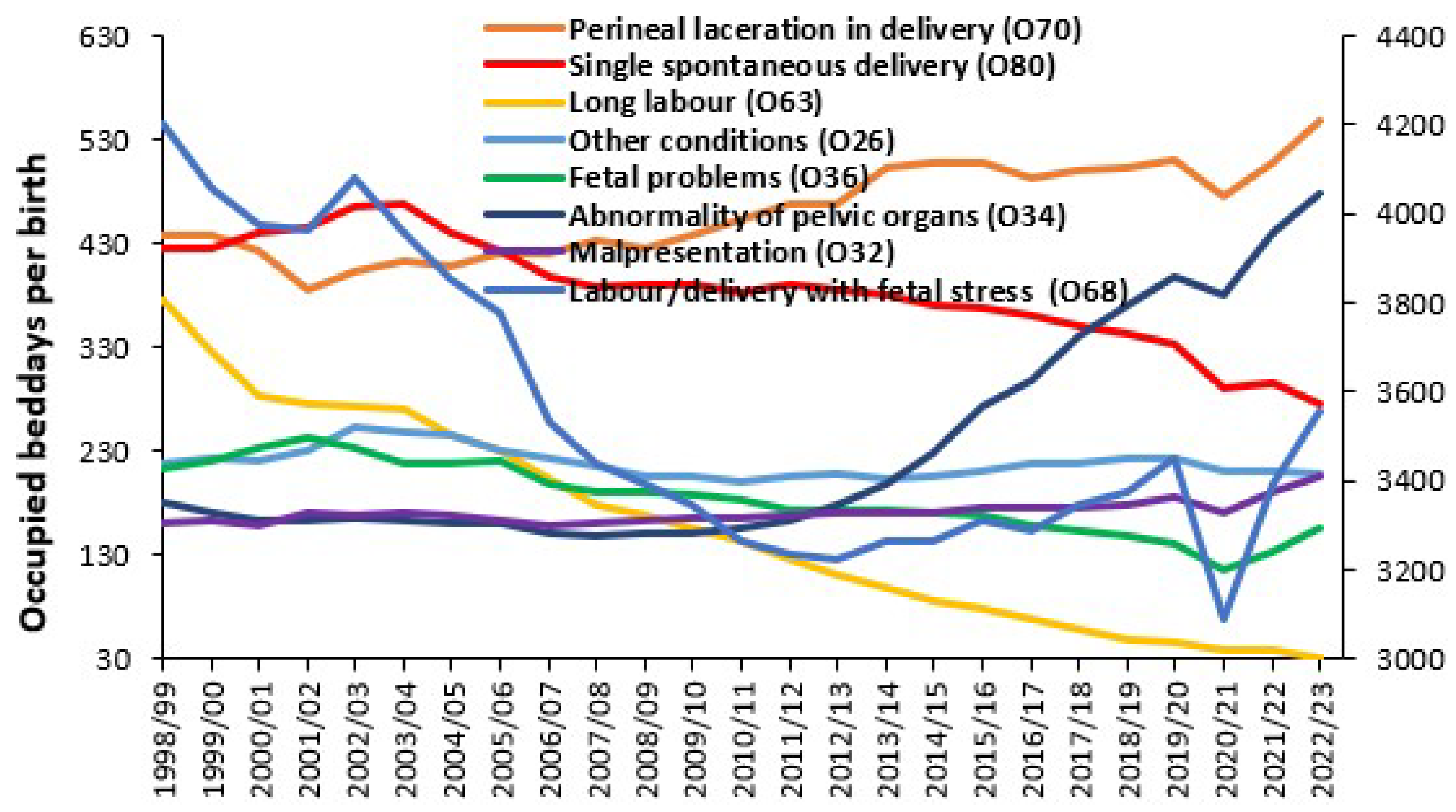
3.6. Trends in Admissions Relating to Pregnancy and Childbirth
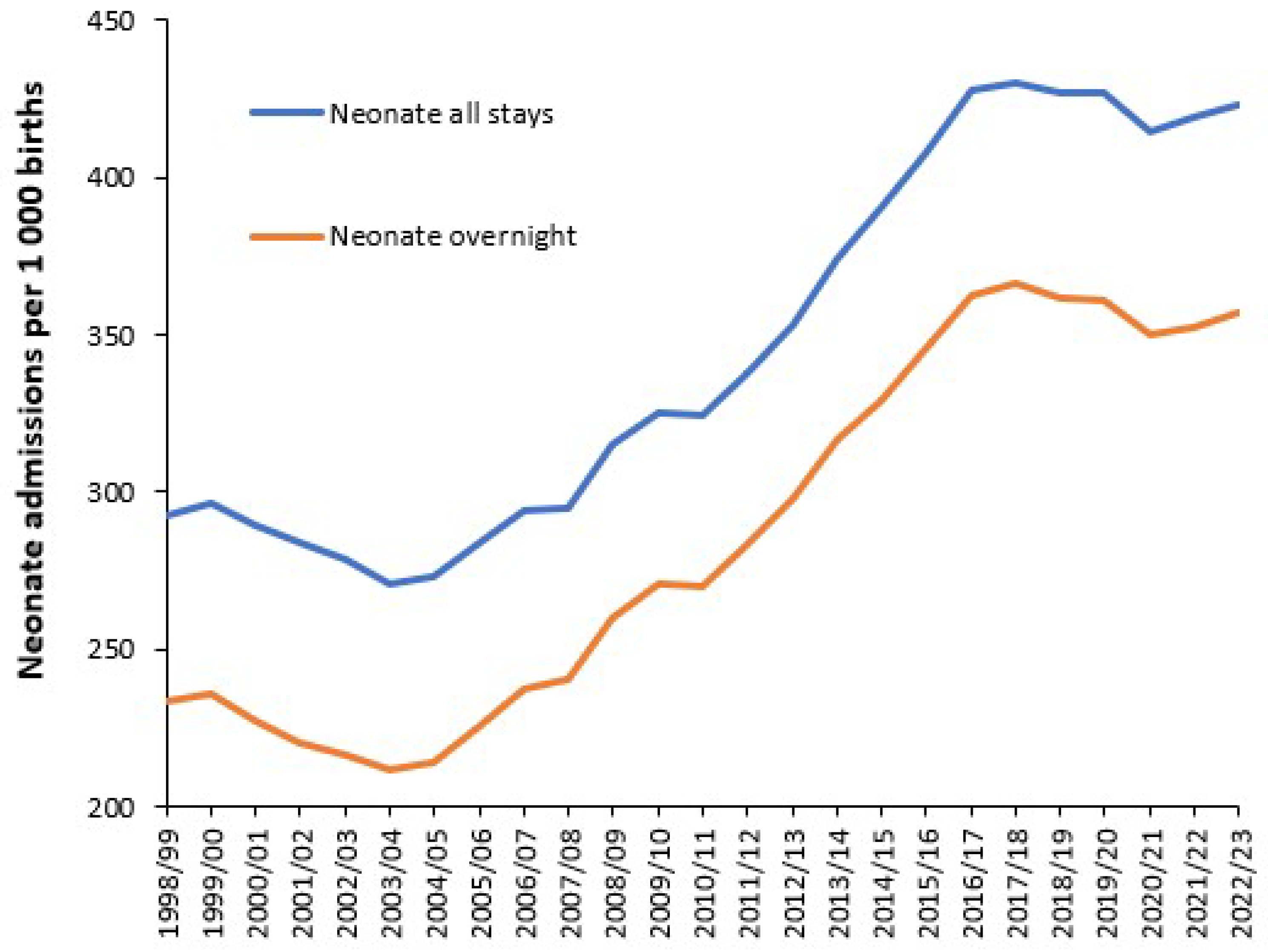
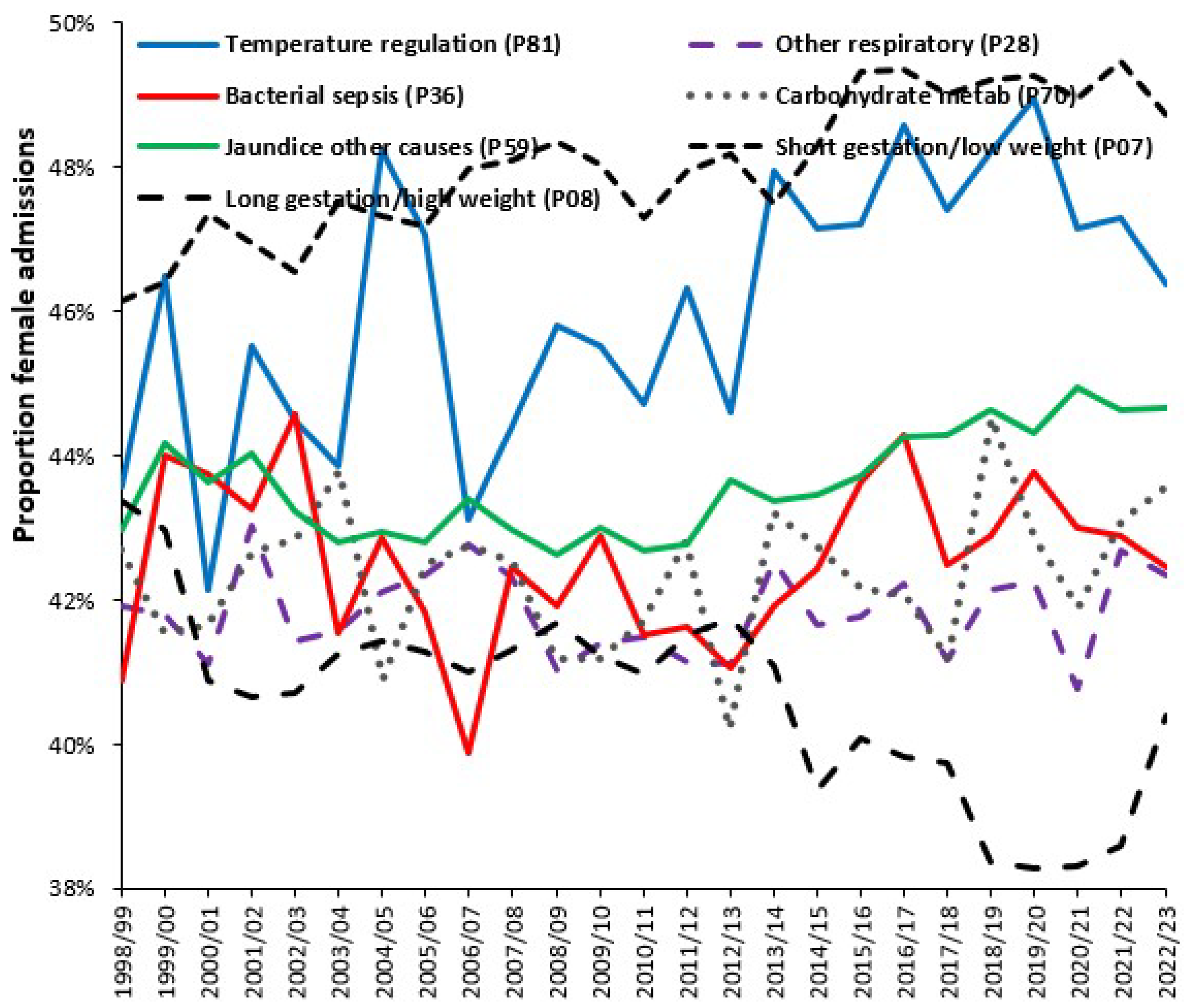
3.7. Effect of the Environment on Neonatal and Congenital Conditions
3.8. An Impending Maternity Crisis?
3.9. A Survey of Capacity Preparedness in England
- Are they aware that the reported bed occupancy at the obstetric unit is higher than may be expected for their size?
- Has any National or Professional Society guidance ever been published on how to correctly size a maternity unit?
- Do they have any planning documents relating to the choice of the current number of maternity beds?
4. Discussion
4.1. General Issues
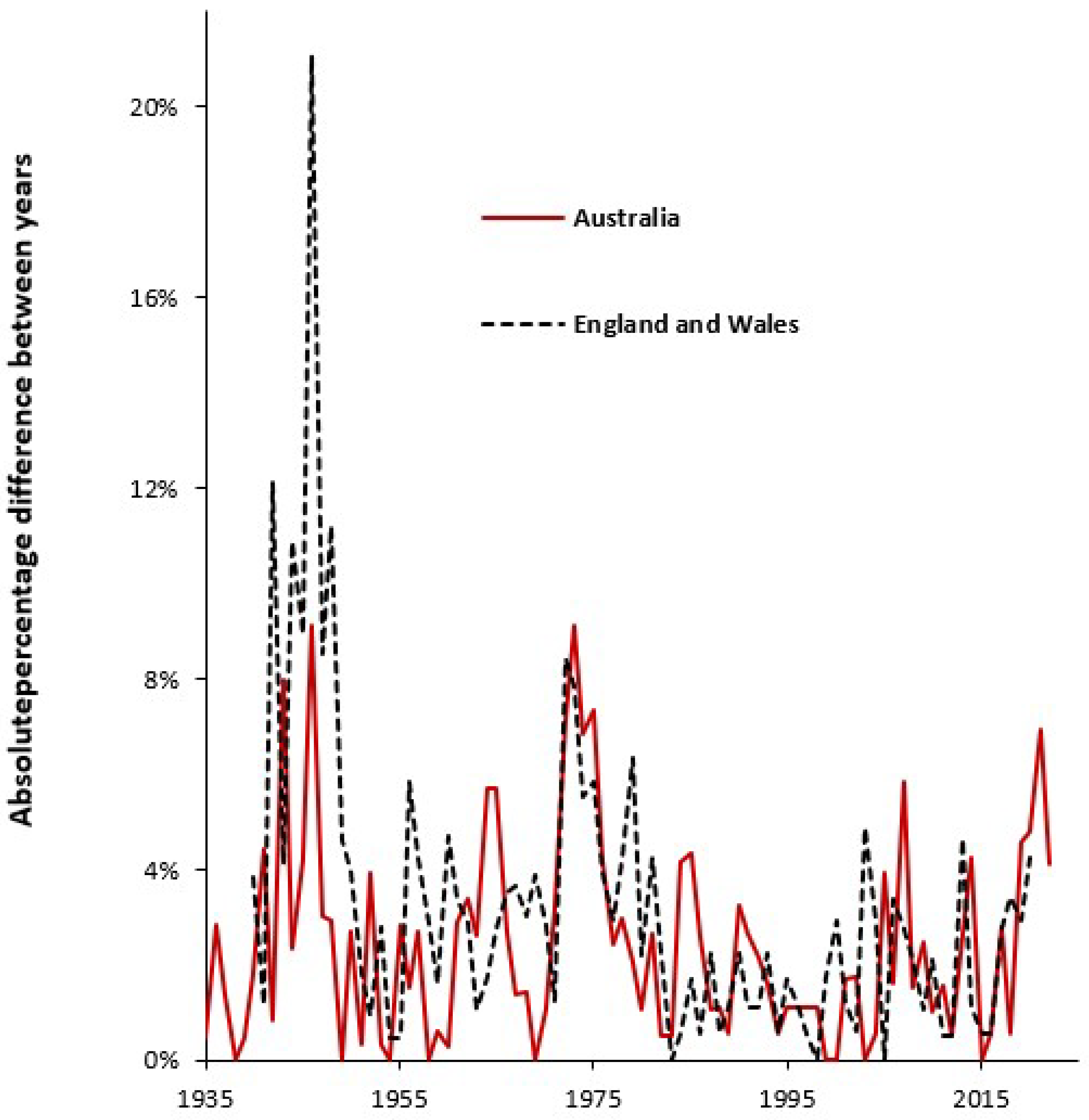
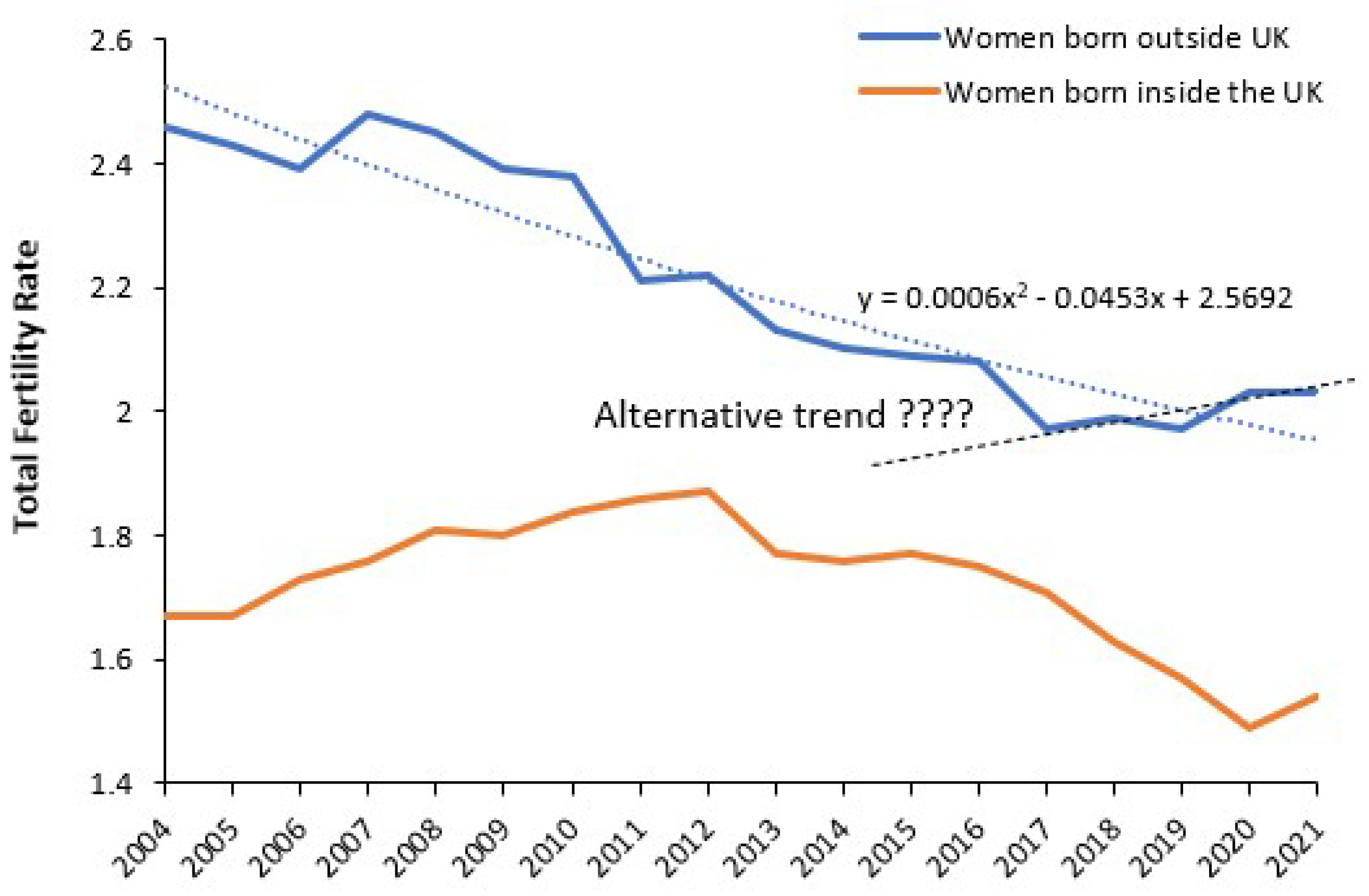
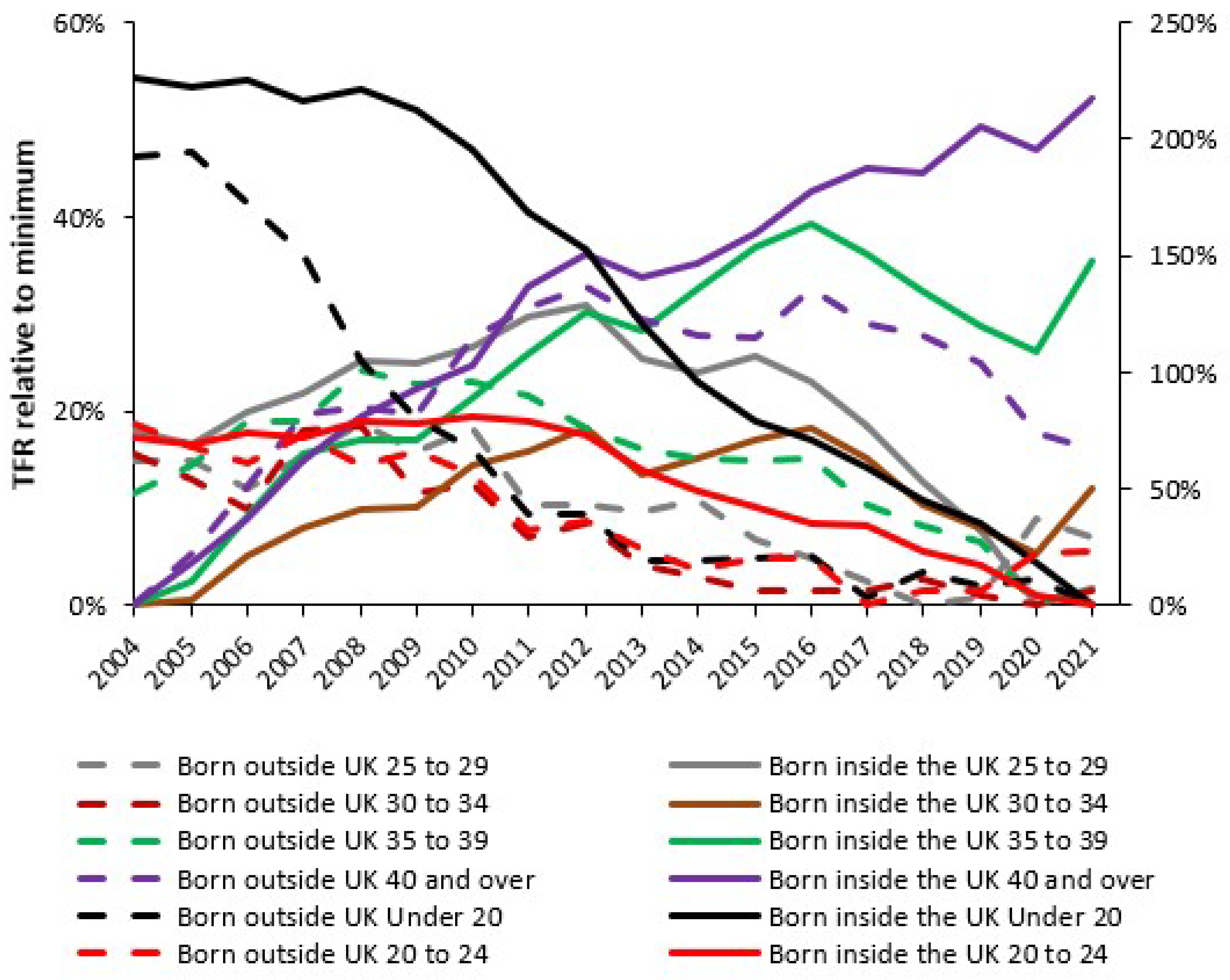
4.2. Forecasting Long-Term Trends in Births
4.3. Seasonality in Births
4.4. 24-h and Weekday Cycles in Bed Occupancy
4.5. Lunar and Solar Cycles
4.6. Infections and Pregnancy
4.7. Risk Factors
4.8. The English NHS National Maternity Review
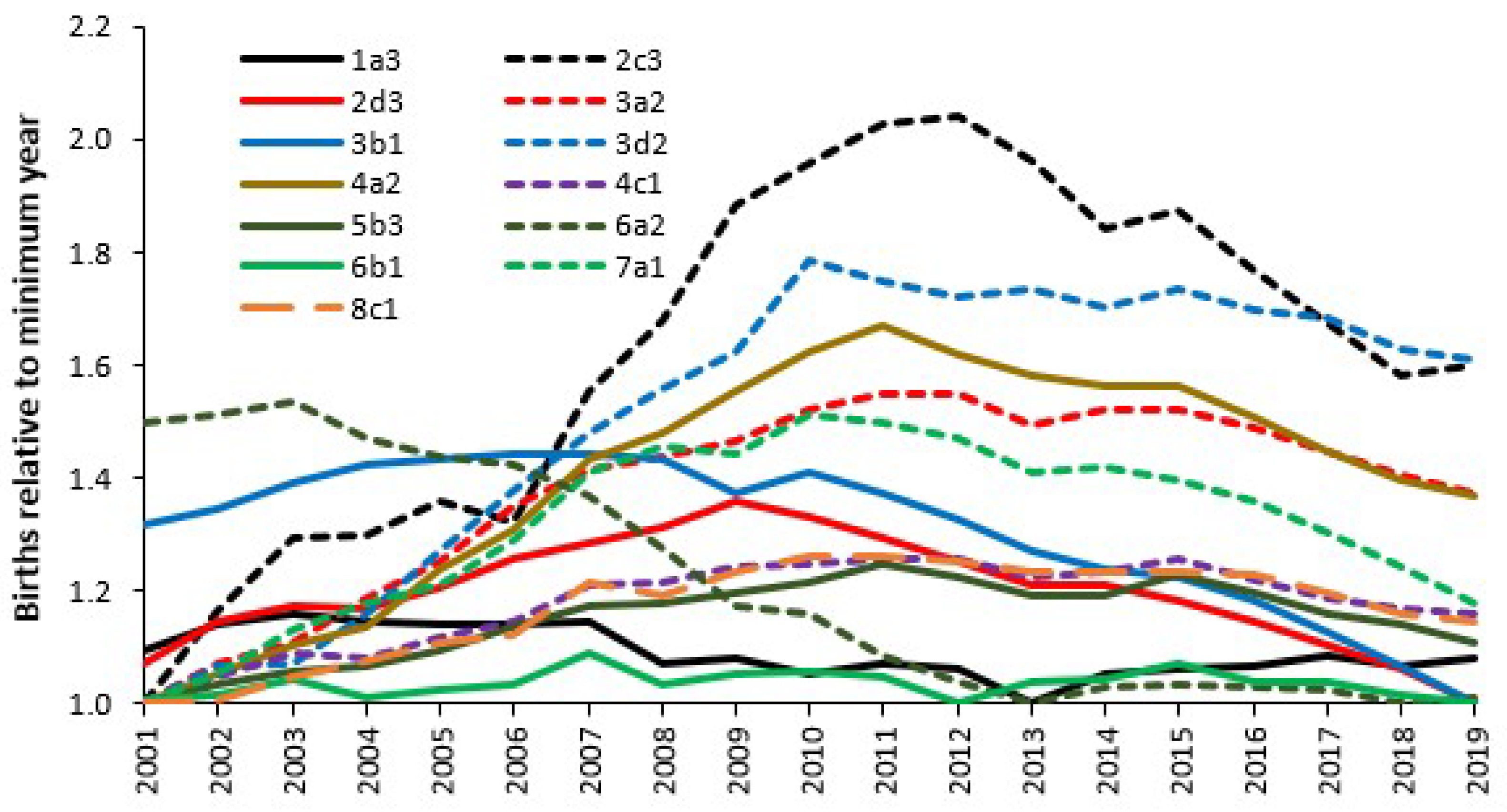
4.9. Issues of Population Density and Distance
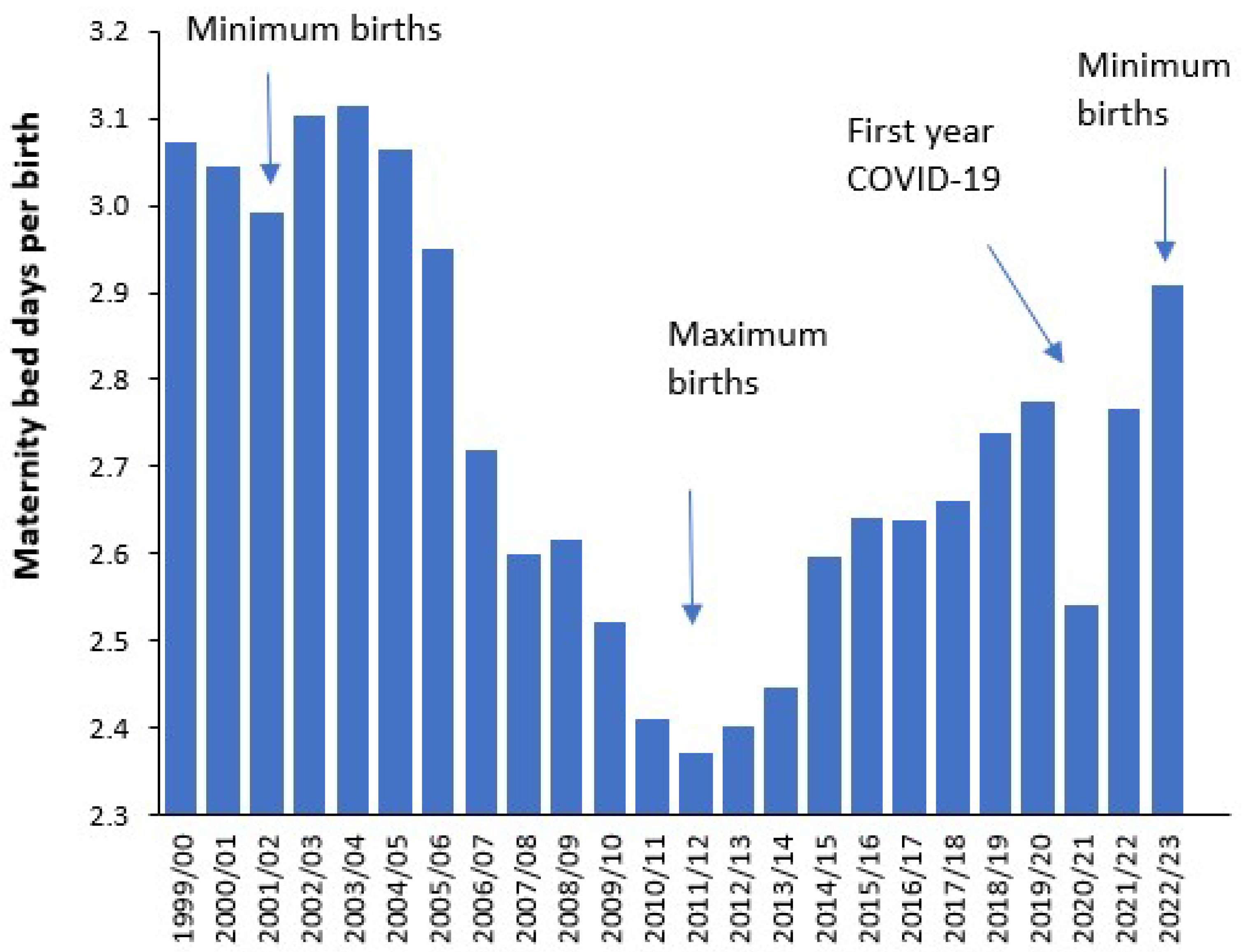
4.10. Does Decreasing Length of Stay (LOS) Actually Reduce Costs
“This is because for both medical and surgical patients, the main costs occur in the first half of the stay when input from staff, investigation, and intervention are at a maximum. Stays in hospital are almost always shortened by reducing lower dependency “cheaper” days, usually in the second half of the stay”. Along similar lines another study noted that “not all hospital days are economically equivalent”[105]
- How are direct maternity costs allocated based on time? Is time-based costing used?
- How are wider hospital overhead costs allocated to the maternity department, and would a change in the apportionment method used for each function have a significant effect on the supposed ‘cost’?
- How do costs behave over time (fixed and variable costs, marginal costs, step increases/reduction due to changes in demand)?
4.11. Pitfalls in Benchmarking Length of Stay (LOS) and Costs
“Reducing the length of time women spend in hospital after birth implies that staff and bed numbers can be reduced. However, the cost savings may be reduced if quality and access to services are maintained. Admission and discharge procedures are relatively fixed and involve high cost, trained staff time. Furthermore, it is important to retain a sufficient bed contingency capacity to ensure a reasonable level of service. If quality of care is maintained, staffing and bed capacity cannot be simply reduced proportionately: reducing average LOS on a typical postnatal ward by six hours or 17% would reduce costs by just 8%. This might still be a significant saving over a high volume service however, earlier discharge results in more women and babies with significant care needs at home. Quality and safety of care would also require corresponding increases in community based postnatal care. Simply reducing staffing in proportion to the LOS increases the workload for each staff member resulting in poorer quality of care and increased staff stress.”
4.12. When Did England Reach the Optimum LOS?
“The hospital stay of the mother and her healthy term newborn infant should be long enough to allow identification of problems and to ensure that the mother is sufficiently recovered and prepared to care for herself and her newborn at home. The LOS should be based on the unique characteristics of each mother-infant dyad, including the health of the mother, the health and stability of the newborn, the ability and confidence of the mother to care for herself and her newborn, the adequacy of support systems at home, and access to appropriate follow-up care in a medical home. Input from the mother and her obstetrical care provider should be considered before a decision to discharge a newborn is made, and all efforts should be made to keep a mother and her newborn together to ensure simultaneous discharge”[115]
4.13. Matching Staffing with Demand
4.14. Size, Statistical Chaos and Income
4.15. Fair Funding for Maternity Units
4.16. Flexible Staffing to Offset the Efect of Size
4.17. A System-Wide View of Maternity Costs
4.18. Key Recommendations
- Government health departments should encourage the use of turn-away for understanding maternity unity capacity preparedness.
- There is reliable evidence that maternity demand is subject to hourly, seasonal and environmental fluctuations, implying that the annual average occupancy should ideally be below 0.01% turn-away.
- Any maternity unit with an annual average turn-away greater than 1% must flag this on the hospital risk register and implement plans to correct this situation.
- Research is required to disentangle the effects of turn-away and poor staffing on safety and outcomes in maternity units.
- Maternity units should monitor bed occupancy and associated turn-away hourly throughout the year in the birthing unit, the postpartum maternity unit, any associated maternity (short stay) assessment unit and any Midwife-led community units. Past trends in such metrics should also be investigated to determine the local fluctuations in demand and ongoing trends. A chart showing daily admissions or births over many years is always a helpful tool.
- Maternity units should refresh their estimates of future demand every two to three years and compare how actual demand compares with past estimates.
- Government regulators should establish guidelines regarding the maximum acceptable turn-away in maternity units.
- Benchmarking of maternity unit minimum acceptable LOS needs to be against nationally agreed levels of quality and safety. High turn-away units should be excluded from the derivation of such benchmarking.
- To compare costs on a like-for-like basis, the cost per HRG/DRG requires the identification of the separate components of cost, namely, depreciation on capital (buildings and equipment), organization-wide apportioned costs for all the non-patient facing departments and the direct costs of care. The direct costs of care per birth will be higher as the unit gets smaller and, on this basis, small midwife-led low risk units are unlikely to be cost effective—although they may be considered desirable by mothers.
- In England, which uses the HRG tariff, all maternity units should receive extra funding based on size to mitigate the unavoidable higher costs relating to smaller size.
- Research is required as to how maternity units cope on days when there are unusually high births arising from Poisson variation, i.e., do they resort to emergency C-section to cope? Due to the skew in the Poisson distribution, this becomes a bigger problem as the unit gets smaller.
- As an extension of #11, the safe staffing levels for maternity units must be formulated with a higher ratio as size decreases.
4.19. Limitations of the Study
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Country of Birth of Mother | 2001 TFR | 2011 TFR | Country of Birth of Mother | 2001 TFR | 2011 TFR |
|---|---|---|---|---|---|
| North Africa | 4.6 | 3.9 | New EU | 2.0 | 2.2 |
| Pakistan | 4.7 | 3.8 | Central Asia | 2.7 | 2.2 |
| Western Africa | 2.7 | 3.3 | Poland | 2.8 | 2.1 |
| Bangladesh | 3.9 | 3.3 | European Union | 1.6 | 1.9 |
| Central Africa | 5.0 | 3.1 | Non-EU Europe | 2.7 | 1.9 |
| Southern Asia | 3.6 | 3.0 | United Kingdom | 1.6 | 1.8 |
| Eastern Africa | 2.3 | 2.6 | Southern Africa | 1.4 | 1.8 |
| Middle East | 3.1 | 2.6 | Central America | 1.7 | 1.8 |
| Sri Lanka | 3.5 | 2.6 | South America | 2.3 | 1.8 |
| Oceania (excl. Australia) | 2.0 | 2.6 | North America | 1.7 | 1.7 |
| India | 2.2 | 2.4 | Eastern Asia | 1.1 | 1.5 |
| Caribbean | 2.8 | 2.3 | South East Asia | 1.5 | 1.5 |
| Australasia | 1.2 | 1.3 |
| Function |
|---|
| Chief Executive, Chairman, Non-executive directors |
| Human Resources (Personnel, recruitment) |
| Media and Communications |
| Procurement |
| Women’s and Children’s Management |
| External advice (Management Consultant fees, Legal costs) |
| State and local government taxes |
| Insurance (buildings, equipment, clinical negligence) |
| Estates and Facilities (buildings and grounds, maintenance) |
| Finance, annual accounts, payroll, debt recovery, etc. |
| Information Technology and supporting software |
| Information Management, monthly reports, etc. |
| Medical Records (non-computerized) |
| Strategy and Planning |
| Portering |
| Patient transport |
| Pathology (blood biochemistry, microbiology, etc.) |
| Radiology |
| Medical Instrumentation |
| Intensive Care |
| Housekeeping (cleaning, etc.) |
| Health and Safety |
| Infection Control |
| Medical Illustration |
| Library |
| Capital costs via depreciation of buildings and equipment |


References
- Jones, R. Addressing the Knowledge Deficit in Hospital Bed Planning and Defining an Optimum Region for the Number of Different Types of Hospital Beds in an Effective Health Care System. Int. J. Environ. Res. Public Health 2023, 20, 7171. [Google Scholar] [CrossRef]
- Jones, R. A New Approach for Understanding International Hospital Bed Numbers and Application to Local Area Bed Demand and Capacity Planning. Int. J. Environ. Res. Public Health 2024, 21, 1035. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, A.M.; Bekker, R.; van Zanten, L.; Koole, G.M. Dimensioning hospital wards using the Erlang loss model. Ann. Oper. Res. 2010, 178, 23–43. [Google Scholar] [CrossRef]
- Lim, G.; Lim, A.J.; Quinn, B.; Carvalho, B.; Zakowski, M.; Lynde, G.C. Obstetric operating room staffing and operating efficiency using queueing theory. BMC Health Serv. Res. 2023, 23, 1147. [Google Scholar] [CrossRef]
- Sülz, S.; Fügener, A.; Becker-Peth, M.; Roth, B. The potential of patient-based nurse staffing—A queuing theory application in the neonatal intensive care setting. Health Care Manag. Sci. 2024, 27, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, N.; Reamer, C.; Reynolds, T.; Howell, L.; Moldenhauer, J.; Day, T. Capacity Planning for Maternal–Fetal Medicine Using Discrete Event Simulation. Am. J. Perinatol. 2014, 32, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, C.; Augusto, V.; Xie, X.; Crenn-Hebert, C. Multi-period capacity planning for maternity facilities in a perinatal network: A queuing and optimization approach. In Proceedings of the IEEE International Conference on Automation Science and Engineering (CASE), Seoul, Republic of Korea, 20–24 August 2012; pp. 137–142. [Google Scholar] [CrossRef]
- Ramalhoto, M. Erlang’s Formulas. In International Encyclopedia of Statistical Science; Lovric, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Joranger, P.; Huitfeldt, A.S.; Bernitz, S.; Blix, E. Cost minimisation analyses of birth care in low-risk women in Norway: A comparison between planned home birth and birth in a standard obstetric unit. BMC Health Serv. Res. 2024, 24, 1150. [Google Scholar] [CrossRef]
- Grzybowski, S.; Stoll, K.; Kornelsen, J. Distance matters: A population based study examining access to maternity services for rural women. BMC Health Serv. Res. 2011, 11, 147. [Google Scholar] [CrossRef]
- NHS England. Bed Availability and Occupancy—KH03. Available online: https://www.england.nhs.uk/statistics/statistical-work-areas/bed-availability-and-occupancy/bed-availability-and-occupancy-kh03/ (accessed on 19 September 2024).
- Northern Ireland Department of Health. Hospital Statistics: Inpatient and Day Case Activity 2023/24. Available online: https://www.health-ni.gov.uk/publications/hospital-statistics-inpatient-and-day-case-activity-202324 (accessed on 12 October 2024).
- Australian Bureau of Statistics. Births, Australia. Available online: https://www.abs.gov.au/statistics/people/population/births-australia/sort (accessed on 13 September 2024).
- Office for National Statistics. Births in England and Wales: Summary Tables. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/datasets/birthsummarytables (accessed on 13 September 2024).
- Office for National Statistics. Birth Characteristics in England and Wales. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthcharacteristicsinenglandandwales/2022 (accessed on 19 October 2024).
- Office for National Statistics. Births in England and Wales: 2020. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthsummarytablesenglandandwales/2020 (accessed on 21 October 2024).
- Office for National Statistics. Vital Statistics in the UK. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/vitalstatisticspopulationandhealthreferencetables (accessed on 1 November 2024).
- Office for National Statistics. Population Projections Incorporating Births, Deaths and Migration for Regions and Local Authorities. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/datasets/componentsofchangebirthsdeathsandmigrationforregionsandlocalauthoritiesinenglandtable5 (accessed on 13 September 2024).
- Office for National Statistics. Live Births by Output Area, England and Wales: Mid-Year Periods (1 July to 30 June) 2001 to 2020. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/adhocs/13952livebirthsbyoutputareaenglandandwalesmidyearperiods1julyto30june2001to2020 (accessed on 13 September 2024).
- Office for National Statistics. 2011 OAC Clusters and Names. Available online: https://www.ons.gov.uk/methodology/geography/geographicalproducts/areaclassifications/2011areaclassifications/datasets (accessed on 13 September 2024).
- NHS England. Hospital Admitted Patient Care Activity. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity (accessed on 16 October 2024).
- Grace, K. Considering climate in studies of fertility and reproductive health in poor countries. Nat. Clim. Change 2017, 7, 479–485. [Google Scholar] [CrossRef]
- National Museum Australia. Postwar Immigration Drive. Available online: https://www.nma.gov.au/defining-moments/resources/postwar-immigration-drive (accessed on 27 September 2024).
- Migration Watch UK. Youth Unemployment and Immigration from the A8 Countries. 2012. Available online: https://www.migrationwatchuk.org/briefing-paper/247 (accessed on 27 September 2024).
- Liao, P.; Dollin, J. Half a century of the oral contraceptive pill: Historical review and view to the future. Can. Fam. Physician 2012, 58, e757–e760. [Google Scholar] [PubMed]
- Office for National Statistics. Parents’ Country of Birth. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/datasets/parentscountryofbirth (accessed on 28 October 2024).
- Office for National Statistics. About the Area Classifications. Available online: https://www.ons.gov.uk/methodology/geography/geographicalproducts/areaclassifications/2011areaclassifications/abouttheareaclassifications (accessed on 29 October 2024).
- Office for National Statistics. Maps. Available online: https://www.ons.gov.uk/methodology/geography/geographicalproducts/areaclassifications/2011areaclassifications/maps (accessed on 1 November 2024).
- World Health Organization. Obesity. Available online: https://www.who.int/health-topics/obesity (accessed on 19 October 2024).
- Creanga, A.; Catalano, P.; Bateman, B. Obesity in pregnancy. N. Engl. J. Med. 2022, 387, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Freaney, P.; Perak, A.; Greenland, P.; Lloyd-Jones, D.; Grobman, W.; Khan, S. Trends in prepregnancy obesity and association with adverse pregnancy outcomes in the United States, 2013 to 2018. J. Am. Heart Assoc. 2021, 10, e020717. [Google Scholar] [CrossRef] [PubMed]
- Rubens, M.; Ramamoorthy, V.; Saxena, A.; McGranaghan, P.; Veledar, E.; Hernandez, A. Obstetric outcomes during delivery hospitalizations among obese pregnant women in the United States. Sci. Rep. 2022, 12, 6862. [Google Scholar] [CrossRef] [PubMed]
- Cavazos-Rehg, P.; Krauss, M.; Spitznagel, E.; Bommarito, K.; Madden, T.; Olsen, M.; Subramaniam, H.; Peipert, J.; Bierut, L. Maternal age and risk of labor and delivery complications. Matern. Child Health J. 2015, 19, 1202–1211. [Google Scholar] [CrossRef]
- Brown, C.; Adams, C.; George, K.; Moore, J. Mental Health Conditions Increase Severe Maternal Morbidity By 50 Percent And Cost $102 Million Yearly In The United States. Health Aff. 2021, 40, 1575–1584. [Google Scholar] [CrossRef]
- Moran, P.; Wuytack, F.; Turner, M.; Normand, C.; Brown, S.; Begley, C.; Daly, D. Economic burden of maternal morbidity—A systematic review of cost-of-illness studies. PLoS ONE 2020, 15, e0227377. [Google Scholar] [CrossRef]
- NHS England. NHS Long Term Workforce Plan. 2023. Available online: https://www.england.nhs.uk/publication/nhs-long-term-workforce-plan/ (accessed on 1 October 2024).
- Department of Health. Children, Young People and Maternity Services. Health Building Note 09-02: Maternity Care Facilities. Available online: https://www.england.nhs.uk/wp-content/uploads/2021/05/HBN_09-02_Final.pdf (accessed on 14 October 2024).
- Bailey, N. Queueing for medical care. J. R. Stat. Soc. Ser. C (Appl. Stat.) 1954, 3, 137–145. [Google Scholar] [CrossRef]
- Jennings, A. Hospital and ward design with particular reference to obstetrical wards. S. Afr. Med. J. 1959, 33, 397–400. [Google Scholar]
- Robards, J. National Statistical Blog—Understanding Our Future Population: Why Projections Are Not Predictions. 2024. Available online: https://blog.ons.gov.uk/2024/01/30/understanding-our-future-population-why-projections-are-not-predictions/ (accessed on 22 December 2024).
- Lee, R. Forecasting Population in an Uncertain World: Approaches, New Uses, and Troubling Limitations. In Population and Development Review; Wiley: Hoboken, NJ, USA, 2024. [Google Scholar] [CrossRef]
- Keilman, N. Uncertainty in Population Forecasts for the Twenty-First Century. Annu. Rev. Resour. Econ. 2020, 12, 449–470. [Google Scholar] [CrossRef]
- Wilson, T. Visualizing the Shelf Life of Population Forecasts: A Simple Approach to Communicating Forecast Uncertainty. J. Off. Stat. 2024, 40, 38–56. [Google Scholar] [CrossRef]
- Grøntved, S.; Kirkeby, M.; Johnsen, S.; Mainz, J.; Valentin, J.; Jensen, C. Towards reliable forecasting of healthcare capacity needs: A scoping review and evidence mapping. Int. J. Med. Inform. 2024, 189, 105527. [Google Scholar] [CrossRef]
- Potančoková, M.; Marois, G. Projecting future births with fertility differentials reflecting women’s educational and migrant characteristics. Vienna Yearb. Popul. Res. 2020, 18, 141–166. [Google Scholar] [CrossRef]
- McDonald, P.; Kippen, R. Forecasting Births Using a Three Parameter Model. Human Fertility Database. Max Planck Institute for Demographic Research (Germany) and Vienna Institute of Demography (Austria). Available online: https://www.humanfertility.org/File/GetDocument/Docs/Symposium/McDonald_Kippen.pdf (accessed on 29 December 2024).
- Comolli, C.; Vignoli, D. Spreading Uncertainty, Shrinking Birth Rates: A Natural Experiment for Italy. Eur. Sociol. Rev. 2021, 37, 555–570. [Google Scholar] [CrossRef]
- Chao, F.; Gerland, P.; Cook, A.; Guilmoto, C.; Alkema, L. Projecting sex imbalances at birth at global, regional and national levels from 2021 to 2100: Scenario-based Bayesian probabilistic projections of the sex ratio at birth and missing female births based on 3.26 billion birth records. BMJ Glob. Health 2021, 6, e005516. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shang, H.; Raymer, J. Forecasting Australian fertility by age, region, and birthplace. Int. J. Forecast. 2024, 40, 532–548. [Google Scholar] [CrossRef]
- Ellison, J.; Dodd, E.; Forster, J. Forecasting of cohort fertility under a hierarchical Bayesian approach. J. R. Stat. Soc. Ser. A Stat. Soc. 2020, 183, 829–856. [Google Scholar] [CrossRef]
- Tzitiridou-Chatzopoulou, M.; Zournatzidou, G.; Kourakos, M. Predicting Future Birth Rates with the Use of an Adaptive Machine Learning Algorithm: A Forecasting Experiment for Scotland. Int. J. Environ. Res. Public Health 2024, 21, 841. [Google Scholar] [CrossRef] [PubMed]
- Consterdine, E. The Huge Political Cost of Blair’s Decision to Allow Eastern European Migrants Unfettered Access to Britain. The Conversation, 16 November 2016. Available online: https://theconversation.com/the-huge-political-cost-of-blairs-decision-to-allow-eastern-european-migrants-unfettered-access-to-britain-66077 (accessed on 14 September 2024).
- Office for National Statistics. National Population Projections, Fertility Assumptions: 2020-Based Interim. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/methodologies/nationalpopulationprojectionsfertilityassumptions2020basedinterim (accessed on 1 November 2024).
- Office for National Statistics. National Population Projections, Migration Assumptions: 2020-Based Interim. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/methodologies/nationalpopulationprojectionsmigrationassumptions2020basedinterim (accessed on 1 November 2024).
- GOV.UK. National Pupil Projections. 2024. Available online: https://explore-education-statistics.service.gov.uk/methodology/national-pupil-projections-methodology (accessed on 14 October 2024).
- Jones, R. Trends in Births and Implications to Maternity Capacity: A Report for Berkshire East PCT. 2008. Available online: https://www.researchgate.net/publication/320548875_Trends_in_Births_and_Implications_to_Maternity_Capacity_A_Report_for_Berkshire_East_PCT (accessed on 23 September 2024).
- Office for National Statistics. House Building Data, UK. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/housing/articles/ukhousebuildingdata/latest (accessed on 1 November 2024).
- Chodick, G.; Flash, S.; Deoitch, Y.; Shalev, V. Seasonality in birth weight: Review of global patterns and potential causes. Hum. Biol. 2009, 81, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Sone, T.; Doi, T.; Kahyo, H. Seasonality of Mean Birth Weight and Mean Gestational Period in Japan. Hum. Biol. 1993, 65, 481–501. [Google Scholar]
- Bobak, M.; Gjonca, A. The seasonality of live birth is strongly influenced by socio-demographic factors. Hum. Reprod. 2001, 16, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.; McDonald, B. Seasonal variation of nutrient intake in pregnancy: Effects on infant measures and possible influence on diseases related to season of birth. Eur. J. Clin. Nutr. 2007, 61, 1271–1280. [Google Scholar] [CrossRef]
- Riala, K.; Hakko, H.; Taanila, A.; Räsänen, P. Season of birth and smoking: Findings from the Northern Finland 1966 Birth Cohort. Chronobiol. Int. 2009, 26, 1660–1672. [Google Scholar] [CrossRef]
- Li, L.; Boland, M.; Miotto, R.; Tatonetti, N.; Dudley, J. Replicating Cardiovascular Condition-Birth Month Associations. Sci. Rep. 2016, 6, 33166. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.; Parhi, P.; Li, L.; Miotto, R.; Carroll, R.; Iqbal, U.; Nguyen, P.; Schuemie, M.; You, S.; Smith, D.; et al. Uncovering exposures responsible for birth season—Disease effects: A global study. J. Am. Med. Inf. Assoc. 2018, 25, 275–288. [Google Scholar] [CrossRef]
- Doyle, M.-A. Seasonal patterns in newborns’ health: Quantifying the roles of climate, communicable disease, economic and social factors. Econ. Hum. Biol. 2023, 51, 101287. [Google Scholar] [CrossRef]
- Kaur, S.; Teoh, A.N.; Shukri, N.; Shafie, S.; Bustami, N.; Takahashi, M.; Lim, P.; Shibata, S. Circadian rhythm and its association with birth and infant outcomes: Research protocol of a prospective cohort study. BMC Pregnancy Childbirth 2020, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Heres, M.; Pel, M.; Borkent-Polet, M.; Treffers, P.; Mirmiran, M. The hour of birth: Comparisons of circadian pattern between women cared for by midwives and obstetricians. Midwifery 2000, 16, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Baykov, D.; Skeldon, A.; Whyte, M. Analysis of bed occupancy data on the acute medical unit. Future Health J. 2020, 7 (Suppl. S1), s84–s85. [Google Scholar] [CrossRef]
- Riahi, V.; Boyle, J.; Hassanzadeh, H.; Yoon, J.; Diouf, I.; Khanna, S.; Samadbeik, M.; Sullivan, C.; Bosley, E.; Staib, A.; et al. Do Midnight Censuses Accurately Portray Hospital Bed Occupancy? In Health. Innovation. Community: It Starts with Us; IOS Press: Amsterdam, The Netherlands, 2024; pp. 36–41. [Google Scholar] [CrossRef]
- Morton-Pradhan, S.; Bay, R.; Coonrod, D. Birth rate and its correlation with the lunar cycle and specific atmospheric conditions. Am. J. Obstet. Gynecol. 2005, 192, 1970–1973. [Google Scholar] [CrossRef]
- Arliss, J.; Kaplan, E.; Galvin, S. The effect of the lunar cycle on frequency of births and birth complications. Am. J. Obstet. Gynecol. 2005, 192, 1462–1464. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.; Bender, S.; Heining, J.; Schmidt, C. The lunar cycle, sunspots and the frequency of births in Germany, 1920–1989. Econ. Hum. Biol. 2013, 11, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.-i.; Shirahashi, K. Novel perspectives on the influence of the lunar cycle on the timing of full-term human births. Chronobiol. Int. 2020, 37, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Mathur, K.; Gupta, A.; Rawat, V.; Sharma, V.; Shakya, S. Chapter 11—Role of noncoding RNAs in host-pathogen interactions: A systems biology approach. In Developments in Microbiology, Systems Biology Approaches for Host-Pathogen Interaction Analysis; Ashraf, M.T., Khan, A.A., Aldakheel, F.M., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 213–249. [Google Scholar] [CrossRef]
- Eichner, H.; Karlsson, J.; Loh, E. The emerging role of bacterial regulatory RNAs in disease. Trends Microbiol. 2022, 30, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Tzur, Y. lncRNAs in fertility: Redefining the gene expression paradigm? Trends Genet. 2022, 38, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Jin, H.; Zhu, Y. The Role of Placental Non-Coding RNAs in Adverse Pregnancy Outcomes. Int. J. Mol. Sci. 2023, 24, 5030. [Google Scholar] [CrossRef]
- Khambata, K.; Modi, D.; Gupta, S. Immunoregulation in the testis and its implication in fertility and infections. Explor. Immunol. 2021, 1, 309–324. [Google Scholar] [CrossRef]
- Pai, M.; Venkatesh, S.; Gupta, P. The role of infections in infertility: A review. Int. J. Acad. Med. 2020, 6, 189–196. [Google Scholar] [CrossRef]
- Giakoumelou, S.; Wheelhouse, N.; Cuschieri, K.; Entrican, G.; Howie, S.E.; Horne, A.W. The role of infection in miscarriage. Hum. Reprod. Update 2016, 22, 116–133. [Google Scholar] [CrossRef]
- Megli, C.J.; Coyne, C.B. Infections at the maternal–fetal interface: An overview of pathogenesis and defence. Nat. Rev. Microbiol. 2022, 20, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Racicot, K.; Kwon, J.; Aldo, P.; Silasi, M.; Mor, G. Understanding the complexity of the immune system during pregnancy. Am. J. Reprod. Immunol. 2014, 72, 107–116. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef] [PubMed]
- Lain, K.; Catalano, P. Metabolic changes in pregnancy. Clin. Obs. Gynecol. 2007, 50, 938–948. [Google Scholar] [CrossRef]
- Légaré, C.; Clément, A.A.; Desgagné, V.; Thibeault, K.; White, F.; Guay, S.P.; Arsenault, B.J.; Scott, M.S.; Jacques, P.É.; Perron, P.; et al. Human plasma pregnancy-associated miRNAs and their temporal variation within the first trimester of pregnancy. Reprod. Biol. Endocrinol. 2022, 20, 14. [Google Scholar] [CrossRef]
- Arya, S.; Mulla, Z.; Plavsic, S. Outcomes of Women Delivering at Very Advanced Maternal Age. J. Women’s Health 2018, 27, 1378–1384. [Google Scholar] [CrossRef]
- Delpisheh, A.; Brabin, L.; Brabin, B. Pregnancy, smoking and birth outcomes. Women’s Health 2006, 2, 389–403. [Google Scholar] [CrossRef]
- McDonald, S.; Han, Z.; Mulla, S.; Beyene, J. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: Systematic review and meta-analyses. BMJ 2010, 20, 341. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, C.; Nassar, N.; Kurinczuk, J.; Bower, C. The effect of maternal alcohol consumption on fetal growth and preterm birth. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 390–400. [Google Scholar] [CrossRef]
- Pinto, S.; Dodd, S.; Walkinshaw, S.; Siney, C.; Kakkar, P.; Mousa, H. Substance abuse during pregnancy: Effect on pregnancy outcomes. Eur. J. Obs. Gyne Reprod. Biol. 2010, 150, 137–141. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency. UK Maps of Radon. Available online: https://www.ukradon.org/information/ukmaps (accessed on 17 October 2024).
- World Health Organization. Radon. Available online: https://www.who.int/news-room/fact-sheets/detail/radon-and-health (accessed on 17 October 2024).
- Papatheodorou, S.; Yao, W.; Vieira, C.; Li, L.; Wylie, B.; Schwartz, J.; Koutrakis, P. Residential radon exposure and hypertensive disorders of pregnancy in Massachusetts, USA: A cohort study. Environ. Int. 2021, 146, 106285. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.; Li, L.; Son, J.; Koutrakis, P.; Bell, M. Associations between gestational residential radon exposure and term low birthweight in Connecticut, USA. Epidemiology 2024, 35, 834–843. [Google Scholar] [CrossRef]
- NHS England (National Maternity Review). Better Births: Improving Outcomes of Maternity Services in England—A Five Year Forward View for Maternity Care. 2016. Available online: https://www.england.nhs.uk/publication/better-births-improving-outcomes-of-maternity-services-in-england-a-five-year-forward-view-for-maternity-care/ (accessed on 14 December 2024).
- NHS England. Maternity Transformation Programme. Available online: https://www.england.nhs.uk/mat-transformation/ (accessed on 14 October 2024).
- NHS England. Three Year Delivery Plan for Maternity and Neonatal Services. Available online: https://www.england.nhs.uk/long-read/three-year-delivery-plan-for-maternity-and-neonatal-services/ (accessed on 23 October 2024).
- GOV.UK. Independent Investigation of the National Health Service in England. Available online: https://assets.publishing.service.gov.uk/media/66f42ae630536cb92748271f/Lord-Darzi-Independent-Investigation-of-the-National-Health-Service-in-England-Updated-25-September.pdf (accessed on 28 October 2024).
- Homer, C.; Biggs, J.; Vaughan, G.; Sullivan, E. Mapping maternity services in Australia: Location, classification and services. Aust. Health Rev. 2011, 35, 222–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pilkington, H.; Blondel, B.; Drewniak, N.; Zeitlin, J. Choice in maternity care: Associations with unit supply, geographic accessibility and user characteristics. Int. J. Health Geogr. 2012, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, M.; Rowe, R.; Schroeder, L.; Puddicombe, D.; MacFarlane, A.; Newburn, M.; McCourt, C.; Sandall, J.; Silverton, L.; Marlow, N. Mapping Maternity Care: The Configuration of Maternity Care in England. Birthplace in England Research Programme (Final Report Part 3 08/1604/140). Southampton, UK: HMSO.2011. Available online: https://openaccess.city.ac.uk/id/eprint/2009/1/SDO_FR3_08-1604-140_V04.pdf (accessed on 17 September 2024).
- Pilkington, H.; Blondel, B.; Papiernik, E.; Cuttini, M.; Charreire, H.; Maier, R.; Petrou, S.; Combier, E.; Künzel, W.; Bréart, G.; et al. Distribution of maternity units and spatial access to specialised care for women delivering before 32 weeks of gestation in Europe. Health Place 2010, 16, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A. Why are we trying to reduce length of stay? Evaluation of the costs and benefits of reducing time in hospital must start from the objectives that govern change. Qual. Health Care 1996, 5, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Taheri, P.; Butz, D.; Greenfield, L. Length of stay has minimal impact on the cost of hospital admission. J. Am. Coll. Surg. 2000, 191, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Caplan, G.; Board, N.; Paten, A.; Tazelaar-Molinia, J.; Crowe, P.; Yap, S.-Y.; Brown, A. Decreasing length of stay: The cost to the community. Aust. N. Z. J. Surg. 1999, 69, 433–437. [Google Scholar] [CrossRef]
- Rauh, S.; Wadsworth, E.; Weeks, W. The fixed-cost dilemma: What counts when counting cost-reduction efforts? Healthc. Financ. Manag. 2010, 64, 60–63. [Google Scholar]
- Campbell, O.; Cegolon, L.; Macleod, D.; Benova, L. Length of Stay After Childbirth in 92 Countries and Associated Factors in 30 Low- and Middle-Income Countries: Compilation of Reported Data and a Cross-sectional Analysis from Nationally Representative Surveys. PLoS Med. 2016, 13, e1001972. [Google Scholar] [CrossRef]
- Grašič, K.; Mason, A.; Street, A. Paying for the quantity and quality of hospital care: The foundations and evolution of payment policy in England. Health Econ. Rev. 2015, 5, 15. [Google Scholar] [CrossRef]
- Crespin, D.; Dworsky, M.; Levin, J.; Ruder, T.; Whaley, C. Upcoding Linked to up to Two-Thirds of Growth in Highest-Intensity Hospital Discharges in 5 States, 2011–2019. Health Aff. 2024, 3, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal-Datta, N.; Chernew, M.; McWilliams, J. Lack Of Persistent Coding In Traditional Medicare May Widen The Risk-Score Gap With Medicare Advantage. Health Aff. 2024, 43, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, A.; Gerardi, M.; Sawchuk, P.; Bihl, I. Boomerang babies: Emergency department utilization by early discharge neonates. Pediatr. Emerg. Care 1997, 13, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, C.; Stewart, M. Factors associated with early neonatal attendance to a paediatric emergency department. Arch. Dis. Child. 2014, 99, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.; Cheyne, H. Reducing the length of postnatal hospital stay: Implications for cost and quality of care. BMC Health Serv. Res. 2015, 16, 16. [Google Scholar] [CrossRef]
- Benitz, W.; Committee on Fetus and Newborn; Watterberg, K.; Aucott, S.; Benitz, W.; Cummings, J.; Eichenwald, E.; Goldsmith, J.; Poindexter, B.; Puopolo, K.; et al. Hospital Stay for Healthy Term Newborn Infants. Pediatrics 2015, 135, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Datar, A.; Sood, N. Impact of postpartum hospital-stay legislation on newborn length of stay, readmission, and mortality in California. Pediatrics 2006, 118, 63–72. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Safe Midwifery Staffing for Maternity Settings. Available online: https://www.nice.org.uk/guidance/ng4 (accessed on 16 October 2024).
- NHS Improvement. Standards and Structures That Underpin Safer, More Personalised, and More Equitable Care. Available online: https://www.england.nhs.uk/wp-content/uploads/2021/04/Developing-workforce-safeguards.pdf (accessed on 23 October 2024).
- Edwards, N.; Vaughan, L.; Palmer, B.; Maternity Services in Smaller Hospitals: A Call to Action. Nuffield Trust Working Paper. August 2020. Available online: https://www.nuffieldtrust.org.uk/sites/default/files/2020-08/maternity-services-in-smaller-hospitals-web.pdf (accessed on 22 December 2024).
- Zbiri, S.; Rozenberg, P.; Goffinet, F.; Milcent, C. Cesarean delivery rate and staffing levels of the maternity unit. PLoS ONE 2018, 13, e0207379. [Google Scholar] [CrossRef] [PubMed]
- Snowden, J.; Kozhimannil, K.; Muoto, I.; Caughey, A.; Connell, J. A ‘busy day’ effect on perinatal complications of delivery on weekends: A retrospective cohort study. BMJ Qual. Saf. 2017, 26, e1. [Google Scholar] [CrossRef] [PubMed]
- Carey, K.; Burgess, J.; Young, G. Economies of scale and scope: The case of specialty hospitals. Contemp. Econ. Policy 2015, 33, 104–117. [Google Scholar] [CrossRef]
- Gaynor, M.; Kleiner, S.; Vogt, W. Analysis of hospital production: An output index approach. J. Appl. Econom. 2015, 30, 398–421. [Google Scholar] [CrossRef]
- Freeman, M.; Savva, N.; Scholtes, S. Economies of Scale and Scope in Hospitals: An Empirical Study of Volume Spillovers. Manag. Sci. 2021, 67, 673–697. [Google Scholar] [CrossRef]
- NHS England. Approved Costing Guidance. Available online: https://www.england.nhs.uk/costing-in-the-nhs/approved-costing-guidance/ (accessed on 1 November 2024).
- NHS England. 2023/25 NHS Payment Scheme (Amended) Annex B: Guidance on Currencies (Section 12). 2024. Available online: https://www.england.nhs.uk/wp-content/uploads/2023/03/23-25NHSPS-amended_Annex-B-Guidance-on-currencies.pdf (accessed on 29 October 2024).
- NHS England. A Guide to the Market Forces Factor. 2023. Available online: https://www.england.nhs.uk/wp-content/uploads/2023/03/23-25NHSPS_Guide-to-the-market-forces-factor.pdf (accessed on 30 October 2024).
- Martin, E.; Ayoub, B.; Miller, Y. A systematic review of the cost-effectiveness of maternity models of care. BMC Pregnancy Childbirth 2023, 23, 859. [Google Scholar] [CrossRef] [PubMed]
- Callander, E.; Fenwick, J.; Donnellan-Fernandez, R.; Toohill, J.; Creedy, D.; Gamble, J.; Fox, H.; Ellwood, D. Cost of maternity care to public hospitals: A first 1000-days perspective from Queensland. Aust. Health Rev. 2019, 43, 556–564. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, R.P. Capacity Planning (Capital, Staff and Costs) of Inpatient Maternity Services: Pitfalls for the Unwary. Int. J. Environ. Res. Public Health 2025, 22, 87. https://doi.org/10.3390/ijerph22010087
Jones RP. Capacity Planning (Capital, Staff and Costs) of Inpatient Maternity Services: Pitfalls for the Unwary. International Journal of Environmental Research and Public Health. 2025; 22(1):87. https://doi.org/10.3390/ijerph22010087
Chicago/Turabian StyleJones, Rodney P. 2025. "Capacity Planning (Capital, Staff and Costs) of Inpatient Maternity Services: Pitfalls for the Unwary" International Journal of Environmental Research and Public Health 22, no. 1: 87. https://doi.org/10.3390/ijerph22010087
APA StyleJones, R. P. (2025). Capacity Planning (Capital, Staff and Costs) of Inpatient Maternity Services: Pitfalls for the Unwary. International Journal of Environmental Research and Public Health, 22(1), 87. https://doi.org/10.3390/ijerph22010087






