The Association between Body Mass Index and Glycohemoglobin (HbA1c) in the US Population’s Diabetes Status
Abstract
1. Introduction
2. Materials and Methods
2.1. NHANES Data
2.2. Study Population
2.3. Measures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 8 March 2024).
- Fan, Y.; Wang, R.; Ding, L.; Meng, Z.; Zhang, Q.; Shen, Y.; Hu, G.; Liu, M. Waist Circumference and Its Changes Are More Strongly Associated with the Risk of Type 2 Diabetes than Body Mass Index and Changes in Body Weight in Chinese Adults. J. Nutr. 2020, 150, 1259–1265. [Google Scholar] [CrossRef]
- Diabetes Quick Facts|Basics|Diabetes|CDC. Available online: https://www.cdc.gov/diabetes/basics/quick-facts.html (accessed on 8 March 2024).
- Parker, E.D.; Lin, J.; Mahoney, T.; Ume, N.; Yang, G.; Gabbay, R.A.; ElSayed, N.A.; Bannuru, R.R. Economic Costs of Diabetes in the US in 2022. Diabetes Care 2024, 47, 26–43. [Google Scholar] [CrossRef]
- Hartemink, N.; Boshuizen, H.C.; Nagelkerke, N.J.D.; Jacobs, M.A.M.; van Houwelingen, H.C. Combining Risk Estimates from Observational Studies with Different Exposure Cutpoints: A Meta-Analysis on Body Mass Index and Diabetes Type 2. Am. J. Epidemiol. 2006, 163, 1042–1052. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gaglia, J.L.; Hilliard, M.E.; Isaacs, D.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2022, 46, S19–S40. [Google Scholar] [CrossRef]
- Ouyang, A.; Hu, K.; Chen, L. Trends and Risk Factors of Diabetes and Prediabetes in US Adolescents, 1999–2020. Diabetes Res. Clin. Pract. 2024, 207, 111022. [Google Scholar] [CrossRef]
- CDC. Diabetes Risk Factors. Available online: https://www.cdc.gov/diabetes/basics/risk-factors.html (accessed on 9 March 2024).
- Guan, X.; Sun, G.; Zheng, L.; Hu, W.; Li, W.; Sun, Y. Associations between Metabolic Risk Factors and Body Mass Index, Waist Circumference, Waist-to-Height Ratio and Waist-to-Hip Ratio in a Chinese Rural Population. J. Diabetes Investig. 2016, 7, 601–606. [Google Scholar] [CrossRef]
- Nyamdorj, R.; Qiao, Q.; Söderberg, S.; Pitkäniemi, J.M.; Zimmet, P.Z.; Shaw, J.E.; Alberti, K.G.M.M.; Pauvaday, V.K.; Chitson, P.; Kowlessur, S.; et al. BMI Compared With Central Obesity Indicators as a Predictor of Diabetes Incidence in Mauritius. Obesity 2009, 17, 342–348. [Google Scholar] [CrossRef]
- Hong, J.W.; Ku, C.R.; Noh, J.H.; Ko, K.S.; Rhee, B.D.; Kim, D.-J. Association between Self-Reported Smoking and Hemoglobin A1c in a Korean Population without Diabetes: The 2011–2012 Korean National Health and Nutrition Examination Survey. PLoS ONE 2015, 10, e0126746. [Google Scholar] [CrossRef]
- CDC. Smoking and Diabetes. Available online: https://www.cdc.gov/diabetes/library/features/smoking-and-diabetes.html (accessed on 9 March 2024).
- Incani, M.; Sentinelli, F.; Perra, L.; Pani, M.G.; Porcu, M.; Lenzi, A.; Cavallo, M.G.; Cossu, E.; Leonetti, F.; Baroni, M.G. Glycated Hemoglobin for the Diagnosis of Diabetes and Prediabetes: Diagnostic Impact on Obese and Lean Subjects, and Phenotypic Characterization. J. Diabetes Investig. 2015, 6, 44–50. [Google Scholar] [CrossRef]
- American Diabetes Association Standards of Medical Care in Diabetes—2011. Diabetes Care 2011, 34, S11–S61. [CrossRef]
- NHANES, 2017–2018 Centers for Disease Control and Prevention—About the National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 10 March 2024).
- NHANES Survey Methods and Analytic Guidelines. Available online: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx (accessed on 10 March 2024).
- Cai, J.; Bidulescu, A. The Association between E-Cigarette Use or Dual Use of E-Cigarette and Combustible Cigarette and Prediabetes, Diabetes, or Insulin Resistance: Findings from the National Health and Nutrition Examination Survey (NHANES). Drug Alcohol Depend. 2023, 251, 110948. [Google Scholar] [CrossRef]
- Jamal, A. Current Cigarette Smoking among Adults—United States, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 1205–1211. [Google Scholar] [CrossRef]
- CDC. Assessing Your Weight. Available online: https://www.cdc.gov/healthyweight/assessing/index.html (accessed on 10 March 2024).
- Managing Overweight and Obesity in Adults: Systematic Evidence Review from the Obesity Expert Panel|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/managing-overweight-obesity-in-adults (accessed on 10 March 2024).
- Lin, W. Association Between Time to First Cigarette Use and Urine Biomarkers of Tobacco Exposure in Adult Smokers. Lung 2024, 202, 217–219. [Google Scholar] [CrossRef]
- Lin, W.; Zhu, J.; Hayes, J.E.; Richie, J.P.; Muscat, J.E. Comparison of Carcinogen Biomarkers in Smokers of Menthol and Nonmenthol Cigarettes: The 2015–2016 National Health and Nutrition Examination Survey Special Sample. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1539–1545. [Google Scholar] [CrossRef]
- Lin, W. Disparities in Healthcare and HBV Vaccination by Smoking Status: Findings from the National Health and Nutrition Examination Survey (NHANES) 2017–2018. Healthcare 2024, 12, 41. [Google Scholar] [CrossRef]
- NHANES Tutorials—Sample Code Module. Available online: https://wwwn.cdc.gov/nchs/nhanes/tutorials/samplecode.aspx (accessed on 11 March 2024).
- Cai, J.; Bidulescu, A. Associations between E-Cigarette Use or Dual Use of E-Cigarette and Combustible Cigarette and Metabolic Syndrome: Results from the National Health and Nutrition Examination Survey (NHANES). Ann. Epidemiol. 2023, 85, 93–99. [Google Scholar] [CrossRef]
- Lin, W. Comparison of Urine Heavy Metals in Exclusive Menthol and Non-Menthol Cigarette Users by Race/Ethnicity: The 2015–2016 National Health and Nutrition Examination Survey Special Sample. Tob. Prev. Cessat. 2023, 9, 22. [Google Scholar] [CrossRef]
- Chan, J.M.; Rimm, E.B.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C. Obesity, Fat Distribution, and Weight Gain as Risk Factors for Clinical Diabetes in Men. Diabetes Care 1994, 17, 961–969. [Google Scholar] [CrossRef]
- Colditz, G.A.; Willett, W.C.; Rotnitzky, A.; Manson, J.E. Weight Gain as a Risk Factor for Clinical Diabetes Mellitus in Women. Ann. Intern. Med. 1995, 122, 481–486. [Google Scholar] [CrossRef]
- Ford, E.S.; Williamson, D.F.; Liu, S. Weight Change and Diabetes Incidence: Findings from a National Cohort of US Adults. Am. J. Epidemiol. 1997, 146, 214–222. [Google Scholar] [CrossRef]
- Hu, G.; Lindström, J.; Valle, T.T.; Eriksson, J.G.; Jousilahti, P.; Silventoinen, K.; Qiao, Q.; Tuomilehto, J. Physical Activity, Body Mass Index, and Risk of Type 2 Diabetes in Patients with Normal or Impaired Glucose Regulation. Arch. Intern. Med. 2004, 164, 892–896. [Google Scholar] [CrossRef]
- Fryar, C.D.; Carroll, M.D.; Afful, J. Prevalence of Overweight, Obesity, and Severe Obesity among Adults Aged 20 and Over: United States, 1960–1962 through 2017–2018. NCHS Health E-Stats. 2020. Available online: https://www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/obesity-adult.htm (accessed on 24 August 2021).
- Elgaddal, N. Physical Activity Among Adults Aged 18 and Over: United States, 2020; National Center for Health Statistics: Hyattsville, MD, USA, 2022. [Google Scholar]
- Abdullah, A.; Peeters, A.; de Courten, M.; Stoelwinder, J. The Magnitude of Association between Overweight and Obesity and the Risk of Diabetes: A Meta-Analysis of Prospective Cohort Studies. Diabetes Res. Clin. Pract. 2010, 89, 309–319. [Google Scholar] [CrossRef]
- Black, E.; Holst, C.; Astrup, A.; Toubro, S.; Echwald, S.; Pedersen, O.; Sørensen, T.I.A. Long-Term Influences of Body-Weight Changes, Independent of the Attained Weight, on Risk of Impaired Glucose Tolerance and Type 2 Diabetes. Diabet. Med. 2005, 22, 1199–1205. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Shaper, A.G. Weight Change and Duration of Overweight and Obesity in the Incidence of Type 2 Diabetes. Diabetes Care 1999, 22, 1266–1272. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. Available online: https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf (accessed on 13 March 2024).
- Leading Health Indicators—Healthy People 2030 | Health.Gov. Available online: https://health.gov/healthypeople/objectives-and-data/leading-health-indicators (accessed on 13 March 2024).
- CDC. Hispanic/Latino Americans and Type 2 Diabetes. Available online: https://www.cdc.gov/diabetes/library/features/hispanic-diabetes.html (accessed on 13 March 2024).
- Han, C.; Liu, Y.; Sun, X.; Luo, X.; Zhang, L.; Wang, B.; Ren, Y.; Zhou, J.; Zhao, Y.; Zhang, D.; et al. Prediction of a New Body Shape Index and Body Adiposity Estimator for Development of Type 2 Diabetes Mellitus: The Rural Chinese Cohort Study. Br. J. Nutr. 2017, 118, 771–776. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; US Department of Health and Human Services: Washington, DC, USA, 2014. [Google Scholar]
- Lin, W. Knowledge of the Health Consequences of Heavy Alcohol Consumption among Individuals with Different Substance Use Statuses: A Cross-Sectional Analysis of 2019 HINT Survey. Chronic Illn. 2023, 8, 17423953231213853. [Google Scholar] [CrossRef]
- Lin, W.; Muscat, J.E. Knowledge and Beliefs Regarding Harm From Specific Tobacco Products: Findings From the H.I.N.T. Survey. Am. J. Health Promot. 2021, 089011712110261. [Google Scholar] [CrossRef]
- Lin, W.; Martinez, S.A.; Ding, K.; Beebe, L.A. Knowledge and Perceptions of Tobacco-Related Harm Associated with Intention to Quit among Cigarette Smokers, E-Cigarette Users, and Dual Users: Findings from the US Population Assessment of Tobacco and Health (PATH) Wave 1. Subst. Use Misuse 2021, 56, 464–470. [Google Scholar] [CrossRef]
- Lin, W.; Krebs, N.M.; Zhu, J.; Foulds, J.; Horn, K.; Muscat, J.E. Comparison between Gradual Reduced Nicotine Content and Usual Nicotine Content Groups on Subjective Cigarette Ratings in a Randomized Double-Blind Trial. Int. J. Environ. Res. Public Health 2020, 17, 7047. [Google Scholar] [CrossRef]
- Goyal, N.; Hennessy, M.; Lehman, E.; Lin, W.; Agudo, A.; Ahrens, W.; Boccia, S.; Brennan, P.; Brenner, H.; Cadoni, G. Risk Factors for Head and Neck Cancer in More and Less Developed Countries: Analysis from the INHANCE Consortium. Oral Dis. 2022, 29, 1565–1578. [Google Scholar] [CrossRef]
- Lin, W.; Hobkirk, A.L.; Zhu, J.; Krebs, N.M.; Hayes, J.E.; Richie, J.P., Jr.; Liao, J.; Horn, K.; Foulds, J.; Muscat, J.E. Effect of Menthol on Nicotine Reduction: Pooled Results from Two Double-Blind Randomized Controlled Trials. Brain Res. Bull. 2022, 189, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Harvard, T.H. Chan School of Public Health. Carbohydrates and Blood Sugar. Available online: https://www.hsph.harvard.edu/nutritionsource/carbohydrates/carbohydrates-and-blood-sugar/ (accessed on 18 April 2024).
- CDC. Prediabetes–Your Chance to Prevent Type 2 Diabetes. Available online: http://bit.ly/2hMpYrt (accessed on 13 March 2024).
- Chen, Y.; Zhang, X.-P.; Yuan, J.; Cai, B.; Wang, X.-L.; Wu, X.-L.; Zhang, Y.-H.; Zhang, X.-Y.; Yin, T.; Zhu, X.-H.; et al. Association of Body Mass Index and Age with Incident Diabetes in Chinese Adults: A Population-Based Cohort Study. BMJ Open 2018, 8, e021768. [Google Scholar] [CrossRef] [PubMed]
- McClure, S.T.; Schlechter, H.; Oh, S.; White, K.; Wu, B.; Pilla, S.J.; Maruthur, N.M.; Yeh, H.-C.; Miller, E.R.; Appel, L.J. Dietary Intake of Adults with and without Diabetes: Results from NHANES 2013–2016. BMJ Open Diab. Res. Care 2020, 8, e001681. [Google Scholar] [CrossRef]
- Diabetes Diet, Eating, & Physical Activity—NIDDK. Available online: https://www.niddk.nih.gov/health-information/diabetes/overview/diet-eating-physical-activity (accessed on 13 March 2024).
- National Diabetes Statistics Report|Diabetes|CDC. Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 13 March 2024).
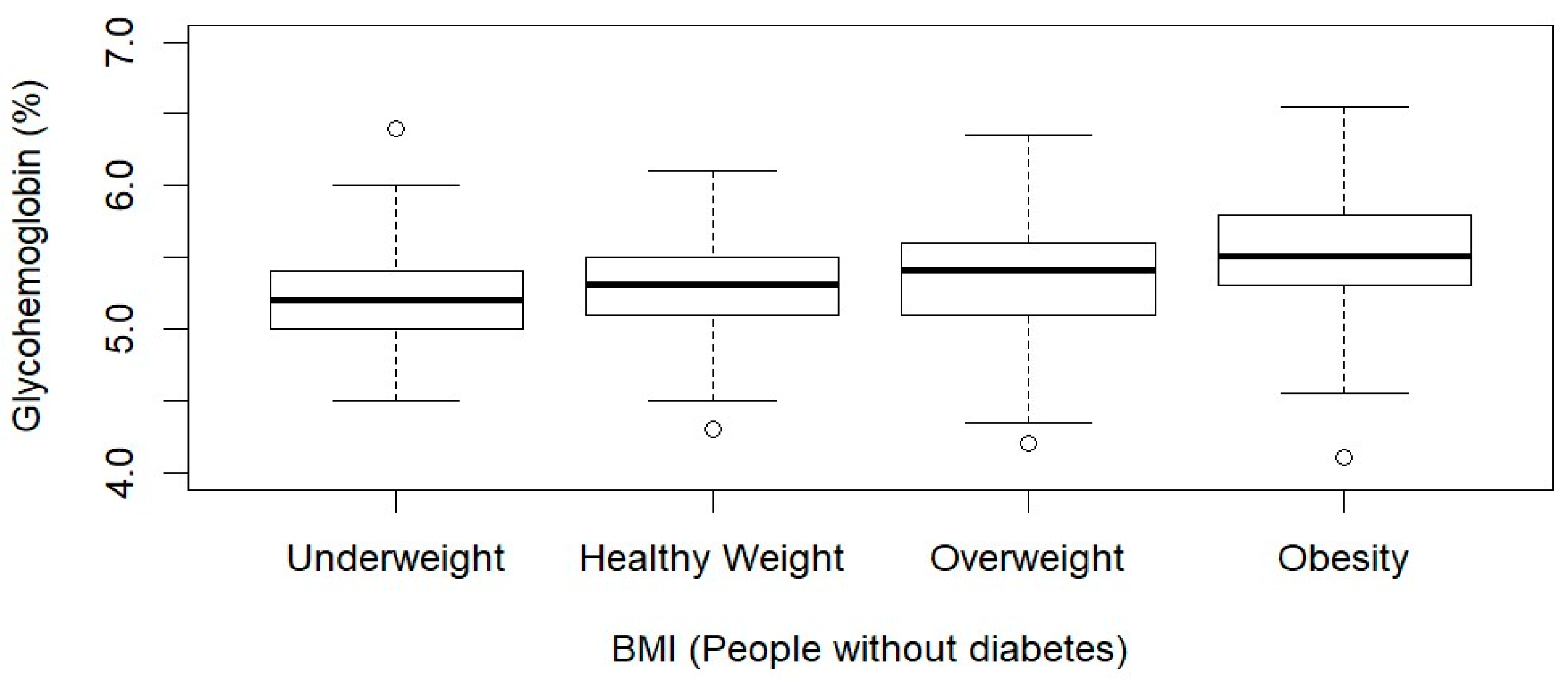
| Diabetes N = 625 (11.3%) | Non-Diabetes N = 3378 (88.7%) | p-Value | |
|---|---|---|---|
| Gender | 0.038 | ||
| Male | 353 (56.8) | 1597 (47.8) | |
| Female | 272 (43.2) | 1781 (52.2) | |
| Race/ethnicity * | 0.85 | ||
| Hispanic | 96 (8.3) | 479 (9.0) | |
| NH-White | 209 (63.8) | 1180 (63.6) | |
| NH-Black | 146 (10.8) | 758 (10.3) | |
| Others | 174 (17.0) | 961 (17.0) | |
| Education | 0.093 | ||
| <High school diploma | 311 (44.5) | 1405 (37.4) | |
| ≥High school diploma | 314 (55.5) | 1973 (62.6) | |
| Place to go for healthcare | <0.001 | ||
| At least one place | 577 (91.8) | 2622 (79.1) | |
| No place | 48 (8.2) | 756 (20.9) | |
| Annual household income | 0.62 | ||
| USD 0 to USD 54.999 | 391 (48.7) | 1943 (46.6) | |
| ≥USD 55,000 | 234 (51.3) | 1435 (53.4) | |
| BMI (kg/m2) | <0.001 | ||
| Underweight (below 18.5) | 1 (0.1) | 54 (1.50) | |
| Healthy Weight (18.5–24.9) | 73 (8.8) | 870 (26.3) | |
| Overweight (25.0–29.9) | 181 (25.1) | 1089 (32.0) | |
| Obesity (30.0 and above) | 370 (65.9) | 1365 (40.3) | |
| Cigarette-smoking status | <0.001 | ||
| Non-smoker | 302 (48.9) | 1996 (58.2) | |
| Former smoker | 229 (38.2) | 713 (23.1) | |
| Current smoker | 94 (12.9) | 669 (18.6) | |
| Alcohol-drinker status | 0.66 | ||
| Non-drinker | 55 (7.7) | 324 (6.8) | |
| Alcohol drinker | 570 (92.3) | 3054 (93.2) | |
| Age, year | 59.9 (0.9) | 45.3 (0.7) | <0.001 |
| BMI (kg/m2) | 33.9 (0.7) | 29.3 (0.2) | <0.001 |
| Glycohemoglobin | 7.3 (0.09) | 5.5 (0.01) | <0.001 |
| Coefficient (95% CI) | p-Value | |
|---|---|---|
| Model 1 (Crude) * | ||
| BMI | 0.028 (0.023, 0.034) | <0.001 |
| Model 2 (Adjusted) | ||
| Gender | 0.03 | |
| Male | [Reference] | |
| Female | −0.09 (−0.16, −0.01) | |
| Race/ethnicity | ||
| Hispanic | [Reference] | |
| NH-White | −0.17 (−0.32, −0.01) | 0.04 |
| NH-Black | 0.09 (−0.05, 0.23) | 0.16 |
| Others | 0.02 (−0.13, 0.16) | 0.76 |
| BMI | 0.025 (0.019, 0.031) | <0.001 |
| Age | 0.018(0.016, 0.021) | <0.001 |
| Model 3 (Adjusted) | ||
| Gender | 0.14 | |
| Male | [Reference] | |
| Female | −0.08 (−0.22, 0.05) | |
| Race/ethnicity | ||
| Hispanic | [Reference] | |
| NH-White | −0.18 (−0.39, 0.03) | 0.07 |
| NH-Black | 0.09 (−0.09, 0.27) | 0.23 |
| Others | 0.005 (−0.18, 0.19) | 0.93 |
| BMI | 0.025 (0.017, 0.034) | 0.002 |
| Age | 0.018 (0.016, 0.021) | <0.001 |
| Coefficient (95% CI) | p-Value | |
|---|---|---|
| Model 1 (Crude) * | ||
| BMI | 0.016 (0.012, 0.019) | <0.001 |
| Model 2 (Adjusted) | ||
| Gender | 0.59 | |
| Male | [Reference] | |
| Female | 0.01 (−0.04, 0.06) | |
| Race/ethnicity | ||
| Hispanic | [Reference] | |
| NH-White | −0.11 (−0.18, −0.04) | 0.006 |
| NH-Black | 0.08 (0.02, 0.14) | 0.017 |
| Others | 0.02 (−0.06, 0.1) | 0.56 |
| BMI | 0.015 (0.011, 0.018) | <0.001 |
| Age | 0.011 (0.010, 0.012) | <0.001 |
| Model 3 (Adjusted) | ||
| Gender | 0.49 | |
| Male | [Reference] | |
| Female | 0.03 (−0.08, 0.13) | |
| Race/ethnicity | ||
| Hispanic | [Reference] | |
| NH-White | −0.13 (−0.22, −0.03) | 0.02 |
| NH-Black | 0.07 (−0.01, 0.16) | 0.08 |
| Others | 0.01 (−0.09, 0.11) | 0.80 |
| BMI | 0.015 (0.01, 0.02) | 0.002 |
| Age | 0.011 (0.01, 0.014) | <0.001 |
| Coefficient (95% CI) | p-Value | |
|---|---|---|
| Model 1 (Crude) * | ||
| BMI | −0.01 (−0.034, 0.024) | 0.71 |
| Model 2 (Adjusted) | ||
| Gender | 0.01 | |
| Male | [Reference] | |
| Female | −0.32 (−0.54, −0.1) | |
| Race/ethnicity | ||
| Hispanic | [Reference] | |
| NH-White | −0.32 (−1.1, 0.4) | 0.34 |
| NH-Black | 0.25 (−0.56, 1.05) | 0.49 |
| Others | −0.24 (−0.97, 0.49) | 0.45 |
| BMI | −0.01 (−0.04, 0.03) | 0.68 |
| Age | −0.013 (−0.03, 0.002) | 0.08 |
| Model 3 (Adjusted) | ||
| Gender | 0.06 | |
| Male | [Reference] | |
| Female | −0.36 (−0.73, 0.02) | |
| Race/ethnicity | ||
| Hispanic | [Reference] | |
| NH-White | −0.31 (−1.3, 0.69) | 0.40 |
| NH-Black | 0.27 (−0.80, 1.34) | 0.48 |
| Others | −0.24 (−1.2, 0.7) | 0.48 |
| BMI | −0.005 (−0.05, 0.04) | 0.74 |
| Age | −0.01 (−0.03, 0.01) | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W. The Association between Body Mass Index and Glycohemoglobin (HbA1c) in the US Population’s Diabetes Status. Int. J. Environ. Res. Public Health 2024, 21, 517. https://doi.org/10.3390/ijerph21050517
Lin W. The Association between Body Mass Index and Glycohemoglobin (HbA1c) in the US Population’s Diabetes Status. International Journal of Environmental Research and Public Health. 2024; 21(5):517. https://doi.org/10.3390/ijerph21050517
Chicago/Turabian StyleLin, Wenxue. 2024. "The Association between Body Mass Index and Glycohemoglobin (HbA1c) in the US Population’s Diabetes Status" International Journal of Environmental Research and Public Health 21, no. 5: 517. https://doi.org/10.3390/ijerph21050517
APA StyleLin, W. (2024). The Association between Body Mass Index and Glycohemoglobin (HbA1c) in the US Population’s Diabetes Status. International Journal of Environmental Research and Public Health, 21(5), 517. https://doi.org/10.3390/ijerph21050517






