Illicit Online Pharmacies: A Scoping Review
Abstract
1. Introduction
2. Methods
2.1. Identifying Research Questions
- RQ1: What are the themes of previous research on illicit online pharmacies?
- RQ1a: What are the themes of previous research on illicit online pharmacies by year?
- RQ1b: What are the themes of previous research on illicit online pharmacies in terms of data collection methods?
- RQ2: How has previous research on illicit online pharmacies changed over time?
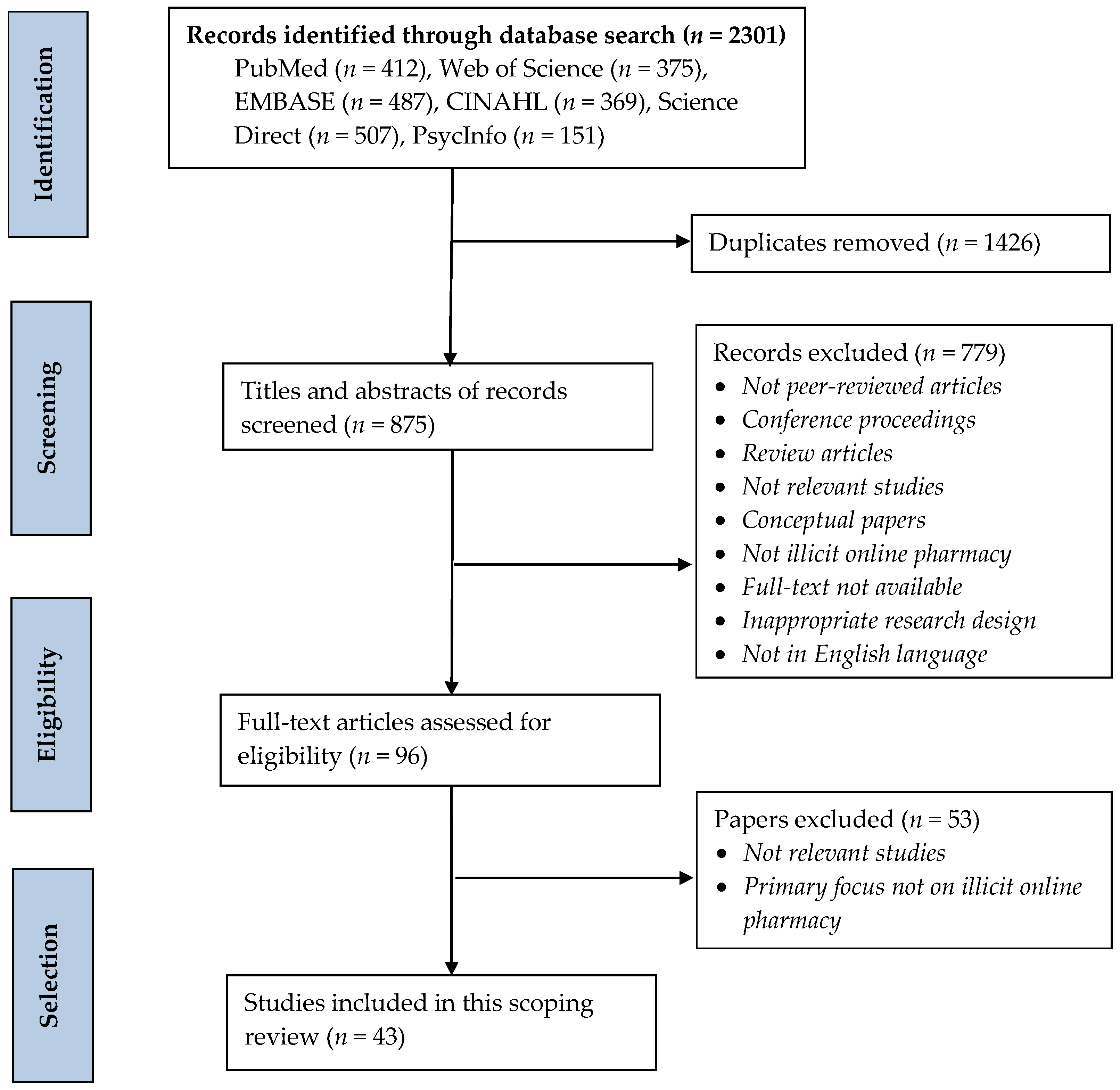
2.2. Identifying Relevant Studies
2.3. Study Selection
2.4. Charting the Data
2.5. Collating, Summarizing and Reporting Results
3. Results
3.1. Characteristics of Studies Included in This Scoping Review
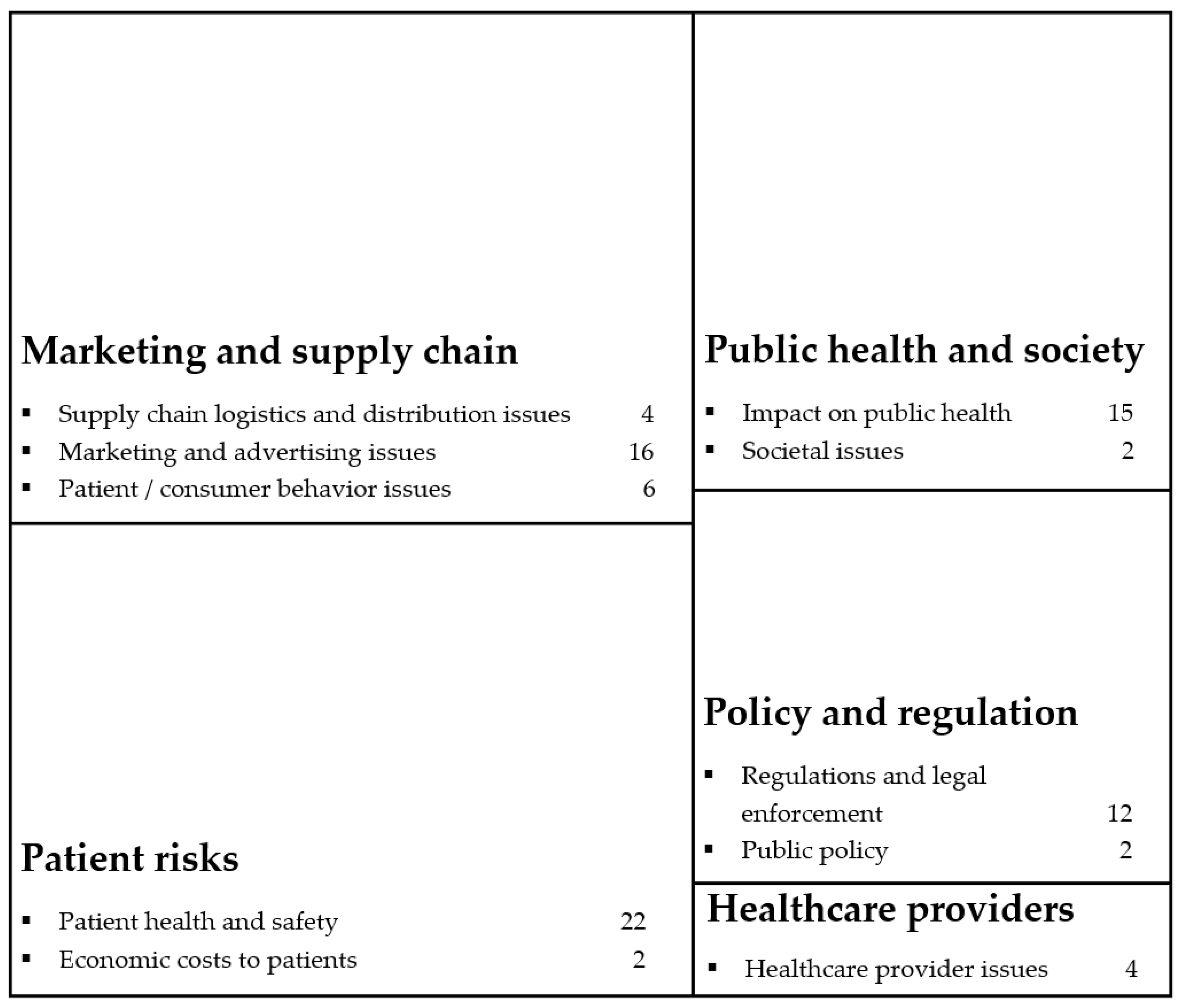
3.2. What Are the Themes of Previous Research on Illicit Online Pharmacies?
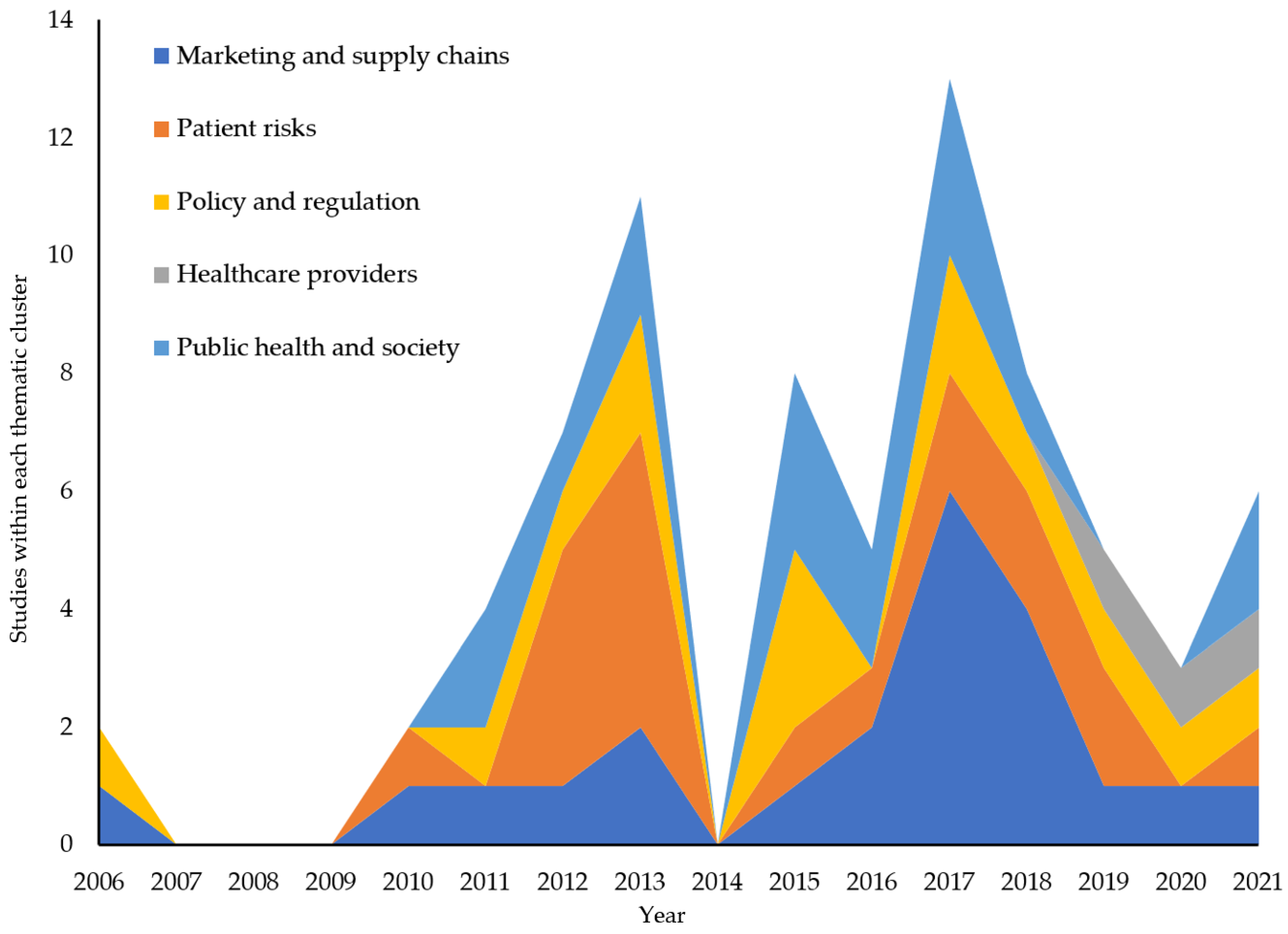
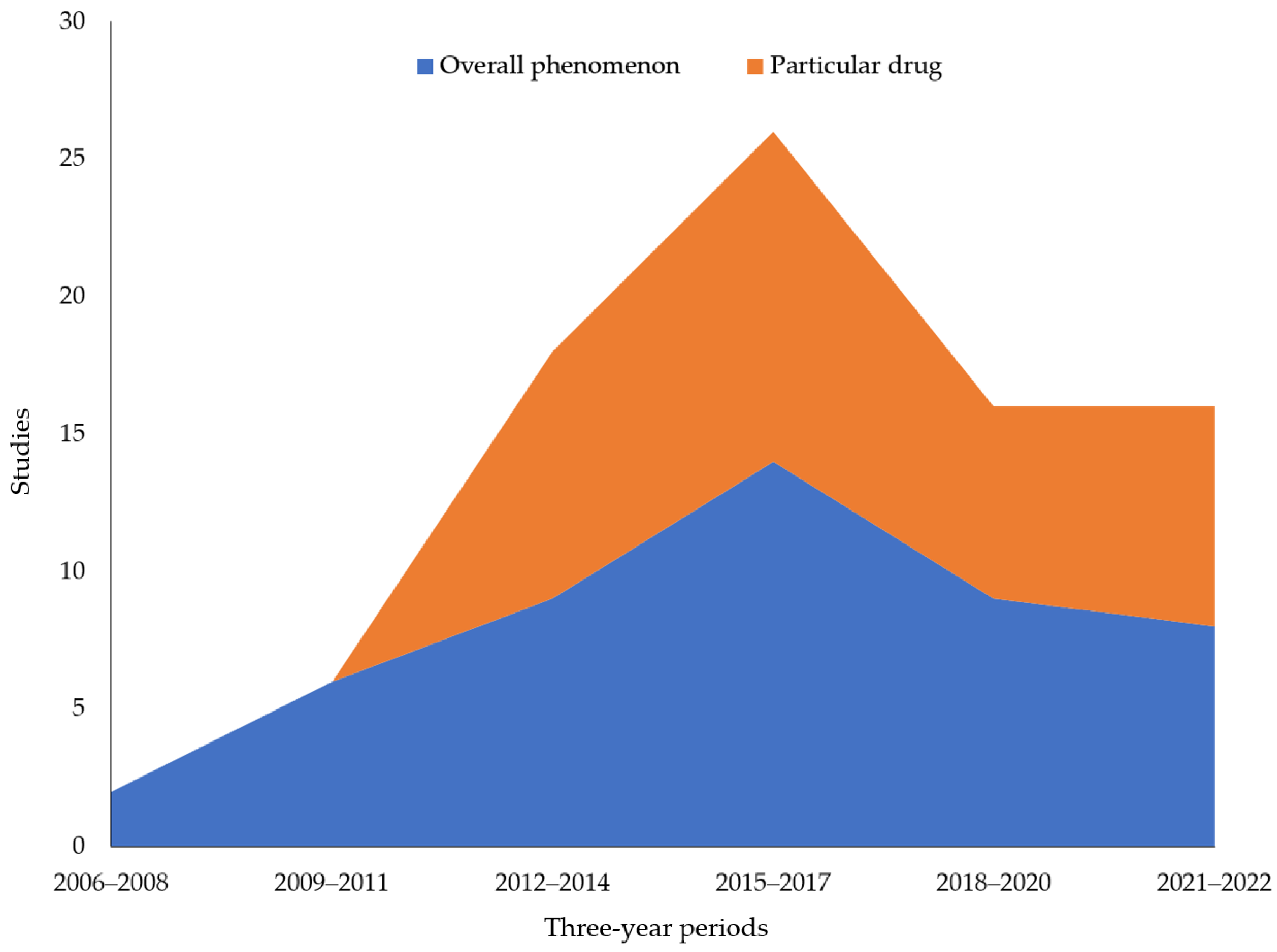
3.3. What Are the Themes of Previous Research on Illicit Online Pharmacies by Year?
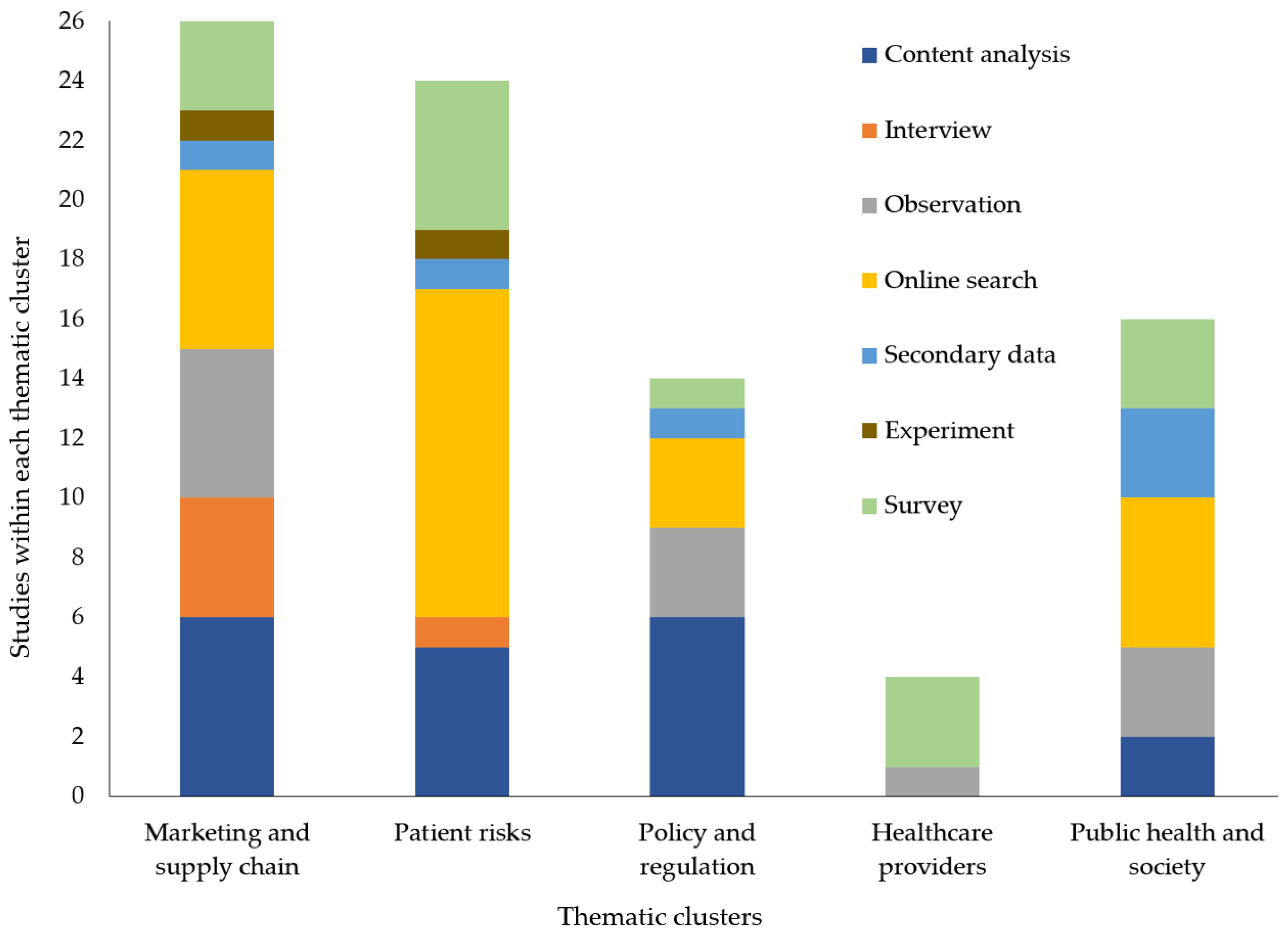
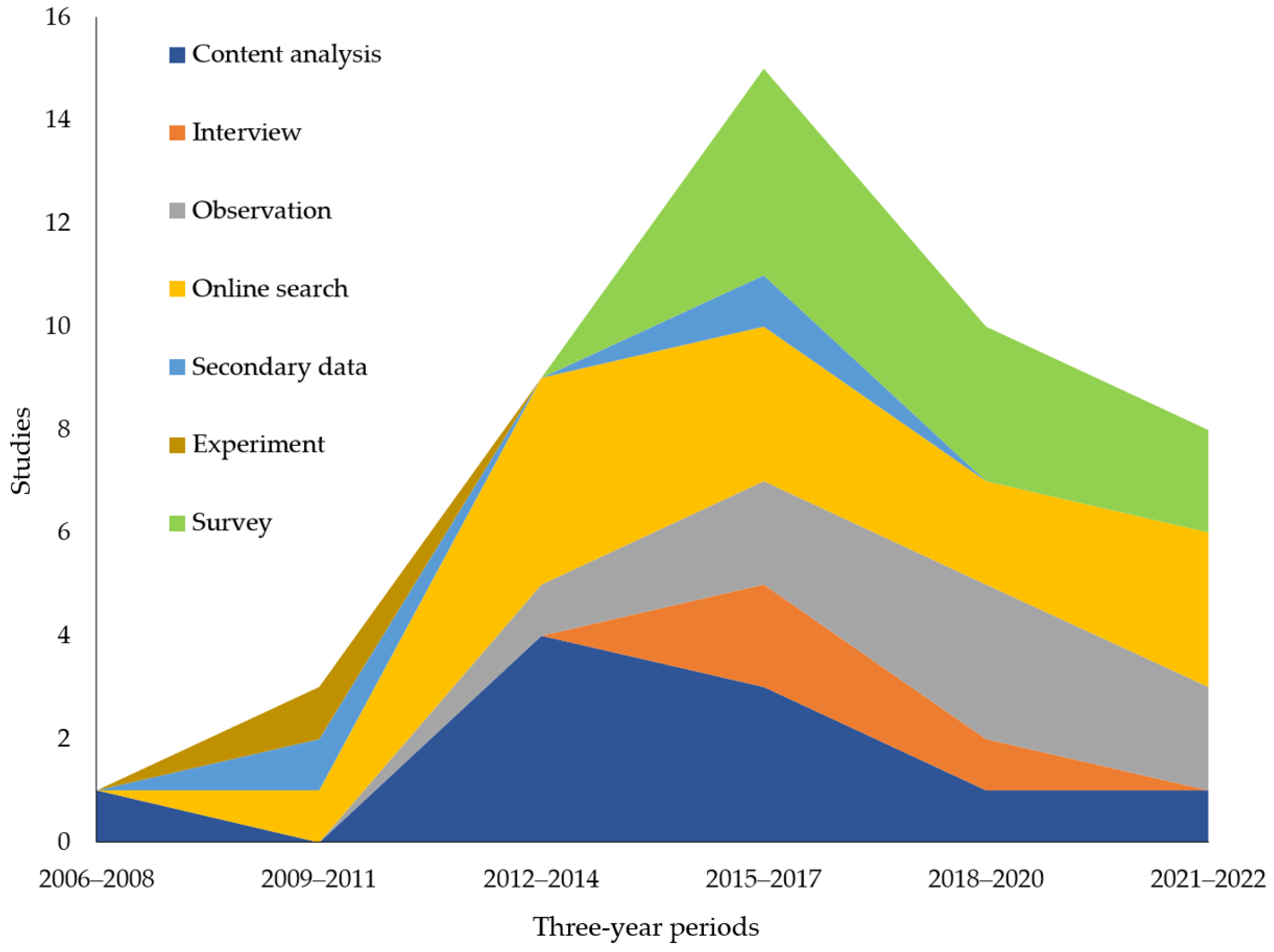
3.4. What Are the Themes of Previous Research on Illicit Online Pharmacies in Terms of Data Collection Method?
3.5. How Has Previous Research on Illicit Online Pharmacies Changed over Time?
4. General Discussion
5. Directions for Future Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Market Insights. E-Pharmacy Market Size by Product Type (OTC Products, Prescription Medicine), Industry Analysis Report, Regional Outlook, Growth Potential, COVID-19 Impact Analysis, Competitive Market Share & Forecast, 2022–2028. 2022. Available online: https://www.gminsights.com/industry-analysis/e-pharmacy-market (accessed on 12 August 2022).
- Arizton Advisory & Intelligence. Online Pharmacy Market—Industry Outlook & Forecast 2021–2026. 2021. Available online: https://www.arizton.com/market-reports/united-states-online-pharmacy-market (accessed on 19 February 2023).
- Mackey, T.K.; Nayyar, G. Digital danger: A review of the global public health, patient safety and cybersecurity threats posed by illicit online pharmacies. Br. Med. Bull. 2016, 118, 110–126. [Google Scholar] [CrossRef]
- Food and Drug Administration. Internet Pharmacy Warning Letters. 2022. Available online: https://www.fda.gov/drugs/drug-supply-chain-integrity/internet-pharmacy-warning-letters (accessed on 18 August 2022).
- Leontiadis, N.; Hutchings, A. Scripting the crime commission process in the illicit online prescription drug trade. J. Cybersecur. 2015, 1, 81–92. [Google Scholar] [CrossRef]
- Penley, B.; Minshew, L.; Chen, H.H.; Eckel, S.; Ozawa, S. Accessibility of low-cost insulin from illegitimate internet pharmacies: Cross-sectional study. J. Med. Internet Res. 2022, 24, e25855. [Google Scholar] [CrossRef]
- Dekker, A.H. What is being done to address the new drug epidemic? J. Osteopath. Med. 2007, 107, E21–E26. [Google Scholar]
- Fittler, A.; Bosze, G.; Botz, L. Evaluating aspects of online medication safety in long-term follow-up of 136 internet pharmacies: Illegal rogue online pharmacies flourish and are long-lived. J. Med. Internet Res. 2013, 15, e199. [Google Scholar] [CrossRef]
- Peltier-Rivest, D.; Pacini, C. Detecting counterfeit pharmaceutical drugs: A multi-stakeholder forensic accounting strategy. J. Financ. Crime 2019, 26, 1027–1047. [Google Scholar] [CrossRef]
- Limbu, Y.B.; Huhmann, B.A. Online but Unlawful Sales of Unapproved and Misbranded Prescription Drugs: Internet Pharmacy Compliance with Food and Drug Administration Warning Letters. J. Consum. Aff. 2023. [Google Scholar] [CrossRef]
- Alliance for Safe Online Pharmacies. Online Pharmacy Behavior Perception Survey Results. 2017. Available online: https://buysaferx.pharmacy/public-awareness-campaigns/drug-importation/factsheets/online-pharmacy-consumer-behavior-and-perception-survey/ (accessed on 19 February 2023).
- Fittler, A.; Adeniye, L.; Katz, Z.; Bella, R. Effect of infodemic regarding the illegal sale of medications on the Internet: Evaluation of demand and online availability of ivermectin during the COVID-19 pandemic. Int. J. Environ. Res. Public Health 2021, 18, 7475. [Google Scholar] [CrossRef]
- Vida, R.G.; Fittler, A.; Mikulka, I.; Ábrahám, E.; Sándor, V.; Kilár, F.; Botz, L. Availability and quality of illegitimate somatropin products obtained from the Internet. Int. J. Clin. Pharm. 2017, 39, 78–87. [Google Scholar] [CrossRef]
- Fincham, J.E. Negative consequences of the widespread and inappropriate easy access to purchasing prescription medications on the Internet. Am. Health Drug Benefits 2021, 14, 22–28. [Google Scholar]
- Orizio, G.; Merla, A.; Schulz, P.J.; Gelatti, U. Quality of online pharmacies and websites selling prescription drugs: A systematic review. J. Med. Internet Res. 2011, 13, e74. [Google Scholar] [CrossRef]
- Mackey, T.K.; Liang, B.A. Global reach of direct-to-consumer advertising using social media for illicit online drug sales. J. Med. Internet Res. 2013, 15, e105. [Google Scholar] [CrossRef]
- Limbu, Y.B.; Huhmann, B.A. Ethical issues in pharmaceutical marketing: A systematic review and future research agenda. J. Glob. Mark. 2022, 35, 1–20. [Google Scholar] [CrossRef]
- Harden, A. Mixed-methods systematic reviews: Integrating quantitative and qualitative findings. Focus 2010, 25, 1–8. [Google Scholar]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for Conducting Systematic Scoping Reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Khalil, H.; Peters, M.; Godfrey, C.M.; McInerney, P.; Soares, C.B.; Parker, D. An evidence-based approach to scoping reviews. Worldviews Evid. Based Nurs. 2016, 13, 118–123. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Forman, R.F.; Block, L.G. The marketing of opioid medications without prescription over the Internet. J. Public Policy Mark. 2006, 25, 133–146. [Google Scholar] [CrossRef]
- Liang, B.A.; Mackey, T.K.; Archer-Hayes, A.N.; Shinn, L.M. Illicit online marketing of lorcaserin before DEA scheduling. Obesity 2013, 21, 861–864. [Google Scholar] [CrossRef]
- Ashames, A.; Bhandare, R.; AlAbdin, S.Z.; Alhalabi, T.; Jassem, F. Public perception toward e-commerce of medicines and comparative pharmaceutical quality assessment study of two different products of furosemide tablets from community and illicit online pharmacies. J. Pharm. Bioallied Sci. 2019, 11, 284. [Google Scholar] [CrossRef]
- Liang, B.A.; Mackey, T.K.; Lovett, K.M. Suspect online sellers and contraceptive access. Contraception 2012, 86, 551–556. [Google Scholar] [CrossRef]
- Bachhuber, M.A.; Cunningham, C.O. Availability of buprenorphine on the Internet for purchase without a prescription. Drug Alcohol Depend. 2013, 130, 238–240. [Google Scholar] [CrossRef]
- Mackey, T.K.; Aung, P.; Liang, B.A. Illicit Internet availability of drugs subject to recall and patient safety consequences. Int. J. Clin. Pharm. 2015, 37, 1076–1085. [Google Scholar] [CrossRef]
- Liang, B.A.; Mackey, T.K.; Lovett, K.M. Illegal “no prescription” internet access to narrow therapeutic index drugs. Clin. Ther. 2013, 35, 694–700. [Google Scholar] [CrossRef]
- Liang, B.A.; Mackey, T.K. Vaccine shortages and suspect online pharmacy sellers. Vaccine 2012, 30, 105–108. [Google Scholar] [CrossRef]
- Koenraadt, R.; van de Ven, K. The Internet and lifestyle drugs: An analysis of demographic characteristics, methods, and motives of online purchasers of illicit lifestyle drugs in the Netherlands. Drugs Educ. Prev. Policy 2018, 25, 345–355. [Google Scholar] [CrossRef]
- Fittler, A.; Vida, R.G.; Káplár, M.; Botz, L. Consumers turning to the internet pharmacy market: Cross-sectional study on the frequency and attitudes of Hungarian patients purchasing medications online. J. Med. Internet Res. 2018, 20, e11115. [Google Scholar] [CrossRef]
- Gaudiano, M.C.; Manna, L.; Rodomonte, A.L.; Bartolomei, M.; Bertocchi, P.; Gallinella, B.; Antoniella, E.; Muleri, N.; Civitelli, G.; Alimonti, S.; et al. A survey on illegal and counterfeit medicines for the treatment of erectile dysfunctions in Italy. J. Sex. Med. 2012, 9, 2130–2137. [Google Scholar] [CrossRef]
- Monteith, S.; Glenn, T.; Bauer, R.; Conell, J.; Bauer, M. Availability of prescription drugs for bipolar disorder at online pharmacies. J. Affect. Disord. 2016, 193, 59–65. [Google Scholar] [CrossRef]
- Ozawa, S.; Billings, J.; Sun, Y.; Yu, S.; Penley, B. COVID-19 Treatments Sold Online Without Prescription Requirements in the United States: Cross-sectional Study Evaluating Availability, Safety and Marketing of Medications. J. Med. Internet Res. 2022, 24, e27704. [Google Scholar] [CrossRef]
- Sun, Y.; Hendrix, A.; Muluneh, B.; Ozawa, S. Online Pharmacy Accessibility of Imatinib, An Oral Chemotherapy Medication. J. Natl. Compr. Cancer Netw. 2022, 20, 808–814. [Google Scholar] [CrossRef]
- Campbell, N.; Clark, J.P.; Stecher, V.J.; Goldstein, I. Internet-ordered Viagra (sildenafil citrate) is rarely genuine. J. Sex. Med. 2012, 9, 2943–2951. [Google Scholar] [CrossRef]
- Ivanitskaya, L.; Brookins-Fisher, J.; O’Boyle, I.; Vibbert, D.; Erofeev, D.; Fulton, L. Dirt cheap and without prescription: How susceptible are young US consumers to purchasing drugs from rogue internet pharmacies? J. Med. Internet Res. 2010, 12, e1520. [Google Scholar] [CrossRef]
- Abanmy, N. The extent of use of online pharmacies in Saudi Arabia. Saudi Pharm. J. 2017, 25, 891–899. [Google Scholar] [CrossRef]
- Fittler, A.; Vida, R.G.; Rádics, V.; Botz, L. A challenge for healthcare but just another opportunity for illegitimate online sellers: Dubious market of shortage oncology drugs. PLoS ONE 2018, 13, e0203185. [Google Scholar] [CrossRef]
- Hertig, J.B.; Kennedy, T.M. Pharmacy Student Perceptions and Knowledge of Online Pharmacy Use. Am. J. Pharm. Educ. 2022, 8, 8933. [Google Scholar] [CrossRef]
- Mackey, T.K.; Kalyanam, J. Detection of illicit online sales of fentanyls via Twitter. F1000Research 2017, 6, 1937. [Google Scholar] [CrossRef]
- Mackey, T.; Kalyanam, J.; Klugman, J.; Kuzmenko, E.; Gupta, R. Solution to detect, classify, and report illicit online marketing and sales of controlled substances via twitter: Using machine learning and web forensics to combat digital opioid access. J. Med. Internet Res. 2018, 20, e10029. [Google Scholar] [CrossRef]
- Anderson, A.C.; Mackey, T.K.; Attaran, A.; Liang, B.A. Mapping of health communication and education strategies addressing the public health dangers of illicit online pharmacies. J. Health Commun. 2016, 21, 397–407. [Google Scholar] [CrossRef]
- Shah, N.; Li, J.; Mackey, T.K. An unsupervised machine learning approach for the detection and characterization of illicit drug-dealing comments and interactions on Instagram. Subst. Abus. 2022, 43, 273–277. [Google Scholar] [CrossRef]
- Tyrawski, J.; De Andrea, D.C. Pharmaceutical companies and their drugs on social media: A content analysis of drug information on popular social media sites. J. Med. Internet Res. 2015, 17, e4357. [Google Scholar] [CrossRef]
- Van de Ven, K.; Koenraadt, R. Exploring the relationship between online buyers and sellers of image and performance enhancing drugs (IPEDs): Quality issues, trust and self-regulation. Int. J. Drug Policy 2017, 50, 48–55. [Google Scholar] [CrossRef]
- Penley, B.; Chen, H.H.; Eckel, S.F.; Ozawa, S. Characteristics of online pharmacies selling Adderall. J. Am. Pharm. Assoc. 2021, 61, e103–e109. [Google Scholar] [CrossRef]
- Mackey, T.K.; Kalyanam, J.; Katsuki, T.; Lanckriet, G. Twitter-based detection of illegal online sale of prescription opioid. Am. J. Public Health 2017, 107, 1910–1915. [Google Scholar] [CrossRef]
- Monteith, S.; Glenn, T. Searching online to buy commonly prescribed psychiatric drugs. Psychiatry Res. 2018, 260, 248–254. [Google Scholar] [CrossRef]
- Katsuki, T.; Mackey, T.K.; Cuomo, R. Establishing a link between prescription drug abuse and illicit online pharmacies: Analysis of Twitter data. J. Med. Internet Res. 2015, 17, e5144. [Google Scholar] [CrossRef]
- Hertig, J.B.; James, S.M.; Hummel, C.J.; Rubin, M.J. Evaluation of pharmacists’ awareness of illegal online pharmacies and perceived impact on safe access to medicines. Med. Access@ Point Care 2021, 5, 23992026211005642. [Google Scholar] [CrossRef]
- Jena, A.B.; Goldman, D.P. Growing Internet use may help explain the rise in prescription drug abuse in the United States. Health Aff. 2011, 30, 1192–1199. [Google Scholar] [CrossRef]
- Liang, B.A.; Mackey, T.K. Prevalence and global health implications of social media in direct-to-consumer drug advertising. J. Med. Internet Res. 2011, 13, e1775. [Google Scholar] [CrossRef]
- Zhao, H.; Muthupandi, S.; Kumara, S. Managing illicit online pharmacies: Web analytics and predictive models study. J. Med. Internet Res. 2020, 22, e17239. [Google Scholar] [CrossRef]
- Li, J.; Xu, Q.; Shah, N.; Mackey, T.K. A machine learning approach for the detection and characterization of illicit drug dealers on Instagram: Model evaluation study. J. Med. Internet Res. 2019, 21, e13803. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Wilson, J.M. Clicking into harm’s way: The decision to purchase regulated goods online. Am. Behav. Sci. 2017, 61, 1358–1386. [Google Scholar] [CrossRef]
- Gong, Y.; Jiang, N.; Chen, Z.; Wang, J.; Zhang, J.; Feng, J.; Lu, Z.; Yin, X. Over-the-counter antibiotic sales in community and online pharmacies, China. Bull. World Health Organ. 2020, 98, 449–457. [Google Scholar] [CrossRef]
- Hussain, R.; Hassali, M.A.; Babar, Z.-U.-D. Quantitative methods in pharmacy practice research. Encycl. Pharm. Pract. Clin. Pharm. 2019, 7, 22–28. [Google Scholar] [CrossRef]
- Kaae, S.; Traulsen, J.M. Qualitative methods in pharmacy practice research. Pharm. Pract. Res. Methods 2015, 31–54. [Google Scholar] [CrossRef]

| Search | Search Terms (Boolean Operators) |
|---|---|
| 1 | “illicit” OR “illegitimate” OR “illegal” OR “rogue” AND “online” AND “pharmac*” |
| 2 | “illicit” OR “illegitimate” OR “illegal” OR “rogue” AND “internet” AND “pharmac*” |
| 3 | “illicit” OR “illegitimate” OR “illegal” OR “rogue” AND “cyber” AND“pharmac*” |
| 4 | “illicit” OR “illegitimate” OR “illegal” OR “rogue” AND “e-pharmac*” |
| Area of Investigation | Description |
|---|---|
| Regulation and enforcement | Which regulatory and enforcement actions most effectively combat IOPs or encourage greater compliance behavior? What enforcement or regulatory changes would best address challenges associated with IOPs? What factors distinguish IOPs that will versus will not seek compliance if subjected to enforcement actions? How can regulatory or enforcement regimes better use these factors to encourage compliance? What regulatory violations or forms of noncompliance are most common (e.g., counterfeiting, unapproved formulation, labeling, drug packaging or package inserts)? To what extent are accreditation and pharmacy licenses leading cross-border operators to become IOPs? How could these barriers of entry be adjusted to encourage more legitimate competition while protecting public health? Are some nations’ regulatory or enforcement agencies better at curbing patient usage of IOPs? If so, why? What are the most significant obstacles to global enforcement? How can these be addressed to help nations better coordinate efforts to combat IOPs? To what extent do IOPs provide drug information required by law, such as usage information or side effects and contraindications? How does this required information’s presence or absence affect the likelihood of enforcement actions? What ethical issues do IOPs or enforcement actions and public health responses to IOPs raise? How do regulatory guidelines effectively address these ethical issues? What is the professional or ethical obligation to ensure access that regulatory or enforcement actions may interrupt? |
| Public health awareness and education | What initiatives and interventions have been designed to communicate and educate the public about IOPs? How effective are they? Why? What challenges do patients have in detecting illicit versus legitimate online pharmacies? How can public health officials improve patients’ accurate identification of IOPs? What message strategies and techniques most effectively raise public awareness of and educate the public about IOPs and the dangers associated with purchasing drugs from IOPs? How should theoretical frameworks developed in communication, marketing or psychology be applied to develop effective campaigns and interventions to raise awareness of and educate the public about the dangers of IOPs? What stakeholders, other than patients or regulatory and enforcement agencies, could play a role or have an ethical obligation to help combat IOPs? How should public awareness and education campaigns best persuade these stakeholders, such as healthcare providers, credit card companies, Internet service providers, search engines and drug manufacturers and suppliers? |
| Healthcare service | How do IOPs impact the delivery of healthcare services by hospitals, physicians and pharmacists? Are the impacts of IOPs on delivering healthcare services or quality of care greater for vulnerable populations? To what extent does access to IOPs encourage patients to abandon legitimate healthcare service providers versus supplement their healthcare services with additional purchases from IOPs? Do IOPs increase access to healthcare services among those otherwise unable to access them? |
| Risks to patients and public health | What are the impacts of products sold by IOPs on patient health and well-being? How do IOPs influence patients’ quality of care? How do IOPs affect public health and global access to effective pharmaceutical treatments? What role do IOPs play in drug abuse and self-medication? What are the privacy and security risks associated with IOPs for patients? What steps can be taken to better protect patients’ information? What is the extent of IOPs’ negative social and personal economic consequences, such as consumer fraud, invasion of privacy and the misuse or sale of personal information? |
| Patients | Who purchases drugs from IOPs in terms of benefits sought, search behavior, preferences, personality, demographics and usage? Based on the characteristics of frequent purchasers, how can public health officials better craft messages and other interventions? What are patients’ attitudes toward IOPs and the sale of pharmaceutical drugs without prescriptions? How do interventions change these attitudes? How do patients’ level of education, literacy and awareness of regulations influence their willingness to purchase from IOPs? What patient segments are at higher risk for purchasing drugs from IOPs? Does direct-to-consumer advertising encourage patients to purchase from IOPs? How do environmental influences, pricing, website design factors or sales promotions (e.g., discounts, coupons and customized offers) affect patient purchases from IOPs? How do social norms and perceived social pressure influence the likelihood of purchasing from IOPs? What theoretical frameworks from psychology, consumer research or sociology could be applied to understand patients’ IOP attitudes and behaviors? |
| Price | Do IOPs offer drug prices that are substantially lower than those of legitimate online pharmacies? If so, how are they able to do so? How transparent are drug prices prior to purchasing from IOPs versus legitimate pharmacies? |
| Product | What is the nature and quality of the unapproved or counterfeit drugs sold by IOPs? Which prescription drugs and brands tend to be sold by IOPs without a prescription? Are most or only a few drugs offered by IOPs counterfeited, adulterated or unapproved? What ethical implications arise from product-related IOP issues (e.g., unapproved and misbranded prescription drugs, drug sales without prescriptions, adequate directions for safe use or warnings about serious health risks)? How do affordability and availability issues complicate these ethical implications? |
| Website design | How do IOPs encourage patients to order? How are patient perceptions, attitudes, usage, and purchase behavior affected by IOP websites’ hedonic aspects, perceived usefulness, ease of use, visual sophistication, resemblance to reputable legitimate pharmacy websites, or signals of trustworthiness (e.g., third-party logos, return policies, purported endorsements of healthcare professionals or other trust cues)? What differences in website features (e.g., shopping carts, free shipping, testimonials or security seals) distinguish legitimate online pharmacies from IOPs? To what extent do IOP websites provide complete contact information? How does the presence or absence of contact information affect patient attitudes toward an IOP or patient behaviors (e.g., the extent of search or purchase)? To what extent do IOP websites include disclosures of policies, such as the terms and conditions of sale, return policy, refund policy, privacy protection policy, website security policy and information collection policy? How does the presence or absence of these policies affect patient attitudes toward the IOP or behaviors? How does including certification seals on IOP websites influence patient attitudes and behaviors? How can theoretical frameworks developed in information systems, communication, marketing or psychology be applied to explain and counteract effective IOP website design? |
| Social media promotion | How has the advent of social media and mobile technology impacted IOPs? How are counterfeit and unapproved drugs or controlled substances trafficked online through social media platforms? How does the social media promotion of IOPs differ across platforms (e.g., TikTok, Facebook or Twitter)? How are social media influencers and live streamers changing the promotion of IOPs? |
| Supply chains and logistics | How have changing supply chains affected IOPs? What is the origin and nature of the supply chain or distribution network of counterfeit and grey market drugs offered by IOPs? What are the roles of drug distributors and retailers in the growth of IOPs? How reliable are IOPs for order fulfillment? What do patients receive when orders are fulfilled (e.g., ordered drugs, counterfeits, adulterated drugs, inert substances or opioids)? How do barriers to entry designed to limit IOPs (e.g., accreditation and pharmacy licenses) increase the complexity and cost of the pharmaceutical supply chain? How does this increased complexity and cost negatively affect legitimate pharmacies and patients? To what degree could blockchain technology validate the pharmaceutical supply chain and reduce purchases of counterfeit drugs via IOPs? |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limbu, Y.B.; Huhmann, B.A. Illicit Online Pharmacies: A Scoping Review. Int. J. Environ. Res. Public Health 2023, 20, 5748. https://doi.org/10.3390/ijerph20095748
Limbu YB, Huhmann BA. Illicit Online Pharmacies: A Scoping Review. International Journal of Environmental Research and Public Health. 2023; 20(9):5748. https://doi.org/10.3390/ijerph20095748
Chicago/Turabian StyleLimbu, Yam B., and Bruce A. Huhmann. 2023. "Illicit Online Pharmacies: A Scoping Review" International Journal of Environmental Research and Public Health 20, no. 9: 5748. https://doi.org/10.3390/ijerph20095748
APA StyleLimbu, Y. B., & Huhmann, B. A. (2023). Illicit Online Pharmacies: A Scoping Review. International Journal of Environmental Research and Public Health, 20(9), 5748. https://doi.org/10.3390/ijerph20095748






