Exploring the Landscape of Breast Cancer Prevention among Chinese Residents in Italy: An In-Depth Analysis of Screening Adherence, Breast Self-Examination (BSE) Practices, the Role of Technological Tools, and Misconceptions Surrounding Risk Factors and Symptoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Survey Instrument
2.3. Ethical Considerations
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limits
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Amuta, A.O.; Mkuu, R.S.; Jacobs, W.; Ejembi, A.Z. Influence of Cancer Worry on Four Cancer Related Health Protective Behaviors among a Nationally Representative Sample: Implications for Health Promotion Efforts. J. Cancer Educ. 2018, 33, 1002–1010. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; Abdulle, A.S.M.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017. JAMA Oncol. 2019, 5, 1749. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. The Global Cancer Observatory 2020 China Fact Sheets. 2021. Available online: https://gco.iarc.fr/today/data/factsheets/populations/160-china-fact-sheets.pdf (accessed on 28 June 2023).
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/today (accessed on 5 February 2022).
- Fan, L.; Zheng, Y.; Yu, K.-D.; Liu, G.-Y.; Wu, J.; Lu, J.-S.; Shen, K.-W.; Shen, Z.-Z.; Shao, Z.-M. Breast Cancer in a Transitional Society over 18 Years: Trends and Present Status in Shanghai, China. Breast Cancer Res. Treat. 2009, 117, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.-K.; Li, J.; Huang, R.; Fan, J.-H.; Zheng, R.-S.; Zhang, B.-N.; Zhang, B.; Tang, Z.-H.; Xie, X.-M.; Yang, H.-J.; et al. Age of Diagnosis of Breast Cancer in China: Almost 10 Years Earlier than in the United States and the European Union. Asian Pac. J. Cancer Prev. 2014, 15, 10021–10025. [Google Scholar] [CrossRef]
- Ding, R.; Xiao, Y.; Mo, M.; Zheng, Y.; Jiang, Y.-Z.; Shao, Z.-M. Breast Cancer Screening and Early Diagnosis in Chinese Women. Cancer Biol. Med. 2022, 19, 450–467. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, S.; Ahumada-Canale, A.; Chen, Z.; Si, L.; Jiang, Y.; Yang, L.; Gu, Y. Breast Cancer Screening Should Embrace Precision Medicine: Evidence by Reviewing Economic Evaluations in China. Adv. Ther. 2023, 40, 1393–1417. [Google Scholar] [CrossRef]
- AIOM-AIRTUM-Siapec-Iap I Numeri Del Cancro in Italia 2022. Available online: https://www.aiom.it/wp-content/uploads/2022/12/2022_AIOM_NDC-web.pdf (accessed on 10 October 2023).
- Levaggi, A.; Poggio, F.; Lambertini, M. The Burden of Breast Cancer from China to Italy. J. Thorac. Dis. 2014, 6, 591–594. [Google Scholar] [CrossRef]
- Italian Ministry of Health Italian Ministry of Health. Piano Nazionale Della Prevenzione 2020–2025. Available online: https://www.salute.gov.it/imgs/C_17_notizie_5029_0_file.pdf (accessed on 28 June 2023).
- Petrelli, A.; Di Napoli, A.; Sebastiani, G.; Rossi, A.; Giorgi Rossi, P.; Demuru, E.; Costa, G.; Zengarini, N.; Alicandro, G.; Marchetti, S.; et al. Italian Atlas of Mortality Inequalities by Education Level. Epidemiol. Prev. 2019, 43, 1–120. [Google Scholar] [CrossRef]
- Petrelli, A.; Giorgi Rossi, P.; Francovich, L.; Giordani, B.; Di Napoli, A.; Zappa, M.; Mirisola, C.; Gargiulo, L. Geographical and Socioeconomic Differences in Uptake of Pap Test and Mammography in Italy: Results from the National Health Interview Survey. BMJ Open 2018, 8, e021653. [Google Scholar] [CrossRef] [PubMed]
- Wegwarth, O.; Widschwendter, M.; Cibula, D.; Sundström, K.; Portuesi, R.; Lein, I.; Rebitschek, F.G. What Do European Women Know about Their Female Cancer Risks and Cancer Screening? A Cross-Sectional Online Intervention Survey in Five European Countries. BMJ Open 2018, 8, e023789. [Google Scholar] [CrossRef]
- Eliassen, A.H.; Colditz, G.A.; Rosner, B.; Willett, W.C.; Hankinson, S.E. Adult Weight Change and Risk of Postmenopausal Breast Cancer. JAMA 2006, 296, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Hulka, B.S.; Moorman, P.G. Breast Cancer: Hormones and Other Risk Factors. Maturitas 2001, 38, 103–113, discussion 113–116. [Google Scholar] [CrossRef] [PubMed]
- Nichols, H.B.; Trentham-Dietz, A.; Egan, K.M.; Titus-Ernstoff, L.; Holmes, M.D.; Bersch, A.J.; Holick, C.N.; Hampton, J.M.; Stampfer, M.J.; Willett, W.C.; et al. Body Mass Index Before and After Breast Cancer Diagnosis: Associations with All-Cause, Breast Cancer, and Cardiovascular Disease Mortality. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Sharma, D. Microbial Alterations and Risk Factors of Breast Cancer: Connections and Mechanistic Insights. Cells 2020, 9, 1091. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.; Whitehead, S.A. Phytoestrogens and Breast Cancer—Promoters or Protectors? Endocr. Relat. Cancer 2006, 13, 995–1015. [Google Scholar] [CrossRef]
- Conte, L.; Lupo, R.; Lezzi, A.; Paolo, V.; Rubbi, I.; Rizzo, E.; Carvello, M.; Calabrò, A.; Botti, S.; De Matteis, E.; et al. A Nationwide Cross-Sectional Study Investigating Adherence to the Mediterranean Diet, Smoking, Alcohol and Work Habits, Hormonal Dynamics between Breast Cancer Cases and Healthy Subjects. Clin. Nutr. Open Sci. 2024. [Google Scholar] [CrossRef]
- Willett, W.C. Diet and Cancer. Oncologist 2000, 5, 393–404. [Google Scholar] [CrossRef]
- Bucholc, M.; Łepecka-Klusek, C.; Pilewska, A.; Kanadys, K. Ryzyko Zachorowania Na Raka Piersi w Opinii Kobiet. Ginekol. Pol. 2001, 72, 1456–1460. [Google Scholar]
- Kim, S.; Ko, Y.; Lee, H.J.; Lim, J.-E. Menopausal Hormone Therapy and the Risk of Breast Cancer by Histological Type and Race: A Meta-Analysis of Randomized Controlled Trials and Cohort Studies. Breast Cancer Res. Treat. 2018, 170, 667–675. [Google Scholar] [CrossRef]
- Zuzak, T.Z.; Hałgas, M.; Kowalska, K.; Gospodarczyk, M.; Wdowiak-Filip, A.; Filip, M.; Zuzak, Z.; Kowaluk, G.; Wdowiak, A. Breast Cancer—The Level of Knowledge about Epidemiology and Prophylaxis among Polish Medical Universities Students. Eur. J. Med. Technol. 2018, 1, 29–34. [Google Scholar]
- Alegre, M.M.; Knowles, M.H.; Robison, R.A.; O’Neill, K.L. Mechanics behind Breast Cancer Prevention—Focus on Obesity, Exercise and Dietary Fat. Asian Pac. J. Cancer Prev. 2013, 14, 2207–2212. [Google Scholar] [CrossRef]
- Kamińska, M.; Ciszewski, T.; Łopacka-Szatan, K.; Miotła, P.; Starosławska, E. Breast Cancer Risk Factors. Menopause Rev. 2015, 3, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, V.-F.; Baban, A.; Dumitrascu, D.L. Psychological Stress and Breast Cancer Incidence: A Systematic Review. Clujul Med. 2018, 91, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.; Tseng, C.; Wu, J.; Yang, J.; Conroy, S.M.; Shariff-Marco, S.; Li, L.; Hertz, A.; Gomez, S.L.; Le Marchand, L.; et al. Association between Ambient Air Pollution and Breast Cancer Risk: The Multiethnic Cohort Study. Int. J. Cancer 2020, 146, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Barlow, W.E. Performance of Diagnostic Mammography for Women With Signs or Symptoms of Breast Cancer. CancerSpectrum Knowl. Environ. 2002, 94, 1151–1159. [Google Scholar] [CrossRef]
- Conte, L.; De Nunzio, G.; Lupo, R.; Mieli, M.; Lezzi, A.; Vitale, E.; Carriero, M.C.; Calabrò, A.; Carvello, M.; Rubbi, I.; et al. Breast Cancer Prevention: The Key Role of Population Screening, Breast Self-Examination (BSE) and Technological Tools. Survey of Italian Women. J. Cancer Educ. 2023, 38, 1728–1742. [Google Scholar] [CrossRef]
- Bashirian, S.; Barati, M.; Mohammadi, Y.; MoaddabShoar, L.; Dogonchi, M. Evaluation of an Intervention Program for Promoting Breast Self-Examination Behavior in Employed Women in Iran. Breast Cancer 2021, 15, 1178223421989657. [Google Scholar] [CrossRef]
- Coughlin, S.S. Epidemiology of Breast Cancer in Women. Adv. Exp. Med. Biol. 2019, 1152, 9–29. [Google Scholar] [CrossRef]
- Goyal, A. Breast Pain. BMJ Clin. Evid. 2011, 2011, 0812. [Google Scholar] [PubMed]
- Italian Ministery of Health Screening Oncologici. Available online: https://www.salute.gov.it/portale/donna/dettaglioContenutiDonna.jsp?id=4511&area=Salute+donna&menu=prevenzione (accessed on 24 July 2022).
- Zhu, S.; Lei, C. Association between Marital Status and All-Cause Mortality of Patients with Metastatic Breast Cancer: A Population-Based Study. Sci. Rep. 2023, 13, 9067. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, A.; Ventura, M.; Spadea, T.; Giorgi Rossi, P.; Bartolini, L.; Battisti, L.; Cacciani, L.; Caranci, N.; Cernigliaro, A.; De Giorgi, M.; et al. Barriers to Accessing Primary Care and Appropriateness of Healthcare Among Immigrants in Italy. Front. Public Health 2022, 10, 817696. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Thames, M.W.; Tom, L.S.; Leung, I.S.; Yang, A.; Simon, M.A. An Examination of the Implementation of a Patient Navigation Program to Improve Breast and Cervical Cancer Screening Rates of Chinese Immigrant Women: A Qualitative Study. BMC Women’s Health 2022, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- Adunlin, G.; Cyrus, J.W.; Asare, M.; Sabik, L.M. Barriers and Facilitators to Breast and Cervical Cancer Screening Among Immigrants in the United States. J. Immigr. Minor. Health 2019, 21, 606–658. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.A.; Al-Marzouki, A.; Otim, M.; Khalil Khayat, N.E.H.; Yousuf, R.; Rahman, P. Awareness about Breast Cancer and Breast Self-Examination among Female Students at the University of Sharjah: A Cross-Sectional Study. Asian Pac. J. Cancer Prev. 2019, 20, 1901–1908. [Google Scholar] [CrossRef]
- Birhane, K.; Alemayehu, M.; Anawte, B.; Gebremariyam, G.; Daniel, R.; Addis, S.; Worke, T.; Mohammed, A.; Negash, W. Practices of Breast Self-Examination and Associated Factors among Female Debre Berhan University Students. Int. J. Breast Cancer 2017, 2017, 8026297. [Google Scholar] [CrossRef]
- Akhtari-Zavare, M.; Juni, M.H.; Ismail, I.Z.; Said, S.M.; Latiff, L.A. Barriers to Breast Self Examination Practice among Malaysian Female Students: A Cross Sectional Study. Springerplus 2015, 4, 692. [Google Scholar] [CrossRef]
- Asmare, K.; Birhanu, Y.; Wako, Z. Knowledge, Attitude, Practice towards Breast Self-Examination and Associated Factors among Women in Gondar Town, Northwest Ethiopia, 2021: A Community-Based Study. BMC Women’s Health 2022, 22, 174. [Google Scholar] [CrossRef]
- Lera, T.; Beyene, A.; Bekele, B.; Abreha, S. Breast Self-Examination and Associated Factors among Women in Wolaita Sodo, Ethiopia: A Community-Based Cross-Sectional Study. BMC Women’s Health 2020, 20, 167. [Google Scholar] [CrossRef]
- McCready, T.; Littlewood, D.; Jenkinson, J. Breast Self-Examination and Breast Awareness: A Literature Review. J. Clin. Nurs. 2005, 14, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Didarloo, A.; Nabilou, B.; Khalkhali, H.R. Psychosocial Predictors of Breast Self-Examination Behavior among Female Students: An Application of the Health Belief Model Using Logistic Regression. BMC Public Health 2017, 17, 861. [Google Scholar] [CrossRef] [PubMed]
- Abo Al-Shiekh, S.S.; Ibrahim, M.A.; Alajerami, Y.S. Breast Cancer Knowledge and Practice of Breast Self-Examination among Female University Students, Gaza. Sci. World J. 2021, 2021, 6640324. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Bui, C.N.; Park, K. Mobile Health Apps for Breast Cancer: Content Analysis and Quality Assessment. JMIR mHealth uHealth 2023, 11, e43522. [Google Scholar] [CrossRef]
- Yusuf, A.; Iskandar, Y.H.P.; Ab Hadi, I.S.; Nasution, A.; Lean Keng, S. Breast Awareness Mobile Apps for Health Education and Promotion for Breast Cancer. Front. Public Health 2022, 10, 951641. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.K.; Heyworth, J.S.; Girschik, J.; Slevin, T.; Saunders, C.; Fritschi, L. Beliefs and Perceptions about the Causes of Breast Cancer: A Case-Control Study. BMC Res. Notes 2014, 7, 558. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef]
- Breslow, R.A.; Sorkin, J.D.; Frey, C.M.; Kessler, L.G. Americans’ Knowledge of Cancer Risk and Survival. Prev. Med. 1997, 26, 170–177. [Google Scholar] [CrossRef]
- Li, L.; Li, S.; Qin, S.; Gao, Y.; Wang, C.; Du, J.; Zhang, N.; Chen, Y.; Han, Z.; Yu, Y.; et al. Diet, Sports, and Psychological Stress as Modulators of Breast Cancer Risk: Focus on OPRM1 Methylation. Front. Nutr. 2021, 8, 747964. [Google Scholar] [CrossRef]
- Youn, H.J.; Han, W. A Review of the Epidemiology of Breast Cancer in Asia: Focus on Risk Factors. Asian Pac. J. Cancer Prev. 2020, 21, 867–880. [Google Scholar] [CrossRef]
- Reményi Kissné, D.; Gede, N.; Szakács, Z.; Kiss, I. Breast Cancer Screening Knowledge among Hungarian Women: A Cross-Sectional Study. BMC Women’s Health 2021, 21, 69. [Google Scholar] [CrossRef]
- Al-Mousa, D.S.; Alakhras, M.; Hossain, S.Z.; Al-Sa’di, A.G.; Al Hasan, M.; Al-Hayek, Y.; Brennan, P.C. Knowledge, Attitude and Practice Around Breast Cancer and Mammography Screening Among Jordanian Women. Breast Cancer 2020, 12, 231–242. [Google Scholar] [CrossRef]
- Renganathan, L.; Ramasubramaniam, S.; Al-Touby, S.; Seshan, V.; Al-Balushi, A.; Al-Amri, W.; Al-Nasseri, Y.; Al-Rawahi, Y. What Do Omani Women Know about Breast Cancer Symptoms? Oman Med. J. 2014, 29, 408–413. [Google Scholar] [CrossRef]
- Azubuike, S.; Okwuokei, S. Knowledge, Attitude and Practices of Women towards Breast Cancer in Benin City, Nigeria. Ann. Med. Health Sci. Res. 2013, 3, 155–160. [Google Scholar] [CrossRef]
- Mazzocco, K.; Masiero, M.; Carriero, M.C.; Pravettoni, G. The Role of Emotions in Cancer Patients’ Decision-Making. Ecancer 2019, 13, 914. [Google Scholar] [CrossRef]
| Section 1: Socio-Demographic Characteristics | Group A: Individuals from the General Population (98%, n = 1118) | Group B: Patients with Breast Cancer (2%, n = 26) | p-Value |
|---|---|---|---|
| Age | |||
| 20–29 | 263 (24) | 2 (8) | <0.001 *** |
| 30–39 | 427 (38) | 5 (19) | |
| 40–49 | 211 (19) | 6 (23) | |
| 50–59 | 160 (14) | 8 (31) | |
| 60–69 | 57 (5) | 5 (19) | |
| Geographical Area | 0.81 | ||
| North | 377 (34) | 18 (69) | |
| Center | 422 (38) | 6 (23) | |
| South and Islands | 319 (29) | 2 (8) | |
| Marital Status | 0.53 | ||
| Married | 528 (47) | 9 (35) | |
| Divorced | 54 (5) | 9 (35) | |
| Single | 446 (40) | 3 (12) | |
| Separated | 73 (7) | 1 (4) | |
| Widowed | 17 (2) | 4 (15) | |
| Educational level | 0.85 | ||
| Degree | 36 (3) | 3 (12) | |
| High school graduation | 184 (16) | 3 (12) | |
| Junior High School Diploma | 696 (62) | 14 (54) | |
| Elementary Education | 160 (14) | 3 (12) | |
| None | 42 (4) | 3 (12) | |
| Occupational Status | 0.56 | ||
| Worker | 310 (28) | 4 (15) | |
| Housewife | 194 (17) | 5 (19) | |
| Public Employee | 62 (6) | 1 (4) | |
| Freelancer | 92 (8) | 2 (8) | |
| Student | 197 (18) | 2 (8) | |
| Retired | 113 (10) | 7 (27) | |
| Other | 150 (13) | 5 (19) | |
| Unemployed | 0 | 0 | |
| Are you currently working? | 0.94 | ||
| No | 466 (42) | 11 (42) | |
| Yes | 652 (58) | 15 (58) | |
| Years in Italy | 0.298 | ||
| Range | 1–50 | 2–44 | |
| Mean | 17.95 | 21.34 | |
| SD | 9.64 | 14.09 |
| Section 2. Access to Health Services | Group A: Individuals from the General Population (98%, n = 1118) | Group B: Patients with Breast Cancer (2%, n = 26) | p-Value |
|---|---|---|---|
| Are you enrolled in the National Health Service (NHS)? | 0.83 | ||
| No | 2 (0) | 0 | |
| Yes | 1116 (100) | 26 (100) | |
| Did you encounter any difficulties in enrollment? | <0.001 *** | ||
| I do not have a residence permit | 43 (4) | 5 (19) | |
| I tried but had difficulty | 17 (2) | 1 (4) | |
| I do not know how to do it | 33 (3) | 2 (8) | |
| I never thought about it | 20 (2) | 1 (4) | |
| I do not care | 16 (1) | 1 (4) | |
| I had no difficulty | 989 (88) | 16 (62) | |
| During the past two years, have you relied on the services of your primary care physician? | 0.27 | ||
| No | 243 (22) | 8 (31) | |
| Yes | 875 (78) | 18 (69) | |
| Over the past two years, have you relied on the services of a pediatrician? | 0.41 | ||
| No | 850 (76) | 8 (31) | |
| Yes | 267 (24) | 18 (69) | |
| Over the past two years, have you relied on the services of the emergency room (ER)? | 0.04 * | ||
| No | 941 (84) | 18 (69) | |
| Yes | 177 (16) | 8 (31) | |
| Over the past two years, have you relied on the services of a hospital? | 0.07 | ||
| No | 923 (83) | 18 (69) | |
| Yes | 195 (17) | 8 (31) | |
| Over the past two years, have you relied on the services of a gynecological consultatory? | 0.70 | ||
| No | 853 (76) | 19 (73) | |
| Yes | 265 (24) | 7 (27) | |
| Over the past two years, have you relied on the services of the centralized booking center (CUP) service? | 0.45 | ||
| No | 923 (83) | 20 (77) | |
| Yes | 195 (17) | 6 (23) | |
| Over the past two years, have you relied on the services of the vaccine outpatient clinic? | 0.01 * | ||
| No | 923 (83) | 8 (31) | |
| Yes | 195 (17) | 18 (69) | |
| Over the past two years, have you relied on anything else? | 0.008 ** | ||
| No | 819 (73) | 13 (50) | |
| Yes | 299 (27) | 13 (50) | |
| Have you chosen your primary care physician? | <0.001 *** | ||
| No | 74 (7) | 7 (27) | |
| Yes | 1044 (93) | 19 (73) | |
| In the past year, how many times have you relied on your family physician? | 0.01 * | ||
| Never | 384 (34) | 4 (15) | |
| 1 time | 241 (22) | 11 (42) | |
| 2–5 times | 399 (36) | 9 (35) | |
| >5 times | 94 (8) | 2 (8) | |
| Are you comfortable with your family physician? | 0.28 | ||
| Not at all | 40 (4) | 1 (4) | |
| Little | 628 (56) | 12 (46) | |
| I do not know | 64 (6) | 3 (12) | |
| Quite | 343 (31) | 8 (31) | |
| Very | 43 (4) | 2 (8) | |
| What problems are there to report about the family physician? | 0.006 ** | ||
| Schedules do not fit | 291 (26) | 4 (15) | |
| I have difficulty understanding the recipes | 36 (3) | 4 (15) | |
| We do not understand each other because of the language | 83 (7) | 4 (15) | |
| I have never had any problems | 29 (3) | 3 (12) | |
| More | 679 (61) | 11 (42) | |
| In the past year, have you relied on the services of the CUP service? | 0.08 | ||
| No | 853 (76) | 16 (62) | |
| Yes | 265 (24) | 10 (38) | |
| What problems are there to report for CUP? | 0.005 ** | ||
| It is not clear how it works | 83 (7) | 8 (31) | |
| It was difficult to book | 138 (12) | 3 (12) | |
| I have never had any problems | 74 (7) | 3 (12) | |
| More | 823 (74) | 12 (46) | |
| In the past year, how many times have you relied on the emergency room? | <0.001 *** | ||
| Never | 793 (71) | 2 (8) | |
| 1 time | 229 (20) | 12 (46) | |
| 2–5 times | 92 (8) | 12 (46) | |
| >5 times | 4 (0) | 0 | |
| Were you satisfied with the service? | 0.01 * | ||
| Not at all | 39 (3) | 3 (12) | |
| Little | 866 (77) | 14 (54) | |
| I do not know. | 114 (10) | 2 (8) | |
| Quite | 65 (6) | 4 (15) | |
| A lot | 34 (3) | 3 (12) | |
| What problems are there to report about the emergency room? | <0.001 *** | ||
| The operators did not have time to explain | 68 (6) | 6 (23) | |
| We did not understand each other because of the language | 87 (8) | 5 (19) | |
| It is unclear how access works | 51 (5) | 2 (8) | |
| Other | 912 (82) | 13 (50) | |
| Do you know that there is a night and holiday medical service? | <0.001 *** | ||
| No | 105 (9) | 8 (31) | |
| Yes | 1013 (91) | 18 (69) | |
| In case of need, would you know how to contact this medical service? | 0.73 | ||
| No | 335 (30) | 7 (27) | |
| Yes | 783 (70) | 19 (73) | |
| Are you aware of the existence of the counseling center? | <0.001 *** | ||
| No | 845 (76) | 12 (46) | |
| Yes | 273 (24) | 14 (54) | |
| Have you ever used the counseling center? | <0.001 *** | ||
| No | 985 (88) | 17 (65) | |
| Yes | 133 (12) | 9 (35) | |
| If yes, for what reason? | <0.001 *** | ||
| Psychological assistance | 17 (2) | 1 (4) | |
| PAP test | 8 (1) | 0 | |
| Contraception | 33 (3) | 2 (8) | |
| Scheduled checks in pregnancy | 47 (4) | 5 (19) | |
| Termination of pregnancy | 13 (1) | 2 (8) | |
| Gynecological examination | 59 (5) | 4 (15) | |
| Other | 941 (84) | 12 (46) | |
| Were you satisfied with the service of the counseling center? | <0.001 *** | ||
| Not at all | 31 (3) | 3 (12) | |
| Little | 75 (7) | 1 (4) | |
| I do not know. | 914 (82) | 13 (50) | |
| Quite | 32 (6) | 6 (23) | |
| A lot | 36 (3) | 3 (12) |
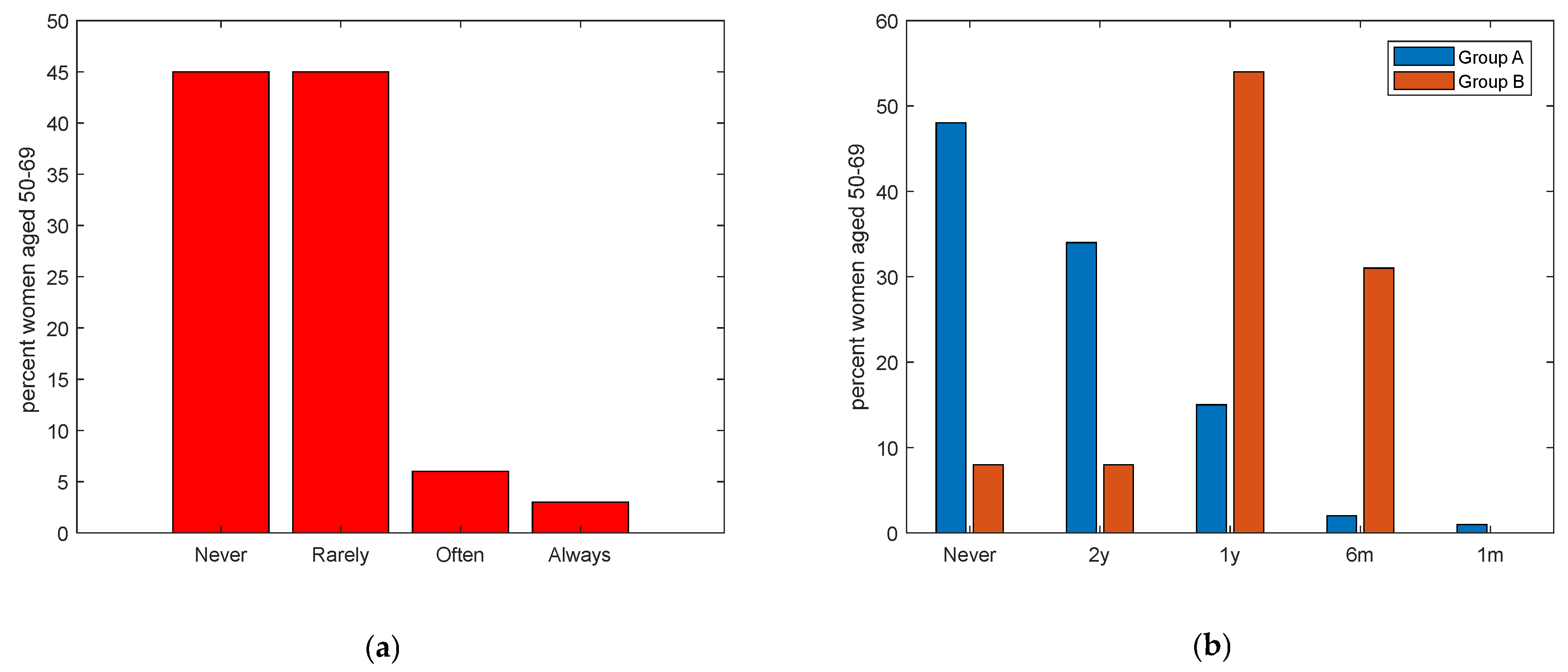
| Section 3: Clinical Breast Cancer Controls and Adherence to Screening Programs, Women Aged 50–69 | Group A Women in the General Population Aged 50–69 (n = 217) N (%) | Group B Women with Cancer Aged 50–69 (n = 13) N (%) | p-Value |
|---|---|---|---|
| Have you ever undertaken clinical controls for the early detection of breast cancer? | <0.001 *** | ||
| Never | 103 (47) | 1 (8) | |
| Rarely | 102 (47) | 2 (15) | |
| Occasionally | 0 | 0 | |
| Often | 7 (3) | 7 (54) | |
| Always | 5 (2) | 2 (23) | |
| If yes, please indicate the frequency | 0.47 | ||
| I have never had a screening exam | 104 (48) | 1 (8) | |
| Every 2 years | 74 (34) | 1 (8) | |
| Every year | 32 (15) | 7 (54) | |
| Every 6 months | 4 (2) | 4 (31) | |
| Every month | 3 (1) | 0 | |
| Have you ever taken advantage of the free screening offered by the region? | 0.04 * | ||
| No | 209 (96) | 11 (85) | |
| Yes | 8 (4) | 2 (15) | |
| I do not know of them | 0 | 0 | |
| Have you ever been called by the local health authority (LHA) for a visit dedicated to prevention? | 0.73 | ||
| Yes, breast cancer (mammography) | 3 (1) | 1 (8) | |
| Yes, for colorectal cancer (stool analysis) | 4 (2) | 1 (8) | |
| Yes, for cervical cancer (PAP test) | 0 | 0 | |
| No, I did not receive the letter | 157 (72) | 6 (46) | |
| I do not know | 53 (24) | 5 (38) | |
| If you received the letter, how understandable was it? | 0.50 | ||
| Not at all | 8 (4) | 1 (8) | |
| Little | 40 (18) | 1 (8) | |
| I do not know | 151 (70) | 7 (54) | |
| Quite | 0 | 0 | |
| A lot | 18 (8) | 4 (31) | |
| Have you ever had a biopsy? | 0.47 | ||
| No | 154 (71) | 8 (62) | |
| Yes | 63 (29) | 5 (38) | |
| Missing | 0 | 0 | |
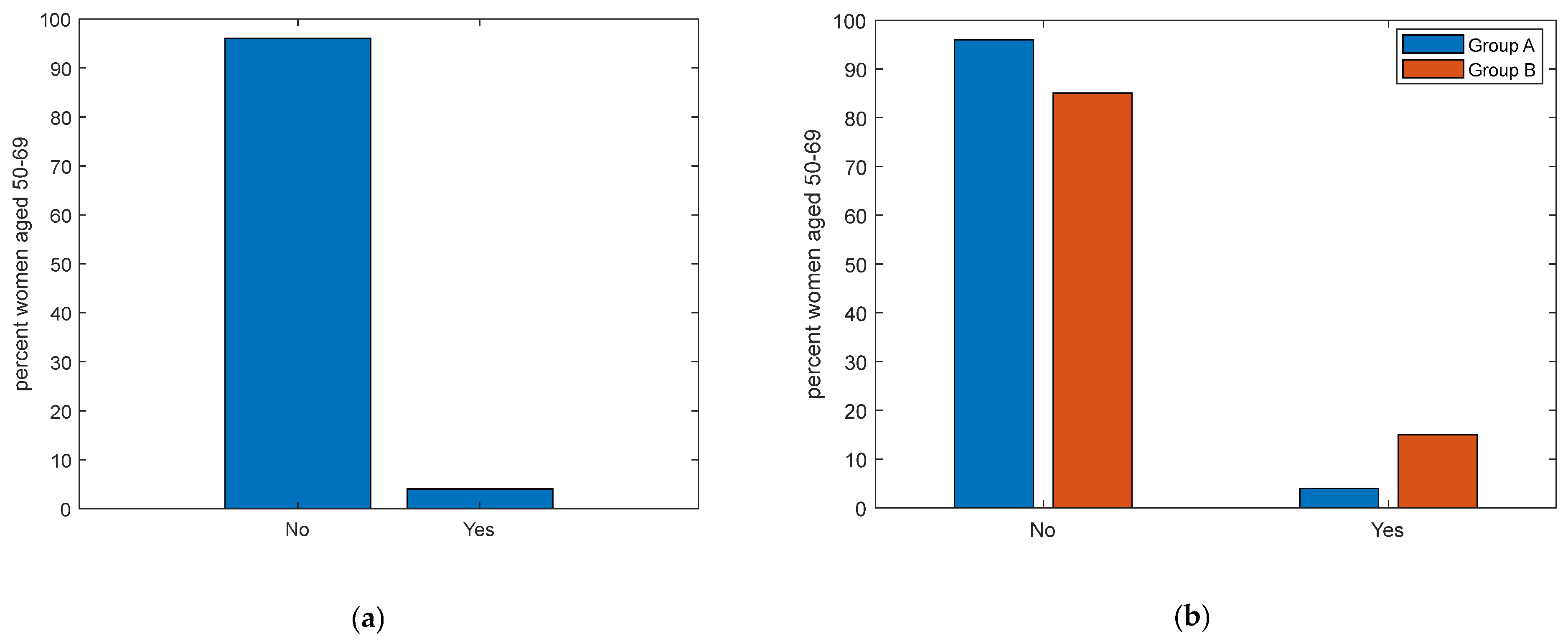
| Have you ever had a mammogram? | <0.01 ** | ||
| No | 154 (71) | 1 (8) | |
| Yes | 63 (29) | 12 (92) | |
| Missing | 0 | 0 | |
| Have you ever had an ultrasound? | <0.001 *** | ||
| No | 191 (88) | 1 (8) | |
| Yes | 26 (12) | 12 (92) | |
| Missing | 0 | 0 | |
| Have you ever had magnetic resonance imaging (MRI)? | <0.001 *** | ||
| No | 201 (93) | 8 (62) | |
| Yes | 16 (7) | 5 (38) | |
| Missing | 0 | 0 |
| Section 4: Approach to Breast Self-Examination (BSE) | Group A Women in The General Population (n = 1118) N (%) | Group B Women with Cancer (n = 26) N (%) | p-Value |
|---|---|---|---|
| Have you ever heard of BSE? | <0.001 *** | ||
| No | 98 (9) | 9 (35) | |
| Yes | 1020 (91) | 17 (65) | |
| In your opinion, what does self-examination consist of? | <0.001 *** | ||
| Breast self-examination | 51 (5) | 3 (12) | |
| Clinical examination of the breast (search for visible and/or palpable findings in the breast and surrounding areas, e.g., areas of lymphatic drainage axilla, neck) | 284 (25) | 7 (27) | |
| Radiological examination of the breast (mammography, ultrasonography, MRI, biopsy, chest X-ray, scintigraphy, CT scan, PET/CT, chest X-ray) | 717 (64) | 9 (35) | |
| I do not know | 50 (4) | 5 (19) | |
| Other | 16 (1) | 2 (8) | |
| Does self-examination help prevent breast cancer? | 0.002 ** | ||
| No | 80 (7) | 6 (23) | |
| Yes | 1038 (93) | 20 (77) | |
| Is self-palpation not necessary if I perform periodic mammography? | <0.001 *** | ||
| Strongly agree | 52 (5) | 4 (15) | |
| Agreed | 194 (17) | 8 (31) | |
| In disagreement | 144 (13) | 11 (42) | |
| Strongly disagree | 5 (0) | 0 | |
| Uncertain | 723 (65) | 3 (12) | |
| Performing a self-examination reduces mortality. | 0.18 | ||
| Strongly agree | 78 (7) | 11 (42) | |
| Agree | 590 (53) | 13 (50) | |
| In disagreement | 6 (1) | 0 | |
| Strongly disagree | 1 (0) | 0 | |
| Uncertain | 443 (40) | 2 (8) | |
| Performing self-examination every month helps me find the nodules | <0.001 *** | ||
| Strongly agree | 34 (3) | 4 (15) | |
| Agree | 104 (9) | 9 (35) | |
| In disagreement | 105 (9) | 2 (8) | |
| Strongly disagree | 7 (1) | 0 | |
| Uncertain | 868 (78) | 11 (42) | |
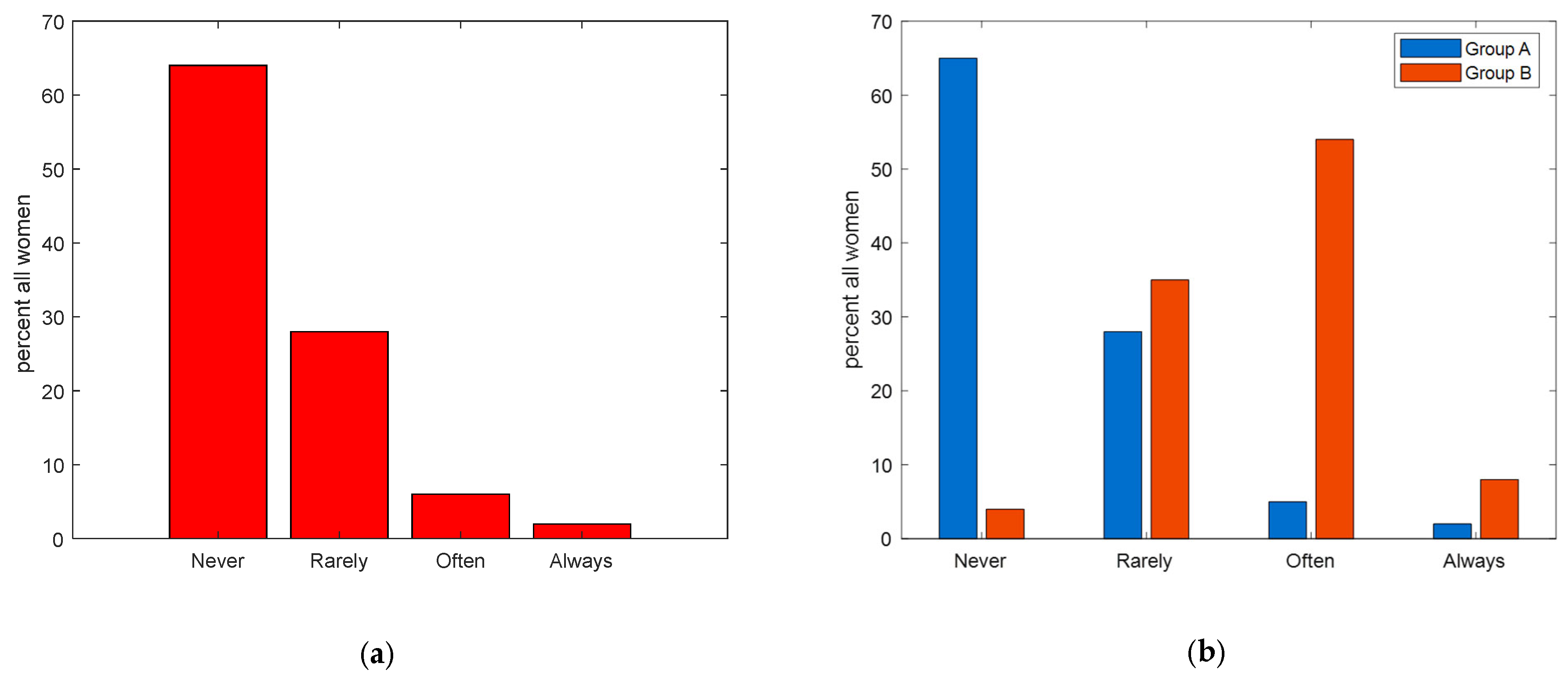
| How often do you perform self-palpation? | <0.001 *** | ||
| Never | 731 (65) | 1 (4) | |
| Rarely | 313 (28) | 9 (35) | |
| Occasionally | 0 | 0 | |
| Often | 57 (5) | 14 (54) | |
| Always | 17 (2) | 2 (8) | |
| If not, state the reason | <0.001 *** | ||
| I perform it | 278 (25) | 12 (46) | |
| I am not at risk | 88 (8) | 3 (12) | |
| I do not remember to run it | 62 (6) | 4 (15) | |
| Fear of ominous prognosis | 101 (9) | 2 (8) | |
| I do not know how to execute it properly | 37 (3) | 4 (15) | |
| I do not know what it is | 32 (3) | 0 | |
| missing | 0 | 1 (4) | |
| When I do self-examination, I take care of myself. | <0.001 *** | ||
| Strongly agree | 75 (7) | 11 (42) | |
| Agree | 997 (89) | 13 (50) | |
| In disagreement | 32 (3) | 1 (4) | |
| Strongly disagree | 11 (1) | 1 (4) | |
| Uncertain | 3 (0) | 0 | |
| Self-palpation is embarrassing | <0.001 *** | ||
| Strongly agree | 38 (3) | 5 (19) | |
| Agree | 103 (9) | 8 (31) | |
| In disagreement | 704 (63) | 1 (4) | |
| Strongly disagree | 267 (24) | 12 (46) | |
| Uncertain | 6 (1) | 0 | |
| Self-examination takes too much time | <0.001 *** | ||
| Strongly agree | 52 (5) | 6 (23) | |
| Agree | 120 (11) | 8 (31) | |
| In disagreement | 715 (64) | 1 (4) | |
| Strongly disagree | 230 (21) | 11 (42) | |
| Uncertain | 1 (0) | 0 | |
| I have more important problems than self-examination | <0.001 *** | ||
| Strongly agree | 48 (4) | 4 (15) | |
| Agree | 90 (8) | 9 (35) | |
| In disagreement | 877 (78) | 7 (27) | |
| Strongly disagree | 101 (9) | 6 (23) | |
| Uncertain | 2 (0) | 0 | |
| I am able to perform self-examination correctly | <0.001 *** | ||
| Strongly agree | 35 (3) | 3 (12) | |
| Agree | 81 (7) | 8 (31) | |
| In disagreement | 843 (75) | 13 (50) | |
| Strongly disagree | 147 (13) | 2 (8) | |
| Uncertain | 12 (1) | 0 | |
| I would like more information about self-examination. | <0.001 *** | ||
| Yes | 1013 (91) | 18 (68) | |
| No | 105 (9) | 8 (31) | |
| Which figure do you find helpful in obtaining information about self-examination? | 0.001 *** | ||
| Primary care physician | 27 (2) | 1 (4) | |
| Nurse | 17 (2) | 9 (35) | |
| Oncologist Psychologist | 271 (24) | 1 (4) | |
| Breast specialist | 777 (69) | 11 (42) | |
| Other | 14 (1) | 3 (12) | |
| Section 5: Knowledge and Use of Apps Dedicated to Prevention | |||
| Do you know or use dedicated applications for self-examination? | <0.001 *** | ||
| No | 1016 (91) | 18 (69) | |
| Yes | 102 (9) | 8 (31) | |
| Do you use BreastTest? | 0.64 | ||
| No | 1091 (98) | 25 (96) | |
| Yes | 27 (2) | 1 (4) | |
| Do you use Igyno? | 0.45 | ||
| No | 1094 (98) | 26 (100) | |
| Yes | 24 (2) | 0 | |
| Do you use Breast Cancer Indicators? | 0.41 | ||
| No | 1090 (97) | 26 (100) | |
| Yes | 28 (3) | 0 | |
| Do you use other apps? | 0.66 | ||
| No | 1090 (97) | 25 (96) | |
| Yes | 28 (3) | 1 (4) | |
| Section 6: Knowledge and Beliefs about the Causes and Symptomatology of Breast Cancer | Group A Women in the General Population (n = 1118) N (%) | Group B Women with Cancer (n = 26) N (%) | p-Value |
|---|---|---|---|
| Do you think you are well informed about breast cancer? | <0.001 *** | ||
| A lot | 26 (2) | 5 (19) | |
| Little | 80 (7) | 13 (50) | |
| Quite | 939 (84) | 6 (23) | |
| Not at all | 73 (7) | 2 (8) | |
| Do you think the cause of breast cancer is genetics? | 0.009 ** | ||
| No | 71(6) | 5 (19) | |
| Yes | 1047 (94) | 21 (81) | |
| Do you think the cause of breast cancer is endocrine? | 0.82 | ||
| No | 155 (14) | 4 (15) | |
| Yes | 963 (86) | 22 (85) | |
| Do you think a cause of breast cancer may be previous breast disease? | 0.50 | ||
| No | 320 (29) | 9 (35) | |
| Yes | 798 (71) | 17 (65) | |
| Do you think one cause of breast cancer may be radiation? | 0.02 * | ||
| No | 951 (85) | 18 (69) | |
| Yes | 167 (15) | 8 (31) | |
| Do you think one cause of breast cancer may be nutrition? | <0.001 *** | ||
| No | 856 (77) | 9 (35) | |
| Yes | 262 (23) | 17 (65) | |
| Do you think a cause of breast cancer may be environmental factors and pollution? | <0.001 *** | ||
| No | 929 (83) | 14 (54) | |
| Yes | 189 (17) | 12 (46) | |
| Do you think a cause of breast cancer may be psychological stress? | 0.004 ** | ||
| No | 532 (48) | 5 (19) | |
| Yes | 586 (52) | 21 (81) | |
| Do you think the cause of breast cancer may be another one? | 0.19 | ||
| No | 573 (51) | 10 (38) | |
| Yes | 545 (49) | 16 (62) | |
| What do you think the symptoms of cancer might be? | 0.44 | ||
| Palpable nodule | 135 (12) | 6 (23) | |
| Change in breast shape and size | 700 (63) | 14 (54) | |
| Nipple secretion | 69 (6) | 2 (8) | |
| Nipple alteration | 70 (6) | 3 (12) | |
| Other | 4 (0) | 0 | |
| I don’t know | 30 (3) | 1 (4) | |
| Section 7: Knowledge and beliefs about breast cancer prevention | |||
| Do you feel that you are well informed about breast cancer prevention? | <0.001 *** | ||
| A lot | 22 (2) | 7 (27) | |
| Quite | 69 (6) | 13 (50) | |
| Little | 955 (85) | 4 (15) | |
| Not at all | 72 (6) | 2 (8) | |
| What does prevention mean to you? | <0.001 *** | ||
| Early detection of cancers | 301 (27) | 11 (42) | |
| Prevention of risk factors | 593 (53) | 5 (19) | |
| Prevention of complications | 159 (14) | 2 (8) | |
| Don’t know | 41 (4) | 6 (23) | |
| More | 24 (2) | 2 (8) | |
| At what age do you think mammography is recommended? | 0.26 | ||
| <20 years old | 6 (1) | 0 | |
| 20–30 | 41 (4) | 0 | |
| 30–40 | 118 (11) | 2 (8) | |
| 40–50 | 199 (18) | 4 (15) | |
| 50–60 | 569 (51) | 15 (58) | |
| 60–70 | 165 (15) | 3 (12) | |
| I don’t know | 20 (2) | 1 (8) | |
| How often do you think mammography is recommended? | 0.0057 | ||
| Based on age/familiarity | 0 | 0 | |
| More than every year | 0 | 0 | |
| Every month | 11 (1) | 0 | |
| Every 6 months | 66 (6) | 2 (8) | |
| Every year | 846 (76) | 13 (50) | |
| Every 2 years | 141 (13) | 5 (19) | |
| I don’t know | 54 (5) | 6 (23) | |
| Do you consider clinical palpation useful as an act of breast cancer prevention? | 0.42 | ||
| No | 117 (10) | 4 (15) | |
| Yes | 1001 (90) | 22 (85) | |
| Do you think Magnetic Resonance Imaging (MRI) is useful for breast cancer prevention? | 0.009 ** | ||
| No | 501 (45) | 5 (19) | |
| Yes | 617 (55) | 21 (81) | |
| Do you consider biopsy useful as an act of breast cancer prevention? | <0.001 *** | ||
| No | 928 (83) | 13 (50) | |
| Yes | 190 (17) | 13 (50) | |
| Do you think computed tomography (CT) is useful for breast cancer prevention? | 0.007 ** | ||
| No | 639 (57) | 8 (31) | |
| Yes | 479 (43) | 18 (69) | |
| Do you think blood tests are useful for breast cancer prevention? | 0.003 ** | ||
| No | 839 (75) | 13 (50) | |
| Yes | 279 (25) | 13 (50) | |
| Do you find the interview with the oncologist useful as an act of breast cancer prevention? | 0.13 | ||
| No | 84 (8) | 4 (15) | |
| Yes | 1034 (92) | 22 (85) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conte, L.; Lupo, R.; Sciolti, S.; Lezzi, A.; Rubbi, I.; Botti, S.; Carvello, M.; Fanizzi, A.; Massafra, R.; Vitale, E.; et al. Exploring the Landscape of Breast Cancer Prevention among Chinese Residents in Italy: An In-Depth Analysis of Screening Adherence, Breast Self-Examination (BSE) Practices, the Role of Technological Tools, and Misconceptions Surrounding Risk Factors and Symptoms. Int. J. Environ. Res. Public Health 2024, 21, 308. https://doi.org/10.3390/ijerph21030308
Conte L, Lupo R, Sciolti S, Lezzi A, Rubbi I, Botti S, Carvello M, Fanizzi A, Massafra R, Vitale E, et al. Exploring the Landscape of Breast Cancer Prevention among Chinese Residents in Italy: An In-Depth Analysis of Screening Adherence, Breast Self-Examination (BSE) Practices, the Role of Technological Tools, and Misconceptions Surrounding Risk Factors and Symptoms. International Journal of Environmental Research and Public Health. 2024; 21(3):308. https://doi.org/10.3390/ijerph21030308
Chicago/Turabian StyleConte, Luana, Roberto Lupo, Serena Sciolti, Alessia Lezzi, Ivan Rubbi, Stefano Botti, Maicol Carvello, Annarita Fanizzi, Raffaella Massafra, Elsa Vitale, and et al. 2024. "Exploring the Landscape of Breast Cancer Prevention among Chinese Residents in Italy: An In-Depth Analysis of Screening Adherence, Breast Self-Examination (BSE) Practices, the Role of Technological Tools, and Misconceptions Surrounding Risk Factors and Symptoms" International Journal of Environmental Research and Public Health 21, no. 3: 308. https://doi.org/10.3390/ijerph21030308
APA StyleConte, L., Lupo, R., Sciolti, S., Lezzi, A., Rubbi, I., Botti, S., Carvello, M., Fanizzi, A., Massafra, R., Vitale, E., & De Nunzio, G. (2024). Exploring the Landscape of Breast Cancer Prevention among Chinese Residents in Italy: An In-Depth Analysis of Screening Adherence, Breast Self-Examination (BSE) Practices, the Role of Technological Tools, and Misconceptions Surrounding Risk Factors and Symptoms. International Journal of Environmental Research and Public Health, 21(3), 308. https://doi.org/10.3390/ijerph21030308












