Frailty in an Adult Acute Hospital Population: Predictors, Prevalence, and Outcomes
Abstract
1. Introduction
2. Materials and Methods
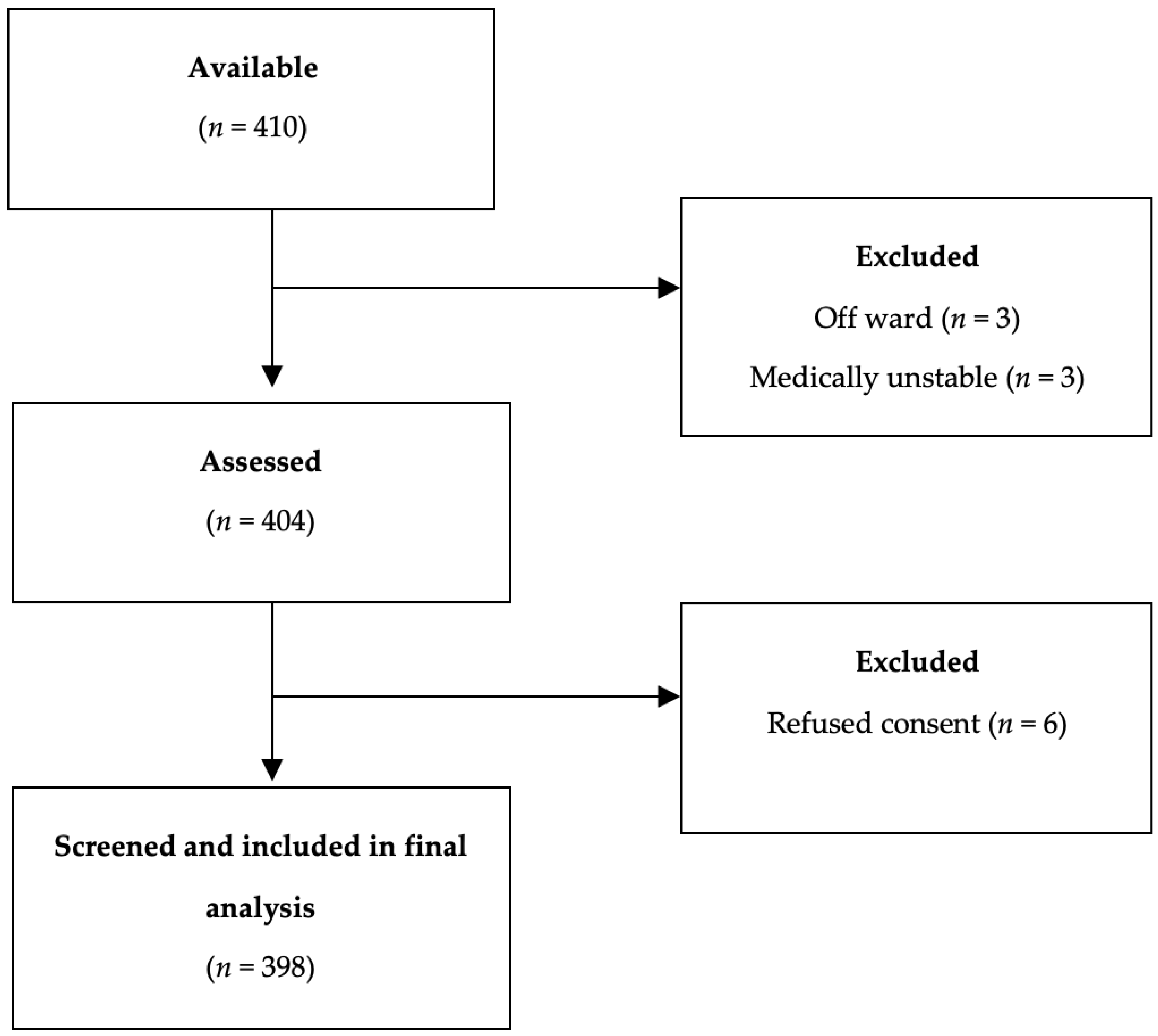
2.1. Inclusion and Exclusion Criteria
2.2. Sampling and Data Collection
2.3. Measures and Outcomes
2.4. Statistical Analysis
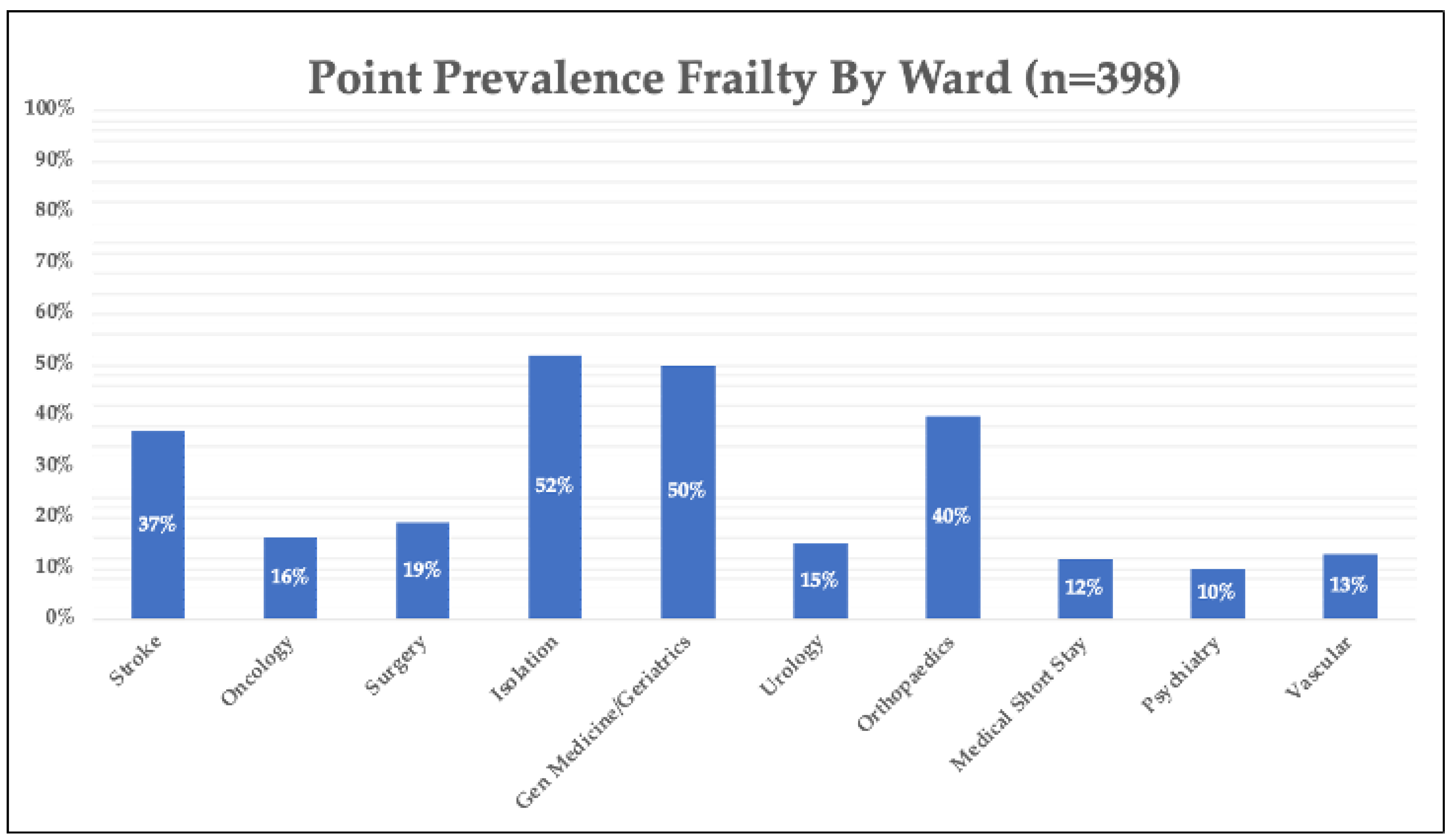
3. Results
4. Discussion
Strengths, Limitations, and Areas for Further Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, D.; Goel, A.; Kumar, U.; Gulati, V.; Narang, R.; Dey, A.B. Frailty is associated with longer hospital stay and increased mortality in hospitalized older patients. J. Nutr. Health Aging 2012, 16, 732–735. [Google Scholar] [CrossRef]
- Boucher, E.L.; Gan, J.M.; Rothwell, P.M.; Shepperd, S.; Pendlebury, S.T. Prevalence and outcomes of frailty in unplanned hospital admissions: A systematic review and meta-analysis of hospital-wide and general (internal) medicine cohorts. EClinicalMedicine 2023, 59, 101947. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, S.; Vella-Azzopardi, R.; Beckwee, D.; Habbig, A.K.; Scafoglieri, A.; Jansen, B.; Bautmans, I.; Verté, D.; Beyer, I.; Petrovic, M.; et al. Frailty and the prediction of negative health outcomes: A meta-analysis. JAMDA 2016, 17, 1163-e1. [Google Scholar] [CrossRef] [PubMed]
- Hartley, P.; Adamson, J.; Cunningham, C.; Embleton, G.; Romero-Ortuno, R. Clinical frailty and functional trajectories in hospitalized older adults: A retrospective observational study. Geriatr. Geront. Int. 2017, 17, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Heppenstall, C.; Wilkinson, T.J.; Hanger, H.C.; Keeling, S. Factors related to care home admission in the year following hospitalisation in frail older adults. Age Ageing 2011, 40, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, D.; O’Donovan, M.; Woo, J.; Bandeen-Roche, K.; Liotta, G.; Fairhall, N.; Rodríguez-Laso, A.; Apóstolo, J.; Clarnette, R.; Holland, C.; et al. Early identification of frailty: Developing an international delphi consensus on pre-frailty. Arch. Gerontol. Geriatr. 2022, 99, 104586. [Google Scholar] [CrossRef]
- Galluzzo, L.; O’Caoimh, R. Epidemiology, surveillance and population screening of frailty. Results from the systematic reviews of the European Joint Action ADVANTAGE. Preface. Ann. Dell’Istituto Super. Sanità 2018, 54, 223–225. [Google Scholar] [CrossRef]
- Liotta, G.; Ussai, S.; Illario, M.; O’Caoimh, R.; Cano, A.; Holland, C.; Roller-Winsberger, R.; Capanna, A.; Grecuccio, C.; Ferraro, M.; et al. Frailty as the Future Core Business of Public Health: Report of the Activities of the A3 Action Group of the European Innovation Partnership on Active and Healthy Ageing (EIP on AHA). Int. J. Environ. Res. Public. Health 2018, 15, 2843. [Google Scholar] [CrossRef]
- Gilardi, F.; Scarcella, P.; Proietti, M.G.; Capobianco, G.; Rocco, G.; Capanna, A.; Mancinelli, S.; Marazzi, M.C.; Palombi, L.; Liotta, G. Frailty as a predictor of mortality and hospital services use in older adults: A cluster analysis in a cohort study. Eur. J. Pub. Health 2018, 28, 842–846. [Google Scholar] [CrossRef]
- Liotta, G.; Gilardi, F.; Orlando, S.; Rocco, G.; Proietti, M.G.; Asta, F.; De Sario, M.; Michelozzi, P.; Mancinelli, S.; Palombi, L.; et al. Cost of hospital care for the older adults according to their level of frailty. A cohort study in the Lazio region, Italy. PloS ONE 2019, 14, e0217829. [Google Scholar] [CrossRef] [PubMed]
- Doody, P.; Asamane, E.A.; Aunger, J.A.; Swales, B.; Lord, J.M.; Greig, C.A.; Whittaker, A.C. The prevalence of frailty and pre-frailty among geriatric hospital inpatients and its association with economic prosperity and healthcare expenditure: A systematic review and meta-analysis of 467,779 geriatric hospital inpatients. Ageing Res. Rev. 2022, 80, 101666. [Google Scholar] [CrossRef] [PubMed]
- Cechinel, C.; Lenardt, M.H.; Rodrigues, J.A.M.; Binotto, M.A.; Aristides, M.M.; Kraus, R. Frailty and delirium in hospitalized older adults: A systematic review with meta-analysis. Rev. Latino-Americana Enferm. 2022, 30, e3687. [Google Scholar] [CrossRef]
- Hubbard, R.E.; Peel, N.M.; Samanta, M.; Gray, L.C.; Mitnitski, A.; Rockwood, K. Frailty status at admission to hospital predicts multiple adverse outcomes. Age. Ageing 2017, 46, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.I.L.; Veronese, N.; de Melo Borges, S.; Ricci, N.A. Frailty as a predictor of adverse outcomes in hospitalized older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2019, 56, 100960. [Google Scholar] [CrossRef] [PubMed]
- Keeble, E.; Roberts, H.C.; Williams, C.D.; Van Oppen, J.; Conroy, S.P. Outcomes of hospital admissions among frail older people: A 2-year cohort study. Br. J. Gen. Pract. 2019, 69, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.; Neuburger, J.; Kraindler, J.; Keeble, E.; Smith, P.; Ariti, C.; Arora, S.; Street, A.; Parker, S.; Roberts, H.C.; et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: An observational study. Lancet 2018, 391, 1775–1782. [Google Scholar] [CrossRef]
- Rezaei-Shahsavarloo, Z.; Atashzadeh-Shoorideh, F.; Gobbens, R.J.J.; Ebadi, A.; Ghaedamini Harouni, G. The impact of interventions on management of frailty in hospitalized frail older adults: A systematic review and meta-analysis. BMC Geriatr. 2020, 20, 526. [Google Scholar] [CrossRef]
- Ellis, G.; Gardner, M.; Tsiachristas, A.; Langhorne, P.; Burke, O.; Harwood, R.H.; Conroy, S.P.; Kircher, T.; Somme, D.; Saltvedt, I.; et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane. Database. Syst. Rev. 2017, 9, CD006211. [Google Scholar] [CrossRef]
- Partridge, J.S.; Harari, D.; Dhesi, J.K. Frailty in the older surgical patient: A review. Age. Ageing 2012, 41, 142–147. [Google Scholar] [CrossRef]
- Soong, J.; Poots, A.J.; Scott, S.; Donald, K.; Woodcock, T.; Lovett, D.; Bell, D. Quantifying the prevalence of frailty in English hospitals. BMJ Open 2015, 5, e008456. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, S.P.; Drumm, B.; Coughlan, T.; Collins, R.; O’Neill, D.; Romero-Ortuno, R. Characteristics and outcomes of older persons attending the emergency department: A retrospective cohort study. QJM 2014, 107, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Gregorevic, K.J.; Hubbard, R.E.; Katz, B.; Lim, W.K. The clinical frailty scale predicts functional decline and mortality when used by junior medical staff: A prospective cohort study. BMC Geriatr. 2016, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Joosten, E.; Demuynck, M.; Detroyer, E.; Milisen, K. Prevalence of frailty and its ability to predict in hospital delirium, falls, and 6-month mortality in hospitalized older patients. BMC Geriatr. 2014, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Wallis, S.J.; Wall, J.; Biram, R.W.; Romero-Ortuno, R. Association of the clinical frailty scale with hospital outcomes. QJM 2015, 108, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Theou, O.; Squires, E.; Mallery, K.; Lee, J.S.; Fay, S.; Goldstein, J.; Armstrong, J.J.; Rockwood, K. What do we know about frailty in the acute care setting? A scoping review. BMC Geriatr. 2018, 18, 139. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Cooney, M.T.; Cooke, J.; O’Shea, D. The challenges of using the Hospital Frailty Risk Score. Lancet 2019, 392, 2693. [Google Scholar] [CrossRef]
- Walston, J.; Buta, B.; Xue, Q.L. Frailty Screening and Interventions: Considerations for Clinical Practice. Clin. Geriatr. Med. 2018, 34, 25–38. [Google Scholar] [CrossRef]
- Liu, X.; Le, M.K.; Lim, A.Y.C.; Koh, E.J.; Nguyen, T.N.; Malik, N.A.; Lien, C.T.C.; Lee, J.E.; Au, L.S.Y.; Low, J.A.Y.H.; et al. Perspectives on frailty screening, management and its implementation among acute care providers in Singapore: A qualitative study. BMC Geriatr. 2022, 22, 58. [Google Scholar] [CrossRef]
- Elliott, A.; Phelps, K.; Regen, E.; Conroy, S.P. Identifying frailty in the Emergency Department-feasibility study. Age Ageing 2017, 46, 840–845. [Google Scholar] [CrossRef]
- Moloney, E.; O’Donovan, M.R.; Sezgin, D.; McGrath, K.; Timmons, S.; O’Caoimh, R. Frailty Knowledge, Use of Screening Tools, and Educational Challenges in Emergency Departments in Ireland: A Multisite Survey. J. Emerg. Nurs. 2023, 50, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Harnett, P.J.; Kennelly, S.; Williams, P. A 10 Step Framework to Implement Integrated Care for Older Persons. Ageing Int. 2020, 45, 288–304. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Theou, O. Using the Clinical Frailty Scale in Allocating Scarce Health Care Resources. Can. Geriatr. J. 2020, 23, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Church, S.; Rogers, E.; Rockwood, K.; Theou, O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020, 20, 393. [Google Scholar] [CrossRef]
- Raîche, M.; Hébert, R.; Dubois, M.F. PRISMA-7: A case-finding tool to identify older adults with moderate to severe disabilities. Arch. Gerontol. Geriatr. 2008, 47, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J. Nutr. Health Aging. 2012, 16, 601–608. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, 146–156. [Google Scholar] [CrossRef]
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; van Kan, G.A.; Ousset, P.J.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; et al. Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J. Nutr. Health Aging 2013, 17, 726–734. [Google Scholar] [CrossRef]
- Makizako, H.; Shimada, H.; Tsutsumimoto, K.; Lee, S.; Doi, T.; Nakakubo, S.; Hotta, R.; Suzuki, T. Social Frailty in Community-Dwelling Older Adults as a Risk Factor for Disability. JAMDA 2014, 16, 1003.e7–1003.e11. [Google Scholar] [CrossRef]
- Eurostat. Hospital Discharges and Length of Stay Statistics. 2018. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Hospital_discharges_and_length_of_stay_statistics (accessed on 28 December 2023).
- Dorner, T.E.; Luger, E.; Tschinderle, J.; Stein, K.V.; Haider, S.; Kapan, A.; Lackinger, C.; Schindler, K.E. Association between nutritional status (MNA®-SF) and frailty (SHARE-FI) in acute hospitalised elderly patients. J Nutr. Health Aging 2014, 18, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.T.T.; Nguyen, T.X.; Nguyen, T.N.; Nguyen, A.T.; Cumming, R.; Hilmer, S.; Pham, T. Prevalence of frailty and its associated factors in older hospitalised patients in Vietnam. BMC Geriatr. 2017, 17, 216. [Google Scholar] [CrossRef]
- Condon, M.; Mannion, E.; Molloy, D.W.; O’Caoimh, R. Urinary and Faecal Incontinence: Point Prevalence and Predictors in a University Hospital. Int. J. Environ. Res. Public. Health 2019, 16, 194. [Google Scholar] [CrossRef]
- O’Donnell, D.; O’Mahony, A.; Doyle, M.; O’Gorman, M.; O’Donoghue, A.; O’Halloran, A.; Mulcahy, R.; Pope, G.; Cooke, J. Point Prevalence of Frailty and Cognitive Impairment Exceeds the Capacity of a Single Ward—Specialist Geriatric Wards to lead Best Practice. Irish. Med. J. 2022, 115, 690. [Google Scholar]
- Richards, S.J.G.; D’Souza, J.; Pascoe, R.; Falloon, M.; Frizelle, F.A. Prevalence of frailty in a tertiary hospital: A point prevalence observational study. PloS ONE 2019, 14, e0219083. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, E.; Jang, I.Y. Frailty and Comprehensive Geriatric Assessment. J. Korean Med. Sci. 2020, 35, e16. [Google Scholar] [CrossRef] [PubMed]
- Hilmer, S.N.; Perera, V.; Mitchell, S.; Murnion, B.P.; Dent, J.; Bajorek, B.; Matthews, S.; Rolfson, D.B. The assessment of frailty in older people in acute care. Australas. J. Ageing 2009, 28, 182–188. [Google Scholar] [CrossRef]
- Al Huraizi, A.R.; Al-Maqbali, J.S.; Al Farsi, R.S.; Al Zeedy, K.; Al-Saadi, T.; Al-Hamadani, N.; Al Alawi, A.M. Delirium and Its Association with Short- and Long-Term Health Outcomes in Medically Admitted Patients: A Prospective Study. J. Clin. Med. 2023, 12, 5346. [Google Scholar] [CrossRef]
- Sahle, B.W.; Pilcher, D.; Litton, E.; Ofori-Asenso, R.; Peter, K.; McFadyen, J.; Bucknall, T. Association between frailty, delirium, and mortality in older critically ill patients: A binational registry study. Ann. Intensiv. Care 2022, 12, 108. [Google Scholar] [CrossRef]
- Bu, F.; Rutherford, A. Dementia, home care and institutionalisation from hospitals in older people. Eur. J. Ageing 2019, 16, 283–291. [Google Scholar] [CrossRef]
- Lin, H.S.; Watts, J.N.; Peel, N.M.; Hubbard, R.E. Frailty and post-operative outcomes in older surgical patients: A systematic review. BMC Geriatr. 2016, 16, 157. [Google Scholar] [CrossRef]
- Hewitt, J.; Carter, B.; McCarthy, K.; Pearce, L.; Law, J.; Wilson, F.V.; Tay, H.S.; McCormack, C.; Stechman, M.J.; Moug, S.J.; et al. Frailty predicts mortality in all emergency surgical admissions regardless of age. An observational study. Age Ageing 2019, 48, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Spiers, G.F.; Kunonga, T.P.; Hall, A.; Beyer, F.; Boulton, E.; Parker, S.; Bower, P.; Craig, D.; Todd, C.; Hanratty, B. Measuring frailty in younger populations: A rapid review of evidence. BMJ Open 2021, 11, e047051. [Google Scholar] [CrossRef]
- Gordon, E.H.; Peel, N.M.; Hubbard, R.E.; Reid, N. Frailty in younger adults in hospital. QJM 2023, 116, 845–849. [Google Scholar] [CrossRef]
- Hogan, D.B.; Maxwell, C.J.; Afilalo, J.; Arora, R.C.; Bagshaw, S.M.; Basran, J.; Bergman, H.; Bronskill, S.E.; Carter, C.A.; Dixon, E.; et al. A Scoping Review of Frailty and Acute Care in Middle-Aged and Older Individuals with Recommendations for Future Research. Can. Geriatr. J. 2017, 20, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Hilmer, S.; Hubbard, R.E. Where next with frailty risk scores in hospital populations? Age Ageing 2022, 51, afab203. [Google Scholar] [CrossRef] [PubMed]
- Jansen-Kosterink, S.; Van Velsen, L.; Frazer, S.; Dekker-van Weering, M.; O’Caoimh, R.; Vollenbroek-Hutten, M. Identification of community-dwelling older adults at risk of frailty using the PERSSILAA screening pathway: A methodological guide and results of a large-scale deployment in the Netherlands. BMC Public. Health 2019, 19, 504. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Laso, Á.; O’Caoimh, R.; Galluzzo, L.; Carcaillon-Bentata, L.; Beltzer, N.; Macijauskiene, J.; Albaina Bacaicoa, O.; Ciutan, M.; Hendry, A.; López-Samaniego, L.; et al. Population screening, monitoring and surveillance for frailty: Three systematic reviews and a grey literature review. Ann. Ist. Super. Sanita. 2018, 54, 253–262. [Google Scholar] [CrossRef]
- Muscedere, J.; Waters, B.; Varambally, A.; Bagshaw, S.M.; Boyd, J.G.; Maslove, D.; Sibley, S.; Rockwood, K. The impact of frailty on intensive care unit outcomes: A systematic review and meta-analysis. Intens. Care. Med. 2017, 43, 1105–1122. [Google Scholar] [CrossRef]
- Pugh, R.J.; Ellison, A.; Pye, K.; Subbe, C.P.; Thorpe, C.M.; Lone, N.I.; Clegg, A. Feasibility and reliability of frailty assessment in the critically ill: A systematic review. Crit. Care. 2018, 22, 49. [Google Scholar] [CrossRef]
- Bellini, G.; Morandi, A.; Di Santo, S.G.; Mazzone, A.; Cherubini, A.; Mossello, E.; Bo, M.; Bianchetti, A.; Rozzini, R.; Zanetti, E.; et al. "Delirium Day": A nationwide point prevalence study of delirium in older hospitalized patients using an easy standardized diagnostic tool. BMC Med. 2016, 14, 106. [Google Scholar] [CrossRef]
- Conroy, S.P.; Stevens, T.; Parker, S.G.; Gladman, J.R. A systematic review of comprehensive geriatric assessment to improve outcomes for frail older people being rapidly discharged from acute hospital: ’interface geriatrics’. Age Ageing 2011, 40, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Ní Shé, É.; McCarthy, M.; O’Donnell, D.; Collins, O.; Hughes, G.; Salter, N.; Cogan, L.; O’Donoghue, C.; McGrath, E.; O’Donovan, J.; et al. The systematic approach to improving care for Frail Older Patients (SAFE) study: A protocol for co-designing a frail older person’s pathway. HRB Open Res. 2018, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Barry, S.; Fhallúin, M.N.; Thomas, S.; Harnett, P.J.; Burke, S. Implementing Integrated Care in Practice—Learning from MDTs Driving the Integrated Care Programme for Older Persons in Ireland. Int. J. Integr. Care. 2021, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Hendry, A.; Vanhecke, E.; Carriazo, A.M.; López-Samaniego, L.; Espinosa, J.M.; Sezgin, D.; O’Donovan, M.; Hammar, T.; Ferry, P.; Vella, A.; et al. Integrated Care Models for Managing and Preventing Frailty: A Systematic Review for the European Joint Action on Frailty Prevention (ADVANTAGE JA). Transl. Med. UniSa. 2019, 19, 5–10. [Google Scholar]
| Predictor | Total (n = 398) Median (Q3–Q1 = +/−IQR) | Frail (n = 120; 30%) Median (Q3–Q1 = +/−IQR) | Non-Frail (n = 278; 70%) Median (Q3–Q1 = +/−IQR) | p Value |
|---|---|---|---|---|
| Age (Years) | 69.5 (80–54 = ±26) | 80 (86–75 = ±9) | 64 (74–44 = ±30) | p < 0.001 * |
| Sex (% Male) | 56% | 49% | 59% | p = 0.08 |
| Medications (Number, any) | 7 (10–5 = ±5) | 8 (11–2 = ±9) | 6 (9–4 = ±5) | p = 0.002 * |
| Polypharmacy (% with ≥5) | 29% | 55% | 18% | p = 0.014 * |
| Cognitive impairment (% documented) | 13% | 36% | 3% | p < 0.001 * |
| Delirium (% documented) | 8% | 19% | 3% | p = 0.005 * |
| CFS (Baseline) | 3 (5–2 = ±3) | 6 (6–5 = ±1) | 2 (3–2 = ±1) | p < 0.001 * |
| CFS (Current) | 4 (6–2 = ±4) | 6 (7–6 = ±1) | 3 (4–2 = ±2) | p < 0.001 * |
| FRAIL Scale | 2 (3–1 = ±2) | 3 (4–2 = ±2) | 1 (2–1 = ±1) | p < 0.001 * |
| PRISMA-7 | 4 (5–2 = ±3) | 5 (6–5 = ±1) | 2 (3–2 = ±1) | p < 0.001 * |
| Predictor | Total Median (Q3–Q1 = +/−IQR) | Frail Median (Q3–Q1 = +/−IQR) | Non-Frail Median (Q3–Q1 = +/−IQR) | p Value |
|---|---|---|---|---|
| LOS (days) | 8 (21–4 = ±17) | 12 (31–5 = ±26) | 6 (15-3 = ±12) | p < 0.001 * |
| Prolonged LOS (% median ≥7 days) | 54% | 66% | 48% | p = 0.001 * |
| 30-day re-admission rate (%) | 12% | 18% | 9% | 0.03 * |
| 90-day re-admission rate (%) | 16% | 24% | 13% | 0.04 * |
| Inpatient mortality (% prior to discharge) | 6% | 10% | 4% | 0.07 |
| Mortality (One-year %) | 27% | 43% | 20% | p < 0.001 * |
| Nursing home admission (One-year %, excluding existing residents) | 6% | 16% | 1% | p < 0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Caoimh, R.; Morrison, L.; Costello, M.; Flannery, A.; Small, C.; O’Reilly, L.; Heffernan, L.; Mannion, E.; Waters, R.; O’Keeffe, S. Frailty in an Adult Acute Hospital Population: Predictors, Prevalence, and Outcomes. Int. J. Environ. Res. Public Health 2024, 21, 273. https://doi.org/10.3390/ijerph21030273
O’Caoimh R, Morrison L, Costello M, Flannery A, Small C, O’Reilly L, Heffernan L, Mannion E, Waters R, O’Keeffe S. Frailty in an Adult Acute Hospital Population: Predictors, Prevalence, and Outcomes. International Journal of Environmental Research and Public Health. 2024; 21(3):273. https://doi.org/10.3390/ijerph21030273
Chicago/Turabian StyleO’Caoimh, Rónán, Laura Morrison, Maria Costello, Antoinette Flannery, Cliona Small, Liam O’Reilly, Laura Heffernan, Edel Mannion, Ruairi Waters, and Shaun O’Keeffe. 2024. "Frailty in an Adult Acute Hospital Population: Predictors, Prevalence, and Outcomes" International Journal of Environmental Research and Public Health 21, no. 3: 273. https://doi.org/10.3390/ijerph21030273
APA StyleO’Caoimh, R., Morrison, L., Costello, M., Flannery, A., Small, C., O’Reilly, L., Heffernan, L., Mannion, E., Waters, R., & O’Keeffe, S. (2024). Frailty in an Adult Acute Hospital Population: Predictors, Prevalence, and Outcomes. International Journal of Environmental Research and Public Health, 21(3), 273. https://doi.org/10.3390/ijerph21030273







