A Review of Wastewater-Based Epidemiology for the SARS-CoV-2 Virus in Rural, Remote, and Resource-Constrained Settings Internationally: Insights for Implementation, Research, and Policy for First Nations in Canada
Abstract
1. Introduction
2. Methods
2.1. Rapid Review
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Analysis
3. Results
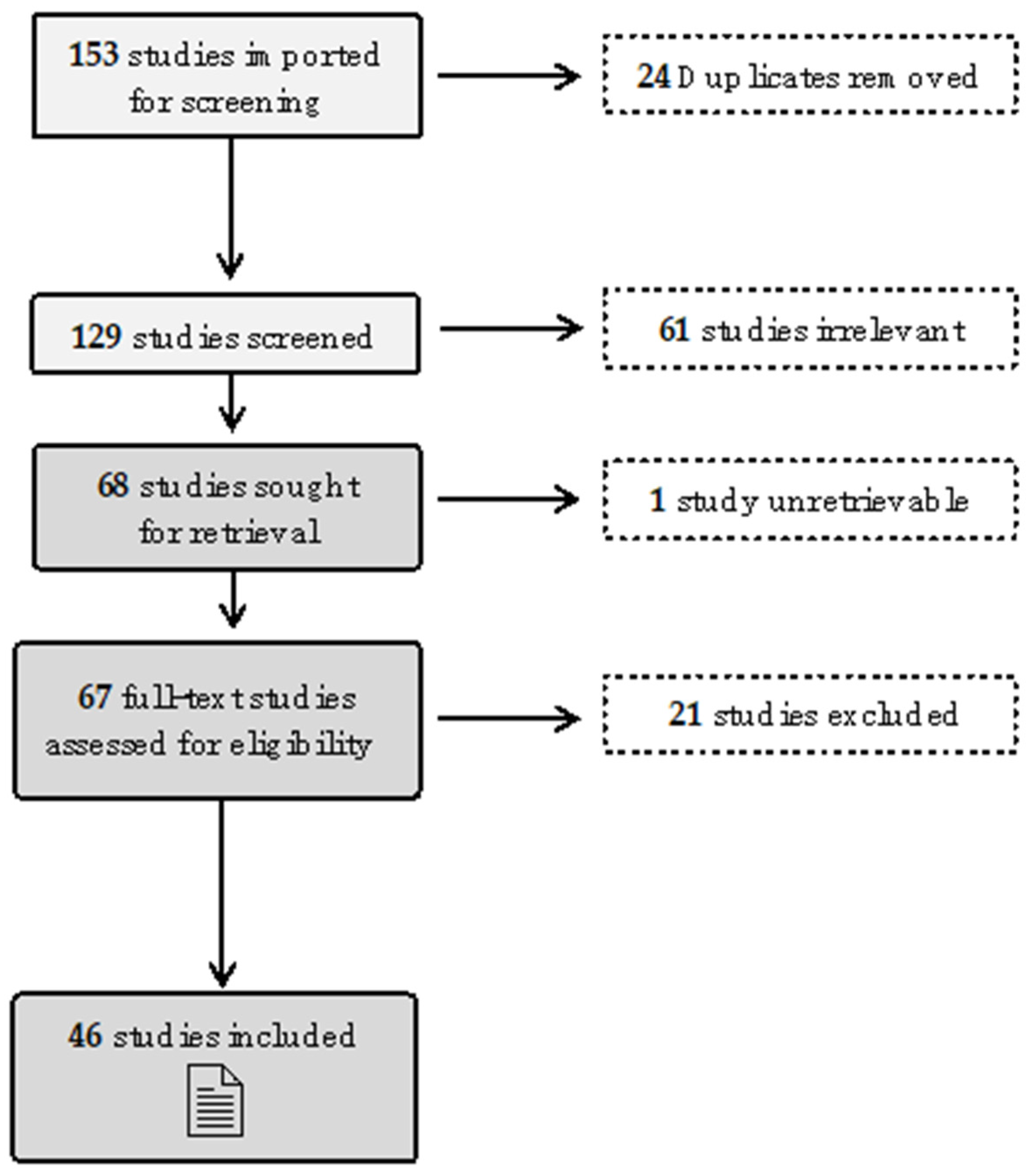
3.1. Rapid Review Literature Analysis Results
3.2. Sampling Protocols and Sampling Technology
3.2.1. Sampling Technology
3.2.2. Distribution System Characteristics
3.2.3. Sampling Frequency
3.3. Laboratory Analysis Protocols
4. Discussion
4.1. Perceived Benefits of WBE for Rural, Remote and Resource-Constrained Communities
Data Exchange, Governance and Sovereignty
4.2. Logistical Considerations and Challenges
4.2.1. Sampler Costs, Precision, and Operation
4.2.2. Community Wastewater Infrastructure
4.2.3. Access to Laboratories
4.3. Equity-Based Implications
Socio-Political and Jurisdictional Barriers
5. Conclusions
5.1. Limitations
5.2. Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Appendix A
| First Author (Year) | Article Type | Target | Setting Category | Area(s) of Focus |
|---|---|---|---|---|
| Acheampong [15] (2023) | Single Study | SARS-Cov-2 Virus | Low Income and/or Resource-constrained Setting(s); Rural and/or Remote Setting(s) | Nupur, India |
| Adhikari [30] (2022) | Literature Review | SARS-Cov-2 Virus, illicit and licit substance use, and the potential to detect various biomarkers | Low Income and/or Resource-constrained Setting(s) | Various |
| Ali [5] (2022) | Single Study | SARS-Cov-2 Virus | Low Income and/or Resource-constrained Setting(s) | Addis Ababa, Ethiopia |
| Barbosa [6] (2022) | Single Study | SARS-CoV-2 Virus | Low Income and/or Resource-constrained Setting(s) | Sao Paulo, Brazil |
| Barnes [16] (2023) | Single Study | SARS-CoV-2 Virus | Low Income and/or Resource-constrained Setting(s) | Blantyre and Lilongwe, Malawi |
| Basu [43] (2022) | Single Study | SARS-CoV-2 Virus | Low Income and/or Resource-constrained Setting(s) | Bengaluru (Bangalore), India |
| Bivins [34] (2022) | Literature Review | SARS-Cov-2 Virus | Low Income and/or Resource-constrained Setting(s) | Various |
| Belmonte-Lopes [17] (2023) | Single Study | SARS-Cov-2 Virus | Low Income and/or Re-source-constrained Setting(s) | Curitiba, Southern Brazil |
| Cancela [18] (2023) | Single Study | SARS-Cov-2 Virus | Low Income and/or Re-source-constrained Setting(s) | Montevideo, Salto, Rivera, Castillos, and Melo, Uruguay |
| Cohen [19] (2023) | Literature Review | SARS-CoV-2 Virus, Norovirus GII, and Pepper mild mottle virus | Rural and/or Remote Setting(s); Low Income and/or Re-source-constrained Setting(s) | Various areas of the United States of America |
| D’Aoust [11](2021) | Single Study | SARS-Cov-2 Virus | Rural and/or Remote Setting(s) | A small sewered community (less than 5000 inhabitants) in Eastern Ontario |
| Daigle [37] (2022) | Single Study | SARS-Cov-2 Virus | Rural and/or Remote Setting(s) | 5 communities in the Northwest Territories (Yellowknife, Hay River, Inuvik, Fort Smith, and Fort Simpson) |
| de Araujo [35] (2021) | Literature Review | SARS-CoV-2 Virus | Low Income and/or Resource-constrained Setting(s) | Various |
| Banadaki [20] (2024) | Single Study | SARS-CoV-2 Virus | Low Income and/or Resource-constrained Setting(s) | 9 WWTPs in eastern Kentucky, USA |
| de Freitas Bueno [36] (2022) | Single Study | SARS-Cov-2 Virus | Low Income and/or Resource-constrained Setting(s) | Sao Paulo and Foz do Iguau, Brazil |
| Donia [7] (2021) | Literature Review | SARS-Cov-2 Virus | Low Income and/or Resource-constrained Setting(s) | Various |
| Driver [32] (2022) | Single Study | Licit and illicit substances | First Nations/Native American Reservation(s) | Undisclosed reservation located in the United States (population > 8000 individuals) |
| Dzinamarira [28] (2022) | Literature Review | SARS-CoV-2 Virus | Low Income and/or Resource-constrained Setting(s) | Africa (various locales) |
| Fongaro [45] (2021) | Single Study | SARS-Cov-2 Virus | Low Income and/or Resource-constrained Setting(s) | Minas Gerais, Brazil |
| Gonçalves [1] (2022) | Literature Review | SARS-CoV-2 Virus | Low Income and/or Resource-constrained Setting(s) | Various |
| Gwenzi [8] (2022) | Literature Review | SARS-Cov-2 Virus | Low Income and/or Resource-constrained Setting(s) | Various |
| Hassard [21] (2023) | Literature Review | SARS-CoV-2 Virus, Influenza, and the potential to detect other infectious diseases | Low Income and/or Resource-constrained Setting(s) | Various |
| Holm [22] (2023a) | Literature Review | SARS-CoV-2 Virus | Low Income and/or Resource-constrained Setting(s); Rural and/or Remote Setting(s) | South Africa, United States of America, |
| Holm [23] (2023b) | Single Study | SARS-CoV-2 Virus | Low Income and/or Re-source-constrained Setting(s) | Louisville, Kentucky; Houston, Texas |
| Hrudey [9] (2022) | Policy Brief | SARS-CoV-2 Virus | Various, including Rural and/or Remote Setting(s) | Canada |
| Jakariya [46] (2022) | Single Study | SARS-Cov-2 Virus | Low Income and/or Resource-constrained Setting(s) | Bangladesh |
| Kolarevic [44] (2022) | Single Study | SARS-Cov-2 Virus | Low Income and/or Resource-constrained Setting(s) | Belgrade, Russia (The Sava River, and the Danube River) |
| Lee [10] (2022) | Single Study | SARS-Cov-2 Virus | Rural and/or Remote Setting(s) | Lincoln Parish, Louisiana (City of Ruston and City of Grambling) |
| Meadows [24] (2023) | Single Study | SARS-Cov-2 Virus | Rural and/or Remote Setting(s) | Various rural communities in Idaho, United States of America |
| Medina [11] (2022) | Literature Review | SARS-Cov-2 Virus | Low Income and/or Resource-constrained Setting(s); Rural and/or Remote Setting(s) | Disadvantaged Communities (DAC) in California |
| Murni [49] (2022) | Single Study | SARS-Cov-2 Virus | Low Income and/or Resource-constrained Setting(s) | Special Region of Yogyakarta Province, Indonesia |
| Oertel [25] (2023) | Single Study | Illicit substances | Rural and/or Remote Setting(s) | 15 German WWTPs of varied sized, including those serving rural areas. |
| Otero [47] (2022) | Single Study | SARS-CoV-2 Virus | Low Income and/or Resource-constrained Setting(s) | Davao City, Philippines |
| Panchal [13] (2021) | Literature Review | SARS-Cov-2 Virus | Low Income and/or Resource-constrained Setting(s) | Various (India, Africa, Bangladesh, Afghanistan, Nepal, Myanmar and Indonesia) |
| Pandey [14] (2021) | Literature Review | SARS-CoV-2 Virus | Low Income and/or Resource-constrained Setting(s) | Developing countries (non-specified) |
| Rojas-Bonilla [31] (2021) | Single Study | Polioviruses | Low Income and/or Resource-constrained Setting(s) | Las Mendozas (La Chorrera), Villa Real (La Chorrera), David (Chiriquí), Las Lomas (Chiriquí), Nuevo Tocumen (Panama City), Panama |
| Salvo [53] (2021) | Single Study | SARS-Cov-2 Virus | Low Income and/or Resource-constrained Setting(s) | Salto, Uruguay |
| Sangsanont [12] (2022) | Single Study | SARS-Cov-2 Virus | Low Income and/or Resource-constrained Setting(s) | Bangkok, Thailand |
| Stockdale [26] (2023) | Single Study | SARS-Cov-2 Virus | Rural and/or Remote Setting (s) | Nagpur district, Central India |
| Street [56] (2020) | Literature Review | SARS-CoV-2 Virus | Low Income and/or Resource-constrained Setting(s) | Africa (sub-Saharan) |
| Tandukar [33] (2022) | Single Study | SARS-CoV-2 Virus | Low Income and/or Resource-constrained Setting(s) | Kathmandu Valley, Nepal |
| Toledo [55] (2022) | Single Study | SARS-CoV-2 Virus | Rural and/or Remote Setting(s) | Northern New England |
| Wardi [27] (2024) | Single Study | SARS-CoV-2 Virus, Influenza A and B, and RSV | Low Income and/or Resource-constrained Setting(s) | Agadir and Inezgane, Morocco |
| Wehrendt [52] (2021) | Single Study | SARS-Cov-2 Virus | Low Income and/or Resource-constrained Setting(s) | Buenos Aires Metropolitan Area, Argentina |
| Wettstone [62] (2023) | Single Study | SARS-Cov-2 Virus | Low Income and/or Re-source-constrained Setting(s) | Dhaka, Bangladesh |
| Zhu [50] (2023) | Single Study | Zika Virus | Low Income and/or Resource-constrained Setting(s) | Salvador, Bahia, Brazil |
References
- Gonçalves, J.; Torres-Franco, A.; Rodriguéz, E.; Diaz, I.; Koritnik, T.; da Silva, P.G.; Mesquita, J.R.; Trkov, M.; Paragi, M.; Muñoz, R.; et al. Centralized and decentralized wastewater-based epidemiology to infer COVID-19 transmission—A brief review. One Health 2022, 15, 100405. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Infectious Diseases. (2023, February 22). PHAC Wastewater Surveillance Program for COVID-19. Available online: https://nccid.ca/wastewater-surveillance-for-covid-19/ (accessed on 27 December 2023).
- Public Health Agency of Canada. Current Federal, Provincial, and Territorial Wastewater Surveillance Networks. Available online: https://nccid.ca/wp-content/uploads/sites/2/2024/07/WW_Map_June2024EN.pdf (accessed on 27 December 2022).
- Hrudey, S.E.; Silva, D.S.; Shelley, J.; Pons, W.; Isaac-Renton, J.; Chik, A.H.-S.; Conant, B. Ethics Guidance for Environmental Scientists Engaged in Surveillance of Wastewater for SARS-CoV-2. Environ. Sci. Technol. 2021, 55, 8484–8491. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Gudina, E.K.; Gize, A.; Aliy, A.; Adankie, B.T.; Tsegaye, W.; Hundie, G.B.; Muleta, M.B.; Chibssa, T.R.; Belaineh, R.; et al. Community Wastewater-Based Surveillance Can Be a Cost-Effective Approach to Track COVID-19 Outbreak in Low-Resource Settings: Feasibility Assessment for Ethiopia Context. Int. J. Environ. Res. Public Health 2022, 19, 8515. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.R.F.; Garcia, S.C.; Bruni, A.d.C.; Machado, F.S.; de Oliveira, R.X.; Dropa, M.; da Costa, A.C.; Leal, E.; Brandão, C.J.; da Silva, R.L.O.; et al. One-year surveillance of SARS-CoV-2 in wastewater from vulnerable urban communities in metropolitan São Paulo, Brazil. J. Water Health 2022, 20, 471–490. [Google Scholar] [CrossRef]
- Donia, A.; Hassan, S.-U.; Zhang, X.; Al-Madboly, L.; Bokhari, H. COVID-19 Crisis Creates Opportunity towards Global Monitoring & Surveillance. Pathogens 2021, 10, 256. [Google Scholar] [CrossRef]
- Gwenzi, W. Wastewater, waste, and water-based epidemiology (WWW-BE): A novel hypothesis and decision-support tool to unravel COVID-19 in low-income settings? Sci. Total Environ. 2022, 806, 150680. [Google Scholar] [CrossRef]
- Hrudey, S.E.; Bischel, H.N.; Charrois, J.; Chik, A.H.; Conant, B.; Delatolla, R.; Dorner, S.; Graber, T.E.; Hubert, C.; Isaac-Renton, J.; et al. Wastewater Surveillance for SARS-CoV-2 RNA in Canada; Royal Society of Canada: Ottawa, ON, Canada, 2022. [Google Scholar]
- Lee, L.; Valmond, L.; Thomas, J.; Kim, A.; Austin, P.; Foster, M.; Matthews, J.; Kim, P.; Newman, J. Wastewater surveillance in smaller college communities may aid future public health initiatives. PLoS ONE 2022, 17, e0270385. [Google Scholar] [CrossRef]
- Medina, C.Y.; Kadonsky, K.F.; Roman, F.A.; Tariqi, A.Q.; Sinclair, R.G.; D’aoust, P.M.; Delatolla, R.; Bischel, H.N.; Naughton, C.C. The need of an environmental justice approach for wastewater based epidemiology for rural and disadvantaged communities: A review in California. Curr. Opin. Environ. Sci. Health 2022, 27, 100348. [Google Scholar] [CrossRef] [PubMed]
- Sangsanont, J.; Rattanakul, S.; Kongprajug, A.; Chyerochana, N.; Sresung, M.; Sriporatana, N.; Wanlapakorn, N.; Poovorawan, Y.; Mongkolsuk, S.; Sirikanchana, K. SARS-CoV-2 RNA surveillance in large to small centralized wastewater treatment plants preceding the third COVID-19 resurgence in Bangkok, Thailand. Sci. Total Environ. 2022, 809, 151169. [Google Scholar] [CrossRef]
- Panchal, D.; Prakash, O.; Bobde, P.; Pal, S. SARS-CoV-2: Sewage surveillance as an early warning system and challenges in developing countries. Environ. Sci. Pollut. Res. 2021, 28, 22221–22240. [Google Scholar] [CrossRef]
- Pandey, D.; Verma, S.; Verma, P.; Mahanty, B.; Dutta, K.; Daverey, A.; Arunachalam, K. SARS-CoV-2 in wastewater: Challenges for developing countries. Int. J. Hyg. Environ. Health 2021, 231, 113634. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, E.; Husain, A.A.; Dudani, H.; Nayak, A.R.; Nag, A.; Meena, E.; Shrivastava, S.K.; McClure, P.; Tarr, A.W.; Crooks, C.; et al. Population infection estimation from wastewater surveillance for SARS-CoV-2 in Nagpur, India during the second pandemic wave. PLoS ONE 2023, 19, e0303529. [Google Scholar] [CrossRef]
- Barnes, K.G.; Levy, J.I.; Gauld, J.; Rigby, J.; Kanjerwa, O.; Uzzell, C.B.; Chilupsya, C.; Anscombe, C.; Tomkins-Tinch, C.; Mbeti, O.; et al. Utilizing river and wastewater as a SARS-CoV-2 surveillance tool in settings with limited formal sewage systems. Nat. Commun. 2023, 14, 7883. [Google Scholar] [CrossRef] [PubMed]
- Belmonte-Lopes, R.; Barquilha, C.E.R.; Kozak, C.; Barcellos, D.S.; Leite, B.Z.; da Costa, F.J.O.G.; Martins, W.L.; Oliveira, P.E.; Pereira, E.H.R.A.; Filho, C.R.M.; et al. 20-Month monitoring of SARS-CoV-2 in wastewater of Curitiba, in Southern Brazil. Environ. Sci. Pollut. Res. 2024, 30, 76687–76701. [Google Scholar] [CrossRef]
- Cancela, F.; Ramos, N.; Smyth, D.S.; Etchebehere, C.; Berois, M.; Rodríguez, J.; Rufo, C.; Alemán, A.; Borzacconi, L.; López, J.; et al. Wastewater surveillance of SARS-CoV-2 genomic populations on a country-wide scale through targeted sequencing. PLoS ONE 2023, 18, e0284483. [Google Scholar] [CrossRef]
- Cohen, A.; Vikesland, P.; Pruden, A.; Krometis, L.-A.; Lee, L.M.; Darling, A.; Yancey, M.; Helmick, M.; Singh, R.; Gonzalez, R.; et al. Making waves: The benefits and challenges of responsibly implementing wastewater-based surveillance for rural communities. Water Res. 2023, 250, 121095. [Google Scholar] [CrossRef]
- Banadaki, M.D.; Torabi, S.; Rockward, A.; Strike, W.D.; Noble, A.; Keck, J.W.; Berry, S.M. Simple SARS-CoV-2 concentration methods for wastewater surveillance in low resource settings. Sci. Total Environ. 2024, 912, 168782. [Google Scholar] [CrossRef]
- Hassard, F.; Bajon-Fernandez, Y.; Castro-Gutierrez, V. Wastewater-based epidemiology for surveillance of infectious diseases in healthcare settings. Curr. Opin. Infect. Dis. 2023, 36, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Holm, R.H.; Pocock, G.; Severson, M.A.; Huber, V.C.; Smith, T.; McFadden, L.M. Using wastewater to overcome health disparities among rural residents. Geoforum 2023, 144, 103816. [Google Scholar] [CrossRef]
- Holm, R.H.; Jelks, N.O.; Schneider, R.; Smith, T. Beyond COVID-19: Designing inclusive public health surveillance by including wastewater monitoring. Health Equity 2023, 7, 377–379. [Google Scholar] [CrossRef]
- Meadows, T.; Coats, E.R.; Narum, S.; Top, E.; Ridenhour, B.J.; Stalder, T. Epidemiological model can forecast COVID-19 outbreaks from wastewater-based surveillance in rural communities. MedRxiv 2024. Preprint. [Google Scholar] [CrossRef]
- Oertel, R.; Schubert, S.; Helm, B.; Mayer, R.; Dumke, R.; El-Armouche, A.; Renner, B. Drug consumption in German cities and municipalities during the COVID-19 lockdown: A wastewater analysis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Stockdale, S.R.; Blanchard, A.M.; Nayak, A.; Husain, A.; Nashine, R.; Dudani, H.; McClure, C.P.; Tarr, A.W.; Nag, A.; Meena, E.; et al. RNA-Seq of untreated wastewater to assess COVID-19 and emerging and endemic viruses for public health surveillance. Lancet Reg. Health. Southeast Asia 2023, 7, 100205. [Google Scholar] [CrossRef] [PubMed]
- Wardi, M.; Belmouden, A.; Aghrouch, M.; Lotfy, A.; Idaghdour, Y.; Lemkhente, Z. Wastewater genomic surveillance to track infectious disease-causing pathogens in low-income countries: Advantages, limitations, and perspectives. Environ. Int. 2024, 192, 109029. [Google Scholar] [CrossRef] [PubMed]
- Garritty, C.; Gartlehner, G.; Nussbaumer-Streit, B.; King, V.J.; Hamel, C.; Kamel, C.; Affengruber, L.; Stevens, A. Cochrane Rapid Reviews Methods Group Offers Evidence-Informed Guidance to Conduct Rapid Reviews. J. Clin. Epidemiol. 2021, 130, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Dobbins, M. Rapid Review Guideline. Steps for Conducting a Rapid Review; National Collaborating Centre for Methods and Tools: Hamilton, ON, Canada, 2017. [Google Scholar]
- Adhikari, S.; Halden, R.U. Opportunities and limits of wastewater-based epidemiology for tracking global health and attainment of UN sustainable development goals. Environ. Int. 2022, 163, 107217. [Google Scholar] [CrossRef]
- Rojas-Bonilla, M.; Coulliette-Salmond, A.; Belgasmi, H.; Wong, K.; Sayyad, L.; Vega, E.; Grimoldi, F.; Oberste, M.S.; Rüttimann, R. Environmental Surveillance for Risk Assessment in the Context of a Phase 2 Clinical Trial of Type 2 Novel Oral Polio Vaccine in Panama. Viruses 2021, 13, 1355. [Google Scholar] [CrossRef]
- Driver, E.M.; Bowes, D.A.; Halden, R.U.; Conroy-Ben, O. Implementing wastewater monitoring on American Indian reservations to assess community health indicators. Sci. Total Environ. 2022, 823, 153882. [Google Scholar] [CrossRef]
- Tandukar, S.; Sthapit, N.; Thakali, O.; Malla, B.; Sherchan, S.P.; Shakya, B.M.; Shrestha, L.P.; Sherchand, J.B.; Joshi, D.R.; Lama, B.; et al. Detection of SARS-CoV-2 RNA in wastewater, river water, and hospital wastewater of Nepal. Sci. Total Environ. 2022, 824, 153816. [Google Scholar] [CrossRef]
- Bivins, A.; Kaya, D.; Ahmed, W.; Brown, J.; Butler, C.; Greaves, J.; Leal, R.; Maas, K.; Rao, G.; Sherchan, S.; et al. Passive sampling to scale wastewater surveillance of infectious disease: Lessons learned from COVID-19. Sci. Total Environ. 2022, 835, 155347. [Google Scholar] [CrossRef]
- de Araujo, J.C.; Gavazza, S.; Leao, T.L.; Florencio, L.; da Silva, H.P.; Albuquerque, J.D.O.; Borges, M.A.D.L.; Alves, R.B.D.O.; Rodrigues, R.H.A.; dos Santos, E.B. SARS-CoV-2 sewage surveillance in low-income countries: Potential and challenges. J. Water Health 2021, 19, 1–19. [Google Scholar] [CrossRef]
- Bueno, R.d.F.; Claro, I.C.M.; Augusto, M.R.; Duran, A.F.A.; Camillo, L.d.M.B.; Cabral, A.D.; Sodré, F.F.; Brandão, C.C.S.; Vizzotto, C.S.; Silveira, R.; et al. Wastewater-based epidemiology: A Brazilian SARS-COV-2 surveillance experience. J. Environ. Chem. Eng. 2022, 10, 108298. [Google Scholar] [CrossRef] [PubMed]
- Daigle, J.; Racher, K.; Hazenberg, J.; Yeoman, A.; Hannah, H.; Duong, D.; Mohammed, U.; Spreitzer, D.; Gregorchuk, B.S.J.; Head, B.M.; et al. A Sensitive and Rapid Wastewater Test for SARS-COV-2 and Its Use for the Early Detection of a Cluster of Cases in a Remote Community. Appl. Environ. Microbiol. 2022, 88, e0174021. [Google Scholar] [CrossRef] [PubMed]
- Schang, C.; Crosbie, N.D.; Nolan, M.; Poon, R.; Wang, M.; Jex, A.; John, N.; Baker, L.; Scales, P.; Schmidt, J.; et al. Passive Sampling of SARS-CoV-2 for Wastewater Surveillance. Environ. Sci. Technol. 2021, 55, 10432–10441. [Google Scholar] [CrossRef] [PubMed]
- Habtewold, J.; McCarthy, D.; McBean, E.; Law, I.; Goodridge, L.; Habash, M.; Murphy, H.M. Passive sampling, a practical method for wastewater-based surveillance of SARS-CoV-2. Environ. Res. 2022, 204, 112058. [Google Scholar] [CrossRef]
- Rafiee, M.; Isazadeh, S.; Mohseni-Bandpei, A.; Mohebbi, S.R.; Jahangiri-Rad, M.; Eslami, A.; Dabiri, H.; Roostaei, K.; Tanhaei, M.; Amereh, F. Moore swab performs equal to composite and outperforms grab sampling for SARS-CoV-2 monitoring in wastewater. Sci. Total Environ. 2021, 790, 148205. [Google Scholar] [CrossRef]
- Hayes, E.K.; Sweeney, C.L.; Anderson, L.E.; Li, B.; Erjavec, G.B.; Gouthro, M.T.; Krkosek, W.H.; Stoddart, A.K.; Gagnon, G.A. A novel passive sampling approach for SARS-CoV-2 in wastewater in a Canadian province with low prevalence of COVID-19. Environ. Sci. Water Res. Technol. 2021, 7, 1576–1586. [Google Scholar] [CrossRef]
- D’Aoust, P.M.; Towhid, S.T.; Mercier, É.; Hegazy, N.; Tian, X.; Bhatnagar, K.; Zhang, Z.; Naughton, C.C.; MacKenzie, A.E.; Graber, T.E.; et al. COVID-19 wastewater surveillance in rural communities: Comparison of lagoon and pumping station samples. Sci. Total Environ. 2021, 801, 149618. [Google Scholar] [CrossRef]
- Basu, P.; Choudhury, S.; Shridhar, V.; Huilgol, P.; Roychoudhury, S.; Nandi, I.; Chaudhuri, A.; Mitra, A. Surveillance of SARS-CoV-2 RNA in open-water sewage canals contaminated with untreated wastewater in resource-constrained regions. Access Microbiol. 2022, 4, 318. [Google Scholar] [CrossRef]
- Kolarević, S.; Micsinai, A.; Szántó-Egész, R.; Lukács, A.; Kračun-Kolarević, M.; Djordjevic, A.; Vojnović-Milutinović, D.; Marić, J.J.; Kirschner, A.K.; Farnleitner, A.A.; et al. Wastewater-based epidemiology in countries with poor wastewater treatment—Epidemiological indicator function of SARS-CoV-2 RNA in surface waters. Sci. Total Environ. 2022, 843, 156964. [Google Scholar] [CrossRef]
- Fongaro, G.; Rogovski, P.; Savi, B.P.; Cadamuro, R.D.; Pereira, J.V.F.; Anna, I.H.S.; Rodrigues, I.H.; Souza, D.S.M.; Saravia, E.G.T.; Rodríguez-Lázaro, D.; et al. SARS-CoV-2 in Human Sewage and River Water from a Remote and Vulnerable Area as a Surveillance Tool in Brazil. Food Environ. Virol. 2021, 14, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Jakariya; Ahmed, F.; Islam, A.; Al Marzan, A.; Hasan, M.N.; Hossain, M.; Ahmed, T.; Hossain, A.; Reza, H.M.; Hossen, F.; et al. Wastewater-based epidemiological surveillance to monitor the prevalence of SARS-CoV-2 in developing countries with onsite sanitation facilities. Environ. Pollut. 2022, 311, 119679. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.C.B.; Murao, L.A.E.; Limen, M.A.G.; Caalim, D.R.A.; Gaite, P.L.A.; Bacus, M.G.; Acaso, J.T.; Miguel, R.M.; Corazo, K.; Knot, I.E.; et al. Multifaceted Assessment of Wastewater-Based Epidemiology for SARS-CoV-2 in Selected Urban Communities in Davao City, Philippines: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 8789. [Google Scholar] [CrossRef]
- Renata, S.; Kattouw, R.; Ahmed, W.; O’Brien, J.W.; Tscharke, B.J.; Choi, P.M.; Thomas, K.V. Wastewater-based epidemiology: Validity and usefulness of a wastewater population normalization approach in Australian and New Zealand settings. Sci. Total Environ. 2022, 828, 154329. [Google Scholar] [CrossRef]
- Murni, I.K.; Oktaria, V.; Handley, A.; McCarthy, D.T.; Donato, C.M.; Nuryastuti, T.; Supriyati, E.; Putri, D.A.D.; Sari, H.M.; Laksono, I.S.; et al. The feasibility of SARS-CoV-2 surveillance using wastewater and environmental sampling in Indonesia. PLoS ONE 2022, 17, e0274793. [Google Scholar] [CrossRef]
- Zhu, K.; Hill, C.; Muirhead, A.; Basu, M.; Brown, J.; Brinton, M.A.; Hayat, M.J.; Venegas-Vargas, C.; Reis, M.G.; Casanovas-Massana, A.; et al. Zika virus RNA persistence and recovery in water and wastewater: An approach for Zika virus surveillance in resource-constrained settings. Water Res. 2023, 241, 120116. [Google Scholar] [CrossRef]
- Mainardi, P.H.; Bidoia, E.D. Challenges and emerging perspectives of an international SARS-CoV-2 epidemiological surveillance in wastewater. Ann. Braz. Acad. Sci. 2021, 93 (Suppl. S4), e20210163. [Google Scholar] [CrossRef]
- Wehrendt, D.P.; Massó, M.G.; Machuca, A.G.; Vargas, C.V.; Barrios, M.E.; Campos, J.; Costamagna, D.; Bruzzone, L.; Cisterna, D.M.; Iglesias, N.G.; et al. A rapid and simple protocol for concentration of SARS-CoV-2 from sewage. J. Virol. Methods 2021, 297, 114272. [Google Scholar] [CrossRef]
- Salvo, M.; Moller, A.; Alvareda, E.; Gamazo, P.; Colina, R.; Victoria, M. Evaluation of low-cost viral concentration methods in wastewaters: Implications for SARS-CoV-2 pandemic surveillances. J. Virol. Methods 2021, 297, 114249. [Google Scholar] [CrossRef]
- Medema, G.; Been, F.; Heijnen, L.; Petterson, S. Implementation of environmental surveillance for SARS-CoV-2 virus to support public health decisions: Opportunities and challenges. Curr. Opin. Environ. Sci. Health 2020, 17, 49–71. [Google Scholar] [CrossRef]
- Toledo, D.M.; Robbins, A.A.; Gallagher, T.L.; Hershberger, K.C.; Barney, R.E.; Salmela, S.M.; Pilcher, D.; Cervinski, M.A.; Nerenz, R.D.; Szczepiorkowski, Z.M.; et al. Wastewater-Based SARS-CoV-2 Surveillance in Northern New England. Microbiol. Spectr. 2022, 10, e0220721. [Google Scholar] [CrossRef] [PubMed]
- Street, R.; Malema, S.; Mahlangeni, N.; Mathee, A. Wastewater surveillance for Covid-19: An African perspective. Sci. Total Environ. 2020, 743, 140719. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.M.; Corston-Pine, E.; Post, Y.; A McBean, E. Insights and Opportunities: Challenges of Canadian First Nations Drinking Water Operators. Int. Indig. Policy J. 2015, 6. [Google Scholar] [CrossRef]
- Walters, D.; Spence, N.; Kuikman, K.; Singh, B. Multi-Barrier Protection of Drinking Water Systems in Ontario: A Comparison of First Nation and Non-First Nation Communities. Int. Indig. Policy J. 2012, 3, 8. [Google Scholar] [CrossRef]
- Lane, K.; Fuller, M.; Stanhope, T.; Stoddart, A. Exploring the Use of a Sanitation Safety Plan Framework to Identify Key Hazards in First Nations Wastewater Systems. Water 2021, 13, 1454. [Google Scholar] [CrossRef]
- Neegan Burnside Ltd. National Assessment of First Nations Water and Wastewater Systems: National Regional Roll-Up Report: Final; Department of Indian and Northern Affairs Canada: Orangeville, ON, Canada, 2011. [Google Scholar]
- Takeda, T.; Kitajima, M.; Abeynayaka, A.; Huong, N.T.T.; Dinh, N.Q.; Sirikanchana, K.; Navia, M.; Sam, A.A.; Tsudaka, M.; Setiadi, T.; et al. Governance of wastewater surveillance systems to minimize the impact of COVID-19 and future epidemics: Cases across Asia-Pacific. In Environmental Resilience and Transformation in Times of COVID-19; Elsevier: Amsterdam, The Netherlands, 2021; pp. 239–244. [Google Scholar] [CrossRef]
- Wettstone, E.G.; Islam, M.O.; Hughlett, L.; Reagen, C.; Shirin, T.; Rahman, M.; Hosan, K.; Hoque, M.R.; Brennhofer, S.A.; Rogawski McQuade, E.T.; et al. Interactive SARS-CoV-2 dashboard for real-time geospatial visualisation of sewage and clinical surveillance data from Dhaka, Bangladesh: A tool for public health situational awareness. BMJ Glob. Health 2023, 8, e010928. [Google Scholar] [CrossRef]
- Hyde, P. Alberta’s Largest Universities Team up to Track Evidence of COVID-19 in Wastewater of 3.2M People across Alberta; University of Calgary: Calgary, AB, Canada, 2021; Available online: https://ucalgary.ca/news/albertas-largest-research-universities-team-track-evidence-covid-19-wastewater-32m-people-across (accessed on 27 December 2022).
- Mashford-Pringle, A.; Skura, C.; Stutz, S.; Yohathasan, T. What We Heard: Indigenous People and COVID-Supplementary Report for the Chief Public Health Officer of Canada’s Report on the State of Public Health in Canada; Waake-Biness-Bryce Institute for Indigenous Health, Dalla Lana School of Public Health, University of Toronto: Toronto, ON, Canada, 2021; Available online: https://www.canada.ca/content/dam/phac-aspc/documents/corporate/publications/chief-public-health-officer-reports-state-public-health-canada/from-risk-resilience-equity-approach-covid-19/indigenous-peoples-covid-19-report/cpho-wwh-report-en.pdf (accessed on 27 December 2022).
- Greenwood, M.; de Leeuw, S.; Ngaroimata Fraser, T. Challenges in health equity for Indigenous peoples in Canada. Lancet 2020, 390, 1717–1735. [Google Scholar] [CrossRef]
| Setting terms | First Nation * | OR “reservation *” OR “Indigenous” OR “American Native” |
| Remote and/or Rural | OR “isolated” OR “country *” | |
| Resource-constrained | OR “low income” OR “refugee” OR “medically underserve*” OR “underserve *” OR “At risk” | |
| Method terms | Wastewater-based epidemiology | OR “WBE” OR “Wastewater testing” OR “wastewater-based monitoring” OR “wastewater surveillance” OR “WWS” OR “Wastewater analysis” OR “WWA” OR “WWW-BE” OR “Sewage” |
| Topic terms | COVID-19 | OR “SARS-CoV-2” OR “Hepatitis *” OR “HVC” OR “Polio” OR “epidemic” OR “pandemic” OR “infectious disease” OR “communicable disease” OR “disease transmission” OR “Infectious disease” |
| Procedure | Considerations |
|---|---|
| Process samples for SARS-CoV-2 RNA concentration Key Techniques/Methods:
|
|
| Procedure | Considerations |
|---|---|
| RNA Extraction Commonly used kits:
Other kits:
|
|
| Procedure | Considerations |
|---|---|
| Detection and Quantification Techniques Standard techniques: The detection and quantification of the SARS-COV-2 virus and other infectious diseases are performed in the lab using the same Polymerase Chain Reaction (PCR) based techniques that are standard in clinical testing. This includes PCR and RT-PCR for basic detection, as well as quantitative PCR (qPCR) and reverse transcription qPCR (RT-qPCR) for quantification, which amplify specific viral genetic material to determine its presence and concentration. Kits used for PCR-based tests:
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annan, J.; Henderson, R.; Gray, M.; Clark, R.G.; Sarin, C.; Black, K. A Review of Wastewater-Based Epidemiology for the SARS-CoV-2 Virus in Rural, Remote, and Resource-Constrained Settings Internationally: Insights for Implementation, Research, and Policy for First Nations in Canada. Int. J. Environ. Res. Public Health 2024, 21, 1429. https://doi.org/10.3390/ijerph21111429
Annan J, Henderson R, Gray M, Clark RG, Sarin C, Black K. A Review of Wastewater-Based Epidemiology for the SARS-CoV-2 Virus in Rural, Remote, and Resource-Constrained Settings Internationally: Insights for Implementation, Research, and Policy for First Nations in Canada. International Journal of Environmental Research and Public Health. 2024; 21(11):1429. https://doi.org/10.3390/ijerph21111429
Chicago/Turabian StyleAnnan, Jessica, Rita Henderson, Mandi Gray, Rhonda Gail Clark, Chris Sarin, and Kerry Black. 2024. "A Review of Wastewater-Based Epidemiology for the SARS-CoV-2 Virus in Rural, Remote, and Resource-Constrained Settings Internationally: Insights for Implementation, Research, and Policy for First Nations in Canada" International Journal of Environmental Research and Public Health 21, no. 11: 1429. https://doi.org/10.3390/ijerph21111429
APA StyleAnnan, J., Henderson, R., Gray, M., Clark, R. G., Sarin, C., & Black, K. (2024). A Review of Wastewater-Based Epidemiology for the SARS-CoV-2 Virus in Rural, Remote, and Resource-Constrained Settings Internationally: Insights for Implementation, Research, and Policy for First Nations in Canada. International Journal of Environmental Research and Public Health, 21(11), 1429. https://doi.org/10.3390/ijerph21111429






