Impaired Well-Being and Insomnia as Residuals of Resolved Medical Conditions: Survey in the Italian Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Presence of Medical Conditions
2.2. Statistical Analysis
3. Results
3.1. Prevalence of Insomnia and Low Level of Well-Being and the Association between Them
3.2. Well-Being and Current and Resolved Medical Conditions Are Positively Associated
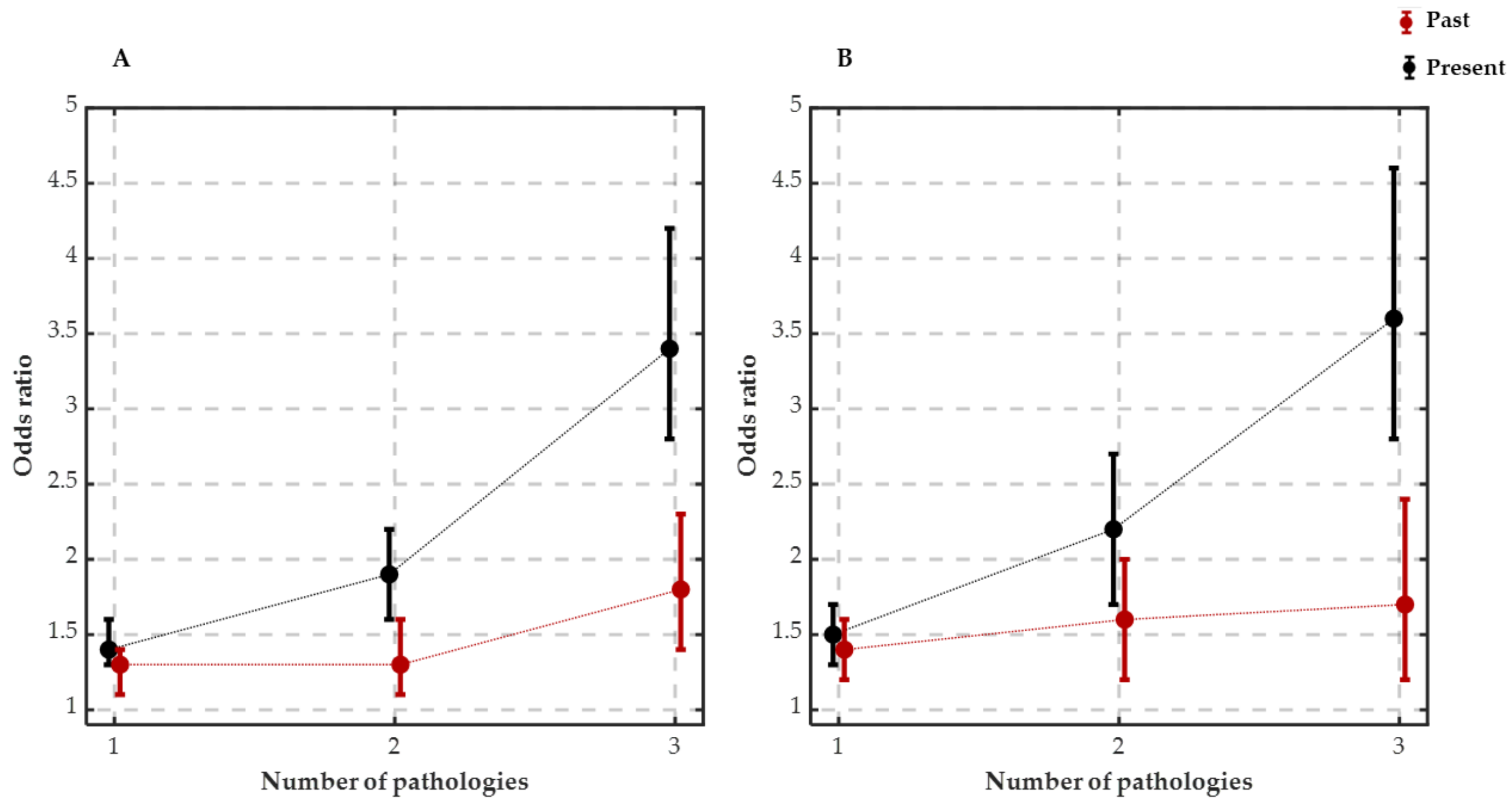
3.3. Insomnia and Current as Well as Resolved Medical Conditions Are Positively Associated
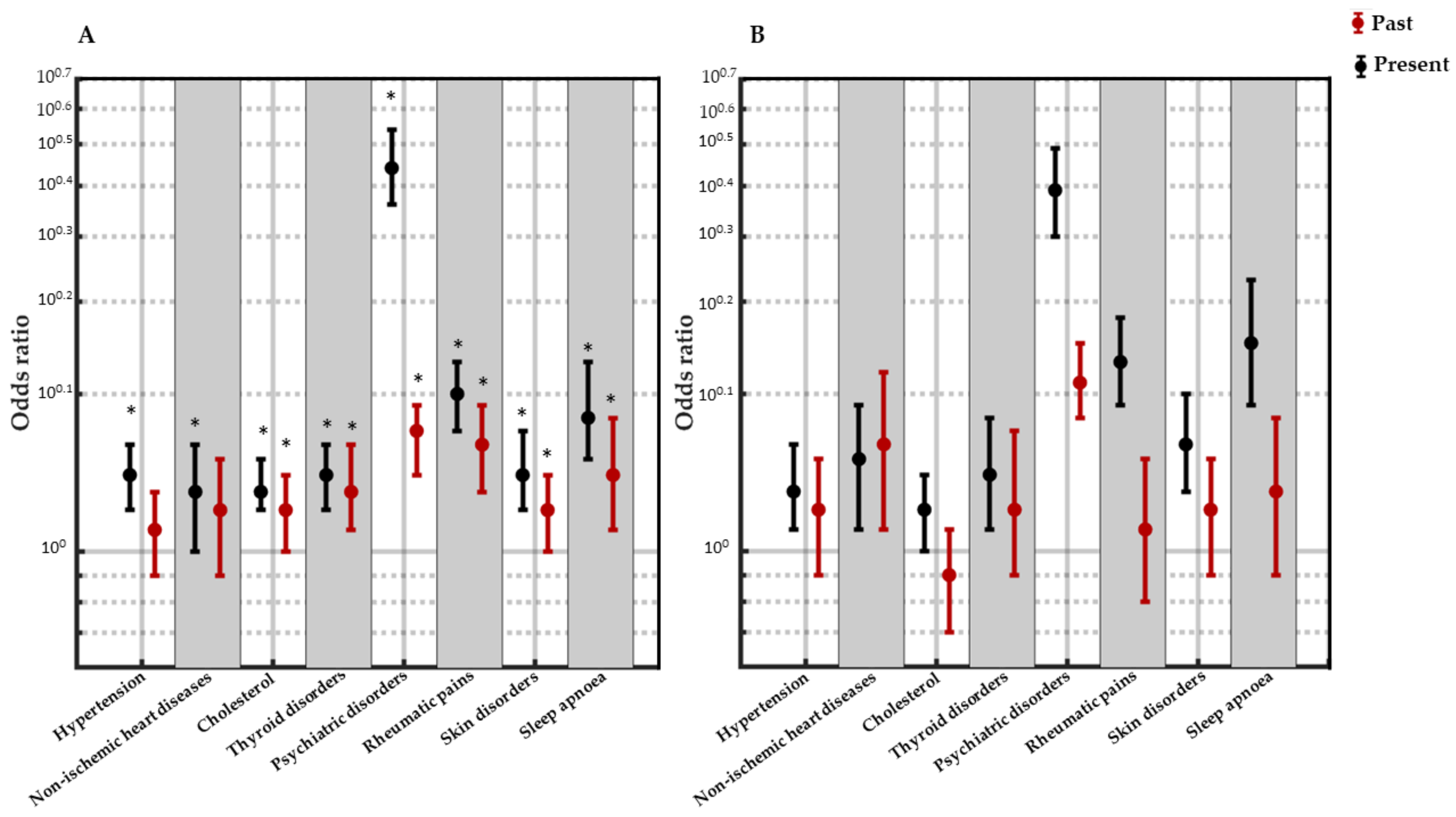
3.4. Association between Each Medical Condition and Insomnia as Well as Low Level of Well-Being
4. Discussion
4.1. Insomnia and Current Pathologies
4.2. Well-Being and Current Pathologies
4.3. Insomnia, Well-Being and Current Psychiatric Disorders: The Vicious Circle That Could Prevent Resolution
4.4. The Presence of Insomnia after Resolution of Pathologies Might Be Sustained by Inflammatory Track
4.5. Well-Being and Resolved Pathologies
4.6. Relationship between Insomnia, Well-Being and Resolved Psychiatric Disorders
5. Limitations and Future Perspectives
- Well-being was assessed using WHO-5, which does not include the evaluation of the socio-relational sphere. Future investigations should also focus on these aspects.
- The assessment of the subjects’ cognitive and psychological characteristics such as coping strategies, personality traits and illness behaviour, and the assessment of medical aspects such as adherence to treatment and treatment side effects, are strongly recommended, since they might act as mediating variables in determining impaired well-being and the development of chronic insomnia.
- Psychological disorders were not well defined in the survey: although the most frequent psychological disorders are depression and anxiety, the ability to speculate about the associations between insomnia and well-being and specific psychological conditions was precluded, since psychological disorders were not divided into specific categories.
- Medical conditions were not defined according to the ICD-10.
- Most of the past pathologies that were investigated in the survey are chronic; therefore, subjects might have reported a resolution because the pathology was controlled by drugs.
- This work does not investigate the effects of chronic diseases on sleep quality and well-being; however, the relevance of the phenomenon should be highlighted: even very specific conditions (e.g., retinal degeneration [116]) can have a global impact on health.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Promoting Well-Being. Available online: https://www.who.int/activities/promoting-well-being (accessed on 5 September 2023).
- Keller, S. What does mental health have to do with well-being? Bioethics 2020, 34, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Marengoni, A.; Angleman, S.; Melis, R.; Mangialasche, F.; Karp, A.; Garmen, A.; Meinow, B.; Fratiglioni, L. Aging with multimorbidity: A systematic review of the literature. Ageing Res. Rev. 2011, 10, 430–439. [Google Scholar] [CrossRef]
- Polis, S.; Fernandez, R. Impact of physical and psychological factors on health-related quality of life in adult patients with liver cirrhosis: A systematic review protocol. JBI Database Syst. Rev. Implement. Rep. 2015, 13, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Groarke, A.; Curtis, R.; Skelton, J.; Groarke, J.M. Quality of life and adjustment in men with prostate cancer: Interplay of stress, threat and resilience. PLoS ONE 2020, 15, e0239469. [Google Scholar] [CrossRef]
- Movsisyan, N.K.; Vinciguerra, M.; Medina-Inojosa, J.R.; Lopez-Jimenez, F. Cardiovascular Diseases in Central and Eastern Europe: A Call for More Surveillance and Evidence-Based Health Promotion. Ann. Glob. Health 2020, 86, 21. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Mental Health Atlas, Geneva. 2020. Available online: https://www.who.int/publications/i/item/9789240036703 (accessed on 6 September 2023).
- Dopheide, J.A. Insomnia overview: Epidemiology, pathophysiology, diagnosis and monitoring, and nonpharmacologic therapy. Am. J. Manag. Care 2020, 26, S76–S84. [Google Scholar] [CrossRef] [PubMed]
- Olfson, M.; Wall, M.; Liu, S.M.; Morin, C.M.; Blanco, C. Insomnia and Impaired Quality of Life in the United States. J. Clin. Psychiatry 2018, 79, 17m12020. [Google Scholar] [CrossRef]
- Proserpio, P.; Biggio, G.; Ferri, R.; Girardi, P.; Agostoni, E.C.; Manni, R.; Minervino, A.; Palagini, L.; Plazzi, G.; Nobili, L.; et al. Insomnia in primary care: A survey conducted on Italian patients older than 50 years-results from the “Sonno e Salute” study. Neurol. Sci. 2022, 43, 6487–6494. [Google Scholar] [CrossRef]
- Terzano, M.G.; Parrino, L.; Cirignotta, F.; Ferini-Strambi, L.; Gigli, G.; Rudelli, G.; Sommacal, S. Studio Morfeo Committee. Studio Morfeo: Insomnia in primary care, a survey conducted on the Italian population. Sleep Med. 2004, 5, 67–75. [Google Scholar] [CrossRef]
- Erman, M.K.; Rosenberg, R.; The, U.S. Modafinil Shift Work Sleep Disorder Study Group. Modafinil for excessive sleepiness associated with chronic shift work sleep disorder: Effects on patient functioning and health-related quality of life. Prim. Care Companion J. Clin. Psychiatry 2007, 9, 188–194. [Google Scholar] [CrossRef]
- Irwin, M.R. Why sleep is important for health: A psychoneuroimmunology perspective. Annu. Rev. Psychol. 2015, 66, 143–172. [Google Scholar] [CrossRef]
- Réus, G.Z.; Fries, G.R.; Stertz, L.; Badawy, M.; Passos, I.C.; Barichello, T.; Kapczinski, F.; Quevedo, J. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 2015, 300, 141–154. [Google Scholar] [CrossRef]
- Riemann, D.; Baglioni, C.; Bassetti, C.; Bjorvatn, B.; Dolenc Groselj, L.; Ellis, J.G.; Espie, C.A.; Garcia-Borreguero, D.; Gjerstad, M.; Gonçalves, M.; et al. European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 2017, 26, 675–700. [Google Scholar] [CrossRef]
- Jarrin, D.C.; Alvaro, P.K.; Bouchard, M.A.; Jarrin, S.D.; Drake, C.L.; Morin, C.M. Insomnia and hypertension: A systematic review. Sleep Med. Rev. 2018, 41, 3–38. [Google Scholar] [CrossRef]
- Riemann, D. Sleep and neuropsychiatric disorders. J. Sleep Res. 2019, 28, e12942. [Google Scholar] [CrossRef]
- Graziani, F.; Tsakos, G. Patient-based outcomes and quality of life. Periodontology 2000 2000, 83, 277–294. [Google Scholar] [CrossRef]
- Irwin, M.R.; Opp, M.R. Sleep Health: Reciprocal Regulation of Sleep and Innate Immunity. Neuropsychopharmacology 2017, 42, 129–155. [Google Scholar] [CrossRef] [PubMed]
- Bertisch, S.M.; Pollock, B.D.; Mittleman, M.A.; Buysse, D.J.; Bazzano, L.A.; Gottlieb, D.J.; Redline, S. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep 2018, 41, zsy047. [Google Scholar] [CrossRef]
- Zheng, B.; Yu, C.; Lv, J.; Guo, Y.; Bian, Z.; Zhou, M.; Yang, L.; Chen, Y.; Li, X.; Zou, J.; et al. China Kadoorie Biobank Collaborative Group. Insomnia symptoms and risk of cardiovascular diseases among 0.5 million adults: A 10-year cohort. Neurology 2019, 93, e2110–e2120. [Google Scholar] [CrossRef] [PubMed]
- Iranzo, A. Sleep and neurological autoimmune diseases. Neuropsychopharmacology 2020, 45, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Ozasa, K.; Mikami, K.; Wakai, K.; Fujino, Y.; Watanabe, Y.; Miki, T.; Nakao, M.; Hayashi, K.; Suzuki, K.; et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: Findings from the Japan collaborative cohort study. Am. J. Epidemiol. 2006, 164, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.; Lightfoot, N.; Kreiger, N. Rotating shift work and risk of prostate cancer. Epidemiology 2007, 18, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Parent, M.É.; El-Zein, M.; Rousseau, M.C.; Pintos, J.; Siemiatycki, J. Night work and the risk of cancer among men. Am. J. Epidemiol. 2012, 176, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Haus, E.L.; Smolensky, M.H. Shift work and cancer risk: Potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med. Rev. 2013, 17, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Asarnow, L.D. Depression and sleep: What has the treatment research revealed and could the HPA axis be a potential mechanism? Curr. Opin. Psychol. 2020, 34, 112–116. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Riemann, D.; Spiegelhalder, K.; Feige, B.; Voderholzer, U.; Berger, M.; Perlis, M.; Nissen, C. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med. Rev. 2010, 14, 19–31. [Google Scholar] [CrossRef]
- Zou, G.; Li, Y.; Liu, J.; Zhou, S.; Xu, J.; Qin, L.; Shao, Y.; Yao, P.; Sun, H.; Zou, Q.; et al. Altered thalamic connectivity in insomnia disorder during wakefulness and sleep. Hum. Brain Mapp. 2021, 42, 259–270. [Google Scholar] [CrossRef]
- Sachs, G.; Anderer, P.; Dantendorfer, K.; Saletu, B. EEG mapping in patients with social phobia. Psychiatry Res. 2004, 131, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, A.K.; Chevrette, T.; Bouvier, H.; Godbout, R. Evening vs. morning wake EEG activity in adolescents with anxiety disorders. J. Anxiety Disord. 2009, 23, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Kasatkin, D.S.; Spirin, N.N. Possible mechanisms of the formation of chronic fatigue syndrome in the clinical picture of multiple sclerosis. Neurosci. Behav. Physiol. 2007, 37, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Kuppuswamy, P.S.; Takala, C.R.; Sola, C.L. Management of psychiatric symptoms in anti-NMDAR encephalitis: A case series, literature review and future directions. Gen. Hosp. Psychiatry 2014, 36, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Silber, M.H. Autoimmune sleep disorders. Handb. Clin. Neurol. 2016, 133, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Gominak, S.C. Vitamin D deficiency changes the intestinal microbiome reducing B vitamin production in the gut. The resulting lack of pantothenic acid adversely affects the immune system, producing a “pro-inflammatory” state associated with atherosclerosis and autoimmunity. Med. Hypotheses 2016, 94, 103–107. [Google Scholar] [CrossRef]
- Blattner, M.S.; de Bruin, G.S.; Bucelli, R.C.; Day, G.S. Sleep disturbances are common in patients with autoimmune encephalitis. J. Neurol. 2019, 266, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.; Dreher, M.; Weeß, H.G.; Staubach, P. Sleep Disturbance in Patients with Urticaria and Atopic Dermatitis: An Underestimated Burden. Acta Derm. Venereol. 2020, 100, adv00073. [Google Scholar] [CrossRef]
- Kalmbach, D.A.; Anderson, J.R.; Drake, C.L. The impact of stress on sleep: Pathogenic sleep reactivity as a vulnerability to insomnia and circadian disorders. J. Sleep Res. 2018, 27, e12710. [Google Scholar] [CrossRef]
- Bower, J.E. Behavioral symptoms in patients with breast cancer and survivors. J. Clin. Oncol. 2008, 26, 768–777. [Google Scholar] [CrossRef]
- Orre, I.J.; Reinertsen, K.V.; Aukrust, P.; Dahl, A.A.; Fosså, S.D.; Ueland, T.; Murison, R. Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J. Psychosom. Res. 2011, 71, 136–141. [Google Scholar] [CrossRef]
- Denoth, F.; Scalese, M.; Siciliano, V.; Di Renzo, L.; De Lorenzo, A.; Molinaro, S. Clustering eating habits: Frequent consumption of different dietary patterns among the Italian general population in the association with obesity, physical activity, sociocultural characteristics and psychological factors. Eat. Weight Disord. 2016, 21, 257–268. [Google Scholar] [CrossRef]
- World Health Organization Regional Office for Europe. In Proceedings of the Wellbeing Measures in Primary Health Care/the DepCare Project: Report on a WHO Meeting, Stockholm, Sweden, 12–13 February 1998. Available online: https://apps.who.int/iris/handle/10665/349766 (accessed on 14 September 2023).
- Topp, C.W.; Ostergaard, S.D.; Sondergaard, S.; Bech, P. The WHO-5 well-being index: A systematic review of the literature. Psychother. Psychosom. 2015, 84, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Sischka, P.E.; Costa, A.P.; Steffgen, G.; Schmidt, A.F. The WHO-5 well-being index–validation based on item response theory and the analysis of measurement invariance across 35 countries. J. Affect. Disord. Rep. 2020, 1, 100020. [Google Scholar] [CrossRef]
- Morin, C.M. Insomnia Severity Index (ISI); Database Record; APA PsycTests: Washington, DC, USA, 1993. [Google Scholar]
- Battagliese, G.; Lombardo, C. Insonnia, Strumenti di Valutazione Psicologica; Chapter L’Insomnia Severity Index; Coradeschi, D., Devoto, A., Eds.; Erickson: Trento, Italy, 2012; pp. 23–31. [Google Scholar]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Kan, K.K.; Yeung, W.F. Assessing insomnia in adolescents: Comparison of Insomnia Severity Index, Athens Insomnia Scale and Sleep Quality Index. Sleep Med. 2011, 12, 463–470. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. IBM SPSS Statistics for Windows, Version 28.0.; IBM Corp.: Armonk, NY, USA, 2015.
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Frange, C.; Hachul, H.; Hirotsu, C.; Tufik, S.; Andersen, M.L. Temporal Analysis of Chronic Musculoskeletal Pain and Sleep in Postmenopausal Women. J. Clin. Sleep Med. 2019, 15, 223–234. [Google Scholar] [CrossRef]
- Sørensen, L.; Jensen, M.S.A.; Rathleff, M.S.; Holden, S. Comorbid insomnia, psychological symptoms and widespread pain among patients suffering from musculoskeletal pain in general practice: A cross-sectional study. BMJ Open 2019, 9, e031971. [Google Scholar] [CrossRef]
- Calcia, M.A.; Bonsall, D.R.; Bloomfield, P.S.; Selvaraj, S.; Barichello, T.; Howes, O.D. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology 2016, 233, 1637–1650. [Google Scholar] [CrossRef]
- Guidi, J.; Lucente, M.; Sonino, N.; Fava, G.A. Allostatic Load and Its Impact on Health: A Systematic Review. Psychother. Psychosom. 2021, 90, 11–27. [Google Scholar] [CrossRef]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef]
- Ren, C.Y.; Rao, J.X.; Zhang, X.X.; Zhang, M.; Xia, L.; Chen, G.H. Changed signals of blood adenosine and cytokines are associated with parameters of sleep and/or cognition in the patients with chronic insomnia disorder. Sleep Med. 2021, 81, 42–51. [Google Scholar] [CrossRef]
- Rodenbeck, A.; Huether, G.; Rüther, E.; Hajak, G. Interactions between evening and nocturnal cortisol secretion and sleep parameters in patients with severe chronic primary insomnia. Neurosci. Lett. 2002, 324, 159–163. [Google Scholar] [CrossRef]
- Nicolaides, N.C.; Kino, T.; Chrousos, G. AIDS and HPA Axis. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya Kathleen Dungan, K., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK279014/ (accessed on 16 January 2024). [PubMed]
- Fraser, R.; Ingram, M.C.; Anderson, N.H.; Morrison, C.; Davies, E.; Connell, J.M. Cortisol effects on body mass, blood pressure, and cholesterol in the general population. Hypertension 1999, 33, 1364–1368. [Google Scholar] [CrossRef]
- Harada, M.; Van Wagoner, D.R.; Nattel, S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ. J. 2015, 79, 495–502. [Google Scholar] [CrossRef]
- Gursoy, M.; Salihoglu, E.; Hatemi, A.C.; Hokenek, A.F.; Ozkan, S.; Ceyran, H. Inflammation and congenital heart disease associated pulmonary hypertension. Heart Surg. Forum 2015, 18, E38–E41. [Google Scholar] [CrossRef]
- Low, A.; George, S.; Howard, L.; Bell, N.; Millar, A.; Tulloh, R.M.R. Lung Function, Inflammation, and Endothelin-1 in Congenital Heart Disease-Associated Pulmonary Arterial Hypertension. J. Am. Heart Assoc. 2018, 7, e007249. [Google Scholar] [CrossRef] [PubMed]
- Salzano, A.; Marra, A.M.; Arcopinto, M.; D’Assante, R.; Triggiani, V.; Coscioni, E.; Pasquali, D.; Rengo, G.; Suzuki, T.; Bossone, E.; et al. Combined effects of growth hormone and testosterone replacement treatment in heart failure. ESC Heart Fail. 2019, 6, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, D.; Díaz, O.; Devesa, P.; Devesa, J. Growth Hormone (GH) and Cardiovascular System. Int. J. Mol. Sci. 2018, 19, 290. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Yanowski, J.; Kaldjian, E.P.; Jaffe, E.S.; LeRoith, T.; Purdue, K.; Cooper, B.D.; Pyle, R.; Adler, W. The effects of growth hormone and insulin-like growth factor I on the immune system of aged female monkeys. Endocrinology 1996, 137, 1071–1079. [Google Scholar] [CrossRef][Green Version]
- Velardi, E.; Tsai, J.J.; van den Brink, M.R.M. T cell regeneration after immunological injury. Nat. Rev. Immunol. 2021, 21, 277–291. [Google Scholar] [CrossRef]
- Redwine, L.; Hauger, R.L.; Gillin, J.C.; Irwin, M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J. Clin. Endocrinol. Metab. 2000, 85, 3597–3603. [Google Scholar] [CrossRef]
- Terán-Pérez, G.; Arana-Lechuga, Y.; Esqueda-León, E.; Santana-Miranda, R.; Rojas-Zamorano, J.Á.; Velázquez Moctezuma, J. Steroid hormones and sleep regulation. Mini Rev. Med. Chem. 2012, 12, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, K.; Holsboer-Trachsler, E.; Eckert, A. BDNF in sleep, insomnia, and sleep deprivation. Ann. Med. 2016, 48, 42–51. [Google Scholar] [CrossRef]
- Shakya, H.; Wang, D.; Zhou, K.; Luo, Z.Y.; Dahal, S.; Zhou, Z.K. Prospective randomized controlled study on improving sleep quality and impact of zolpidem after total hip arthroplasty. J. Orthop. Surg. Res. 2019, 14, 289. [Google Scholar] [CrossRef]
- Peng, W.; Wu, Z.; Song, K.; Zhang, S.; Li, Y.; Xu, M. Regulation of sleep homeostasis mediator adenosine by basal forebrain glutamatergic neurons. Science 2020, 369, eabb0556. [Google Scholar] [CrossRef]
- Simard, T.; Jung, R.; Labinaz, A.; Faraz, M.A.; Ramirez, F.D.; Di Santo, P.; Pitcher, I.; Motazedian, P.; Gaudet, C.; Rochman, R.; et al. Adenosine as a Marker and Mediator of Cardiovascular Homeostasis: A Translational Perspective. Cardiovasc. Hematol. Disord. Drug Targets 2019, 19, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Grossfeld, D.; Kasselman, L.J.; Renna, H.A.; Vernice, N.A.; Drewes, W.; Konig, J.; Carsons, S.E.; DeLeon, J. Adenosine and the Cardiovascular System. Am. J. Cardiovasc. Drugs 2019, 19, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Pedroni, M.N.; Hirotsu, C.; Porro, A.M.; Tufik, S.; Andersen, M.L. The role of sleep in pemphigus: A review of mechanisms and perspectives. Arch. Dermatol. Res. 2017, 309, 659–664. [Google Scholar] [CrossRef]
- Kaaz, K.; Szepietowski, J.C.; Matusiak, Ł. Influence of Itch and Pain on Sleep Quality in Patients with Hidradenitis Suppurativa. Acta Derm. Venereol. 2018, 98, 757–761. [Google Scholar] [CrossRef]
- Mostaghimi, L.; Hetzel, S. Insomnia and other sleep complaints in inflammatory versus noninflammatory skin disorders: An observational case-control study. Int. J. Dermatol. 2019, 58, 976–981. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int. J. Mol. Sci. 2019, 20, 459. [Google Scholar] [CrossRef] [PubMed]
- Dauden, E.; Blasco, A.J.; Bonanad, C.; Botella, R.; Carrascosa, J.M.; González-Parra, E.; Jodar, E.; Joven, B.; Lázaro, P.; Olveira, A.; et al. Position statement for the management of comorbidities in psoriasis. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2058–2073. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ortega, J.M.; Nogueras, P.; Muñoz-Negro, J.E.; Gutiérrez-Rojas, L.; González-Domenech, P.; Gurpegui, M. Quality of life, anxiety and depressive symptoms in patients with psoriasis: A case-control study. J. Psychosom. Res. 2019, 124, 109780. [Google Scholar] [CrossRef] [PubMed]
- Elenkov, I.J.; Iezzoni, D.G.; Daly, A.; Harris, A.G.; Chrousos, G.P. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation 2005, 12, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Faugere, M.; Micoulaud-Franchi, J.A.; Faget-Agius, C.; Lançon, C.; Cermolacce, M.; Richieri, R. Quality of life is associated with chronic inflammation in depression: A cross-sectional study. J. Affect. Disord. 2018, 227, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Soo, J.; Kubzansky, L.D.; Chen, Y.; Zevon, E.S.; Boehm, J.K. Psychological well-being and restorative biological processes: HDL-C in older English adults. Soc. Sci. Med. 2018, 209, 59–66. [Google Scholar] [CrossRef]
- Nowakowski, A.C. Chronic inflammation and quality of life in older adults: A cross-sectional study using biomarkers to predict emotional and relational outcomes. Health Qual. Life Outcomes 2014, 12, 141. [Google Scholar] [CrossRef]
- Hood, M.M.; Wilson, R.; Gorenz, A.; Jedel, S.; Raeisi, S.; Hobfoll, S.; Keshavarzian, A. Sleep Quality in Ulcerative Colitis: Associations with Inflammation, Psychological Distress, and Quality of Life. Int. J. Behav. Med. 2018, 25, 517–525. [Google Scholar] [CrossRef]
- ISTAT. La Salute Mentale Nelle Varie Fasi Della Vita, Istat, Roma (Italian only). 2018. Available online: https://www.istat.it/it/files/2018/07/Report_Salute_mentale.pdf (accessed on 7 September 2023).
- Davis, M.; Whalen, P.J. The amygdala: Vigilance and emotion. Mol. Psychiatry 2001, 6, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.; Rossetti, A.C.; Racagni, G.; Gass, P.; Riva, M.A.; Molteni, R. Brain-derived neurotrophic factor: A bridge between inflammation and neuroplasticity. Front. Cell Neurosci. 2004, 8, 430. [Google Scholar] [CrossRef]
- Sanada, K.; Montero-Marin, J.; Barceló-Soler, A.; Ikuse, D.; Ota, M.; Hirata, A.; Yoshizawa, A.; Hatanaka, R.; Valero, M.S.; Demarzo, M.; et al. Effects of Mindfulness-Based Interventions on Biomarkers and Low-Grade Inflammation in Patients with Psychiatric Disorders: A Meta-Analytic Review. Int. J. Mol. Sci. 2020, 21, 2484. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, M.A.; Thong, M.S.; Bartels, M.; Barsevick, A.; Ordoñana, J.; Shi, Q.; Wang, X.S.; Klepstad, P.; Wierenga, E.A.; Singh, J.A.; et al. GeneQol Consortium. Biological pathways, candidate genes, and molecular markers associated with quality-of-life domains: An update. Qual. Life Res. 2014, 23, 1997–2013. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Billiard, M.; Bentley, A. Is insomnia best categorized as a symptom or a disease? Sleep Med. 2004, 1, S35–S40. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. Aging of the Immune System. Mechanisms and Therapeutic Targets. Ann. Am. Thorac. Soc. 2016, 13, S422–S428. [Google Scholar] [CrossRef]
- Johnsson, H.; Panarelli, M.; Cameron, A.; Sattar, N. Analysis and modelling of cholesterol and high-density lipoprotein cholesterol changes across the range of C-reactive protein levels in clinical practice as an aid to better understanding of inflammation-lipid interactions. Ann. Rheum. Dis. 2014, 73, 1495–1499. [Google Scholar] [CrossRef]
- Aksoy, H.; Aksoy, Ü.; Karadağ, Ö.İ.; Hacimusalar, Y.; Açmaz, G.; Aykut, G.; Çağlı, F.; Yücel, B.; Aydın, T.; Babayiğit, M.A. Depression levels in patients with hyperemesis gravidarum: A prospective case-control study. Springerplus 2015, 4, 34. [Google Scholar] [CrossRef]
- Kumari, B.; Kumar, P.; Chaudhary, R.K.P. Evaluation of Anti-Cyclic Citrullinated Peptide Autoantibodies and C-Reactive Protein in Common Autoimmune Skin Diseases with and without Arthritis. J. Clin. Diagn. Res. 2017, 11, BC06–BC08. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Nehring, S.M.; Goyal, A.; Patel, B.C. C Reactive Protein. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441843/ (accessed on 16 January 2024).
- Koc, H.C.; Xiao, J.; Liu, W.; Li, Y.; Chen, G. Long COVID and its Management. Int. J. Biol. Sci. 2022, 18, 4768–4780. [Google Scholar] [CrossRef]
- Irwin, M.R. Sleep and inflammation: Partners in sickness and in health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Weinert, D.; Gubin, D. The Impact of Physical Activity on the Circadian System: Benefits for Health, Performance and Wellbeing. Appl. Sci. 2022, 12, 9220. [Google Scholar] [CrossRef]
- Csoma, B.; Bikov, A. The Role of the Circadian Rhythm in Dyslipidaemia and Vascular Inflammation Leading to Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 14145. [Google Scholar] [CrossRef]
- Kim, M.; Vu, T.H.; Maas, M.B.; Braun, R.I.; Wolf, M.S.; Roenneberg, T.; Daviglus, M.L.; Reid, K.J.; Zee, P.C. Light at night in older age is associated with obesity, diabetes, and hypertension. Sleep 2023, 46, zsac130. [Google Scholar] [CrossRef]
- Shen, Y.; Lv, Q.K.; Xie, W.Y.; Gong, S.-Y.; Zhuang, S.; Liu, J.-Y.; Mao, C.-J.; Liu, C.-F. Circadian disruption and sleep disorders in neurodegeneration. Transl. Neurodegener. 2023, 12, 8. [Google Scholar] [CrossRef]
- Malhotra, R.K. Neurodegenerative Disorders and Sleep. Sleep Med. Clin. 2018, 13, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Purvis, T.E.; Hu, K.; Scheer, F.A. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E1402–E1411. [Google Scholar] [CrossRef]
- Khayyat, S.M.; Mohamed, M.M.A.; Khayyat, S.M.S.; Hyat Alhazmi, R.S.; Korani, M.F.; Allugmani, E.B.; Saleh, S.F.; Mansouri, D.A.; Lamfon, Q.A.; Beshiri, O.M.; et al. Association between medication adherence and quality of life of patients with diabetes and hypertension attending primary care clinics: A cross-sectional survey. Qual. Life Res. 2019, 28, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, A.; Malefakis, D. Resilience, Trauma, and Coping. Psychodyn. Psychiatry 2018, 46, 81–113. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.J.; Benca, R.M. Sleep in mood disorders. Psychiatr. Clin. N. Am. 2006, 29, 1009–1032. [Google Scholar] [CrossRef]
- Jha, M.K.; Trivedi, M.H. Experimental Therapies for Treatment-Resistant Depression: “How do you decide when to go to an unproven or experimental therapy with patients that are treatment-resistant depression?”. Focus 2018, 16, 279–284. [Google Scholar] [CrossRef]
- Harvey, K.; Welch, Z.; Kovala, A.T.; Garcia, J.G.; English, D. Comparative analysis of in vitro angiogenic activities of endothelial cells of heterogeneous origin. Microvasc. Res. 2002, 63, 316–326. [Google Scholar] [CrossRef]
- Levenson, J.C.; Kay, D.B.; Buysse, D.J. The pathophysiology of insomnia. Chest 2015, 147, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.G.; Malishevskaya, T.N.; Astakhov, Y.S.; Astakhov, S.Y.; Cornelissen, G.; Kuznetsov, W.A.; Weinert, D. Progressive retinal ganglion cell loss in primary open-angle glaucoma is associated with temperature circadian rhythm phase delay and compromised sleep. Chronobiol. Int. 2019, 36, 564–577. [Google Scholar] [CrossRef]
- Feng, H.; Yang, L.; Ai, S.; Liu, Y.; Zhang, W.; Lei, B.; Chen, J.; Liu, Y.; Chan, J.W.Y.; Chan, N.Y.; et al. Association between accelerometer-measured amplitude of rest-activity rhythm and future health risk: A prospective cohort study of the UK Biobank. Lancet Healthy Longev. 2023, 4, e200–e210. [Google Scholar] [CrossRef]
- Bufano, P.; Laurino, M.; Said, S.; Tognetti, A.; Menicucci, D. Digital Phenotyping for Monitoring Mental Disorders: Systematic Review. J. Med. Internet Res. 2023, 25, e46778. [Google Scholar] [CrossRef]
| Sample Characteristics | N (%) | |
|---|---|---|
| Gender | Males | Females |
| 2685 (48.3) | 2876 (51.7) | |
| Age, years | Males | Females |
| <20 | 122 (4.7) | 122 (4.4) |
| 20–29 | 361 (13.9) | 503 (18.0) |
| 30–39 | 363 (13.9) | 487 (1.4) |
| 40–49 | 499 (19.2) | 576 (20.6) |
| 50–59 | 601 (23.1) | 574 (20.5) |
| 60–69 | 500 (19.2) | 407 (14.6) |
| >70 | 159 (6.1) | 126 (4.5) |
| Marital status | ||
| Unmarried | 2145 (39.1) | |
| Married | 2828 (51.5) | |
| Separate | 177 (3.2) | |
| Divorced | 219 (4.0) | |
| Widower | 199 (2.2) | |
| Education | ||
| None | 7 (0.1) | |
| Primary Education | 82 (1.5) | |
| Secondary Education | 615 (11.2) | |
| Tertiary Education | 366 (6.1) | |
| High School | 2189 (39.8) | |
| Bachelor’s Degree | 443 (8.1) | |
| Master’s Degree | 1358 (24.7) | |
| Post-Graduation | 470 (8.5) | |
| Employed | ||
| No | 2114 (38.4) | |
| Yes | 3392 (61.6) | |
| N(%) | ||
|---|---|---|
| WHO-5 | Males | Females |
| >50 | 1642 (61.2) | 1503 (52.3) |
| ≤50 | 1043 (38.8) | 1373 (47.7) |
| ISI | Males | Females |
| <10 | 2168 (80.7) | 2137 (74.3) |
| ≥10 | 517 (19.3) | 739 (25.7) |
| Present pathologies | Males | Females |
| No | 205 (8.8) | 228 (9.4) |
| One | 295 (12.7) | 295 (12.1) |
| Two | 604 (25.9) | 596 (24.5) |
| Three or more | 1225 (52.6) | 1317 (54.1) |
| Past pathologies | Males | Females |
| No | 90 (3.9) | 93 (3.8) |
| One | 221 (9.5) | 222 (9.1) |
| Two | 521 (22.4) | 684 (28.1) |
| Three or more | 1497 (64.3) | 1437 (59.0) |
| N | Mean | S.D. | ||
|---|---|---|---|---|
| WHO-5 | present pathologies | 2223 | 48.66 | 21.06 |
| no present pathologies | 2542 | 55.87 | 19.29 | |
| ISI | present pathologies | 2223 | 6.92 | 4.92 |
| no present pathologies | 2542 | 5.49 | 4.13 | |
| N | Mean | S.D. | ||
|---|---|---|---|---|
| WHO-5 | past pathologies | 1831 | 50.10 | 20.43 |
| no past pathologies | 2934 | 54.01 | 20.32 | |
| ISI | past pathologies | 1831 | 6.80 | 4.71 |
| no past pathologies | 2934 | 5.75 | 4.43 | |
| Regression Weights | |||
|---|---|---|---|
| Variables (d.f.) | O.R. | 95% CI | p Value |
| Age (1) | 0.99 | 0.98–1.00 | 0.43 |
| Gender (1) | 1.18 | 0.88–1.58 | 0.24 |
| Age × gender (1) | 1.00 | 0.99–1.00 | 0.44 |
| Only present (1) | 1.75 | 1.58–1.94 | <0.0001 |
| Only past (1) | 1.33 | 1.21–1.47 | <0.0001 |
| Regression Weights | |||
|---|---|---|---|
| Variables (d.f.) | O.R. | 95% CI | p Value |
| Age (1) | 0.98 | 0.97–0.99 | 0.02 |
| Gender (1) | 0.84 | 0.55–1.29 | 0.43 |
| Age × gender (1) | 1.01 | 1.00–1.02 | 0.01 |
| Only present (1) | 1.90 | 1.63–2.20 | <0.0001 |
| Only past (1) | 1.43 | 1.24–1.60 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menicucci, D.; Bastiani, L.; Malloggi, E.; Denoth, F.; Gemignani, A.; Molinaro, S. Impaired Well-Being and Insomnia as Residuals of Resolved Medical Conditions: Survey in the Italian Population. Int. J. Environ. Res. Public Health 2024, 21, 129. https://doi.org/10.3390/ijerph21020129
Menicucci D, Bastiani L, Malloggi E, Denoth F, Gemignani A, Molinaro S. Impaired Well-Being and Insomnia as Residuals of Resolved Medical Conditions: Survey in the Italian Population. International Journal of Environmental Research and Public Health. 2024; 21(2):129. https://doi.org/10.3390/ijerph21020129
Chicago/Turabian StyleMenicucci, Danilo, Luca Bastiani, Eleonora Malloggi, Francesca Denoth, Angelo Gemignani, and Sabrina Molinaro. 2024. "Impaired Well-Being and Insomnia as Residuals of Resolved Medical Conditions: Survey in the Italian Population" International Journal of Environmental Research and Public Health 21, no. 2: 129. https://doi.org/10.3390/ijerph21020129
APA StyleMenicucci, D., Bastiani, L., Malloggi, E., Denoth, F., Gemignani, A., & Molinaro, S. (2024). Impaired Well-Being and Insomnia as Residuals of Resolved Medical Conditions: Survey in the Italian Population. International Journal of Environmental Research and Public Health, 21(2), 129. https://doi.org/10.3390/ijerph21020129







