Reference Values, Determinants and Regression Equation for the Timed-Up and Go Test (TUG) in Healthy Asian Population Aged 21 to 85 Years
Abstract
1. Introduction
2. Material & Methods
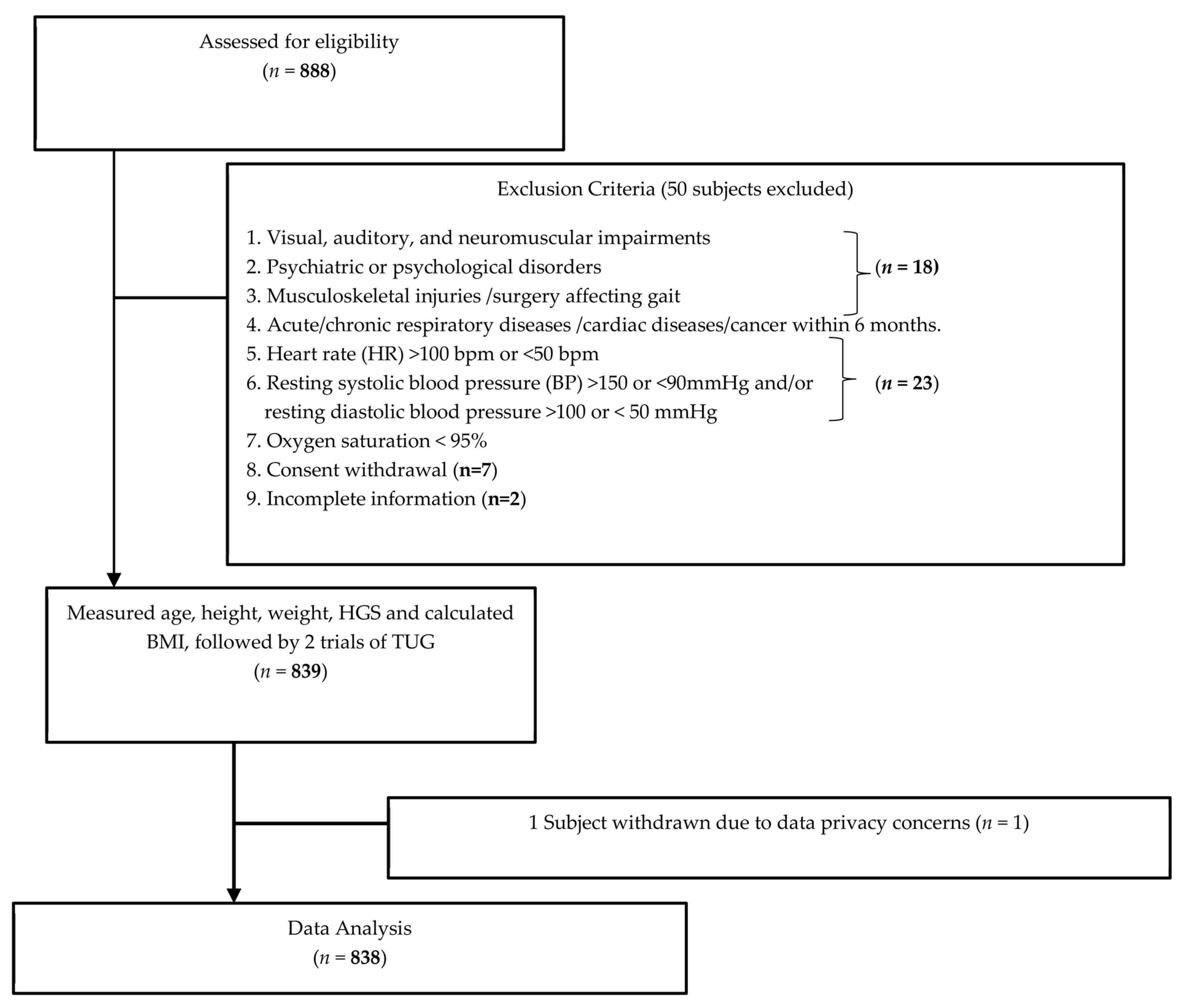
2.1. Study Design and Recruitment Process
2.2. Subjects
2.3. Timed Up and Go Test (TUG)
2.4. Grip Strength Test
2.5. Statistical Analysis
3. Results
3.1. Reliability
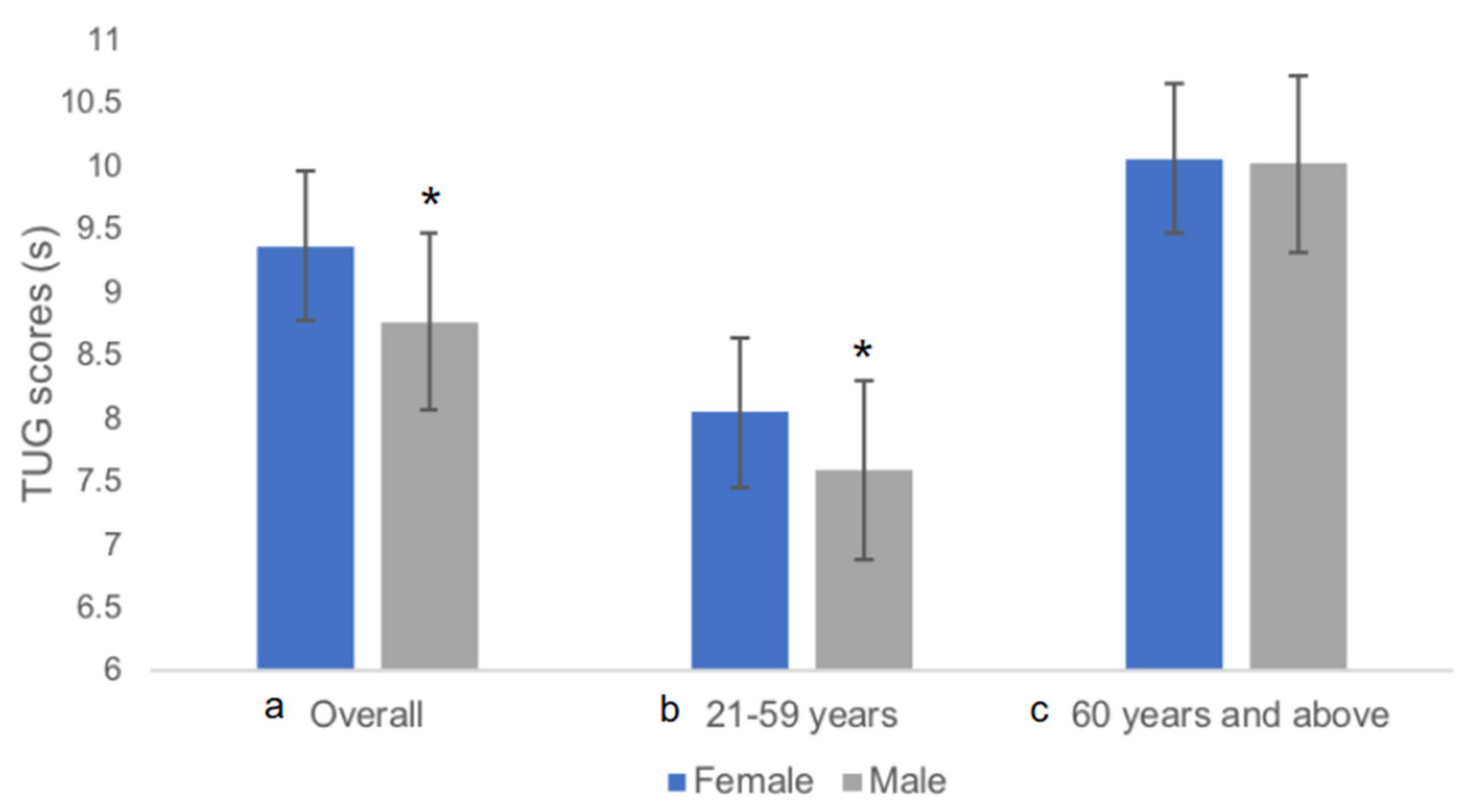
3.2. Subject Characteristics and TUG
3.3. Relationship between TUG and Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathias, S.; Nayak, U.; Isaacs, B. Balance in elderly patients: The “get-up and go” test. Arch. Phys. Med. Rehabil. 1986, 67, 387–389. [Google Scholar] [PubMed]
- Herman, T.; Giladi, N.; Hausdorff, J.M. Properties of the ‘timed up and go’ test: More than meets the eye. Gerontology 2011, 57, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [PubMed]
- Bohannon, R.W. Reference values for the timed up and go test: A descriptive meta-analysis. J. Geriatr. Phys. Ther. 2006, 29, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Christopher, A.; Kraft, E.; Olenick, H.; Kiesling, R.; Doty, A. The reliability and validity of the Timed Up and Go as a clinical tool in individuals with and without disabilities across a lifespan: A systematic review. Disabil. Rehabil. 2019, 43, 1799–1813. [Google Scholar] [CrossRef]
- Kear, B.M.; Guck, T.P.; McGaha, A.L. Timed Up and Go (TUG) Test: Normative Reference Values for Ages 20 to 59 Years and Relationships With Physical and Mental Health Risk Factors. J. Prim. Care Community Health 2017, 8, 9–13. [Google Scholar] [CrossRef]
- Zampieri, C.; Salarian, A.; Carlson-Kuhta, P.; Aminian, K.; Nutt, J.G.; Horak, F.B. The instrumented timed up and go test: Potential outcome measure for disease modifying therapies in Parkinson’s disease. J. Neurol. Neurosurg. Psych. 2010, 81, 171–176. [Google Scholar] [CrossRef]
- Whitney, S.L.; Marchetti, G.F.; Schade, A.; Wrisley, D.M. The sensitivity and specificity of the Timed “Up & Go” and the Dynamic Gait Index for self-reported falls in persons with vestibular disorders. J. Vestib. Res. 2004, 14, 397–409. [Google Scholar]
- Long, J.; Cai, T.; Huang, X.; Zhou, Y.; Kuang, J.; Wu, L. Reference value for the TUGT in healthy older people: A systematic review and meta-analysis. Geriatr. Nurs. 2020, 41, 325–330. [Google Scholar] [CrossRef]
- Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J. Am. Geriatr Soc. 2011, 59, 148–157. [Google Scholar] [CrossRef]
- Hartholt, K.A.; Lee, R.; Burns, E.R.; van Beeck, E.F. Mortality from Falls Among US Adults Aged 75 Years or Older, 2000–2016. JAMA 2019, 321, 2131–2133. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.; Galvin, R.; Keogh, C.; Horgan, F.; Fahey, T. Is the Timed Up and Go test a useful predictor of risk of falls in community dwelling older adults: A systematic review and meta-analysis. BMC Geriatr. 2014, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H.A.; Stahelin, H.B.; Monsch, A.U.; Iversen, M.D.; Weyh, A.; von Dechend, M.; Akos, R.; Conzelmann, M.; Dick, W.; Theiler, R. Identifying a cutoff point for normal mobility: A comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing 2003, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Junior, H.J.; Rodrigues, B.; Goncalves, I.O.; Asano, R.Y.; Uchida, M.C.; Marzetti, E. The physical capabilities underlying timed “Up and Go” test are time-dependent in community-dwelling older women. Exp. Gerontol. 2018, 104, 138–146. [Google Scholar] [CrossRef]
- Kamide, N.; Takahashi, K.; Shiba, Y. Reference values for the Timed Up and Go test in healthy Japanese elderly people: Determination using the methodology of meta-analysis. Geriatr. Gerontol. Int. 2011, 11, 445–451. [Google Scholar] [CrossRef]
- Kenny, R.A.; Coen, R.F.; Frewen, J.; Donoghue, O.A.; Cronin, H.; Savva, G.M. Normative values of cognitive and physical function in older adults: Findings from the Irish Longitudinal Study on Ageing. J. Am. Geriatr. Soc. 2013, 61 (Suppl. S2), S279–S290. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.J. Normative data: Their definition, interpretation, and importance for primary care physicians. Fam. Med. 1990, 22, 307–311. [Google Scholar]
- Ibrahim, A.; Singh, D.K.A.; Shahar, S. ‘Timed Up and Go’ test: Age, gender and cognitive impairment stratified normative values of older adults. PLoS ONE 2017, 12, e0185641. [Google Scholar] [CrossRef]
- Gusi, N.; Prieto, J.; Olivares, P.R.; Delgado, S.; Quesada, F.; Cebrian, C. Normative fitness performance scores of community-dwelling older adults in Spain. J. Aging Phys. Act. 2012, 20, 106–126. [Google Scholar] [CrossRef]
- Thaweewannakij, T.; Wilaichit, S.; Chuchot, R.; Yuenyong, Y.; Saengsuwan, J.; Siritaratiwat, W.; Amatachaya, S. Reference values of physical performance in Thai elderly people who are functioning well and dwelling in the community. Phys. Ther. 2013, 93, 1312–1320. [Google Scholar] [CrossRef]
- Roubenoff, R. Sarcopenia: A major modifiable cause of frailty in the elderly. J. Nutr. Health Aging 2000, 4, 140–142. [Google Scholar] [PubMed]
- Dart, A.M.; Du, X.J.; Kingwell, B.A. Gender, sex hormones and autonomic nervous control of the cardiovascular system. Cardiovasc. Res. 2002, 53, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Pondal, M.; del Ser, T. Normative data and determinants for the timed “up and go” test in a population-based sample of elderly individuals without gait disturbances. J. Geriatr. Phys. Ther. 2008, 31, 57–63. [Google Scholar] [CrossRef]
- Riebe, D.; Blissmer, B.J.; Greaney, M.L.; Garber, C.E.; Lees, F.D.; Clark, P.G. The relationship between obesity, physical activity, and physical function in older adults. J. Aging Health 2009, 21, 1159–1178. [Google Scholar] [CrossRef] [PubMed]
- Forhan, M.; Gill, S.V. Obesity, functional mobility and quality of life. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 129–137. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Dos-Santos-Silva, I.; Leon, D.A.; Douglas, I.J.; Smeeth, L. Association of BMI with overall and cause-specific mortality: A population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. 2018, 6, 944–953. [Google Scholar] [CrossRef]
- Fjeldstad, C.; Fjeldstad, A.S.; Acree, L.S.; Nickel, K.J.; Gardner, A.W. The influence of obesity on falls and quality of life. Dyn. Med. 2008, 7, 4. [Google Scholar] [CrossRef]
- Marsh, A.P.; Rejeski, W.J.; Espeland, M.A.; Miller, M.E.; Church, T.S.; Fielding, R.A.; Gill, T.M.; Guralnik, J.M.; Newman, A.B.; Pahor, M. Muscle strength and BMI as predictors of major mobility disability in the Lifestyle Interventions and Independence for Elders pilot (LIFE-P). J. Gerontol. A Biol. Sci. Med Sci. 2011, 66, 1376–1383. [Google Scholar] [CrossRef]
- Stenholm, S.; Head, J.; Aalto, V.; Kivimaki, M.; Kawachi, I.; Zins, M.; Goldberg, M.; Platts, L.G.; Zaninotto, P.; Magnusson Hanson, L.L.; et al. Body mass index as a predictor of healthy and disease-free life expectancy between ages 50 and 75: A multicohort study. Int. J. Obes. 2017, 41, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, M.S.; Sim, S.; Park, B.; Choi, H.G. Association Between Obesity and Falls Among Korean Adults: A Population-Based Cross-Sectional Study. Medicine 2016, 95, e3130. [Google Scholar] [CrossRef]
- Sayer, A.A.; Kirkwood, T.B. Grip strength and mortality: A biomarker of ageing? Lancet 2015, 386, 226–227. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2018, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Martyn, C.N.; Cooper, C.; Sayer, A.A. Grip strength, body composition, and mortality. Int. J. Epidemiol. 2006, 36, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Handgrip Dynamometry Predicts Future Outcomes in Aging Adults. J. Geriatr. Phys. Ther. 2008, 31, 3–10. [Google Scholar] [CrossRef]
- Rantanen, T.; Harris, T.; Leveille, S.G.; Visser, M.; Foley, D.; Masaki, K.; Guralnik, J.M. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M168–M173. [Google Scholar] [CrossRef]
- Chua, K.Y.; Lim, W.S.; Lin, X.; Yuan, J.M.; Koh, W.P. Handgrip Strength and Timed Up-and-Go (TUG) Test are Predictors of Short-Term Mortality among Elderly in a Population-Based Cohort in Singapore. J. Nutr. Health Aging 2020, 24, 371–378. [Google Scholar] [CrossRef]
- Rothstein, J.M.; Echternach, J.L. Primer on Measurement: An Introductory Guide to Measurement Issues, Featuring the American Physical Therapy Association’s Standards for Tests and Measurements in Physical Therapy Practice; American Physical Therapy Association: Alexandria, VA, USA, 1993. [Google Scholar]
- Merchant, R.A.; Chen, M.Z.; Tan, L.W.L.; Lim, M.Y.; Ho, H.K.; van Dam, R.M. Singapore Healthy Older People Everyday (HOPE) Study: Prevalence of Frailty and Associated Factors in Older Adults. J. Am. Med. Dir. Assoc. 2017, 18, 734.e9–734.e14. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar]
- Coldham, F.; Lewis, J.; Lee, H. The reliability of one vs. three grip trials in symptomatic and asymptomatic subjects. J. Hand Ther. 2006, 19, 318–326, quiz 27. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Weber, K.; Volland, G.; Kashman, N. Reliability and validity of grip and pinch strength evaluations. J. Hand Surg. Am. 1984, 9, 222–226. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Khant, N.; Dani, V.B.; Patel, P.; Rathod, R. Establishing the reference value for “timed up-and-go” test in healthy adults of Gujarat, India. J. Educ. Health Promot. 2018, 7, 62. [Google Scholar] [PubMed]
- Dai, W.; Tham, Y.C.; Chee, M.L.; Tan, N.Y.Q.; Wong, K.H.; Majithia, S.; Sabanayagam, C.; Lamoureux, E.; Wong, T.Y.; Cheng, C.Y. Falls and Recurrent Falls among Adults in A Multi-ethnic Asian Population: The Singapore Epidemiology of Eye Diseases Study. Sci. Rep. 2018, 8, 7575. [Google Scholar] [CrossRef] [PubMed]
- Vereeck, L.; Wuyts, F.; Truijen, S.; Van de Heyning, P. Clinical assessment of balance: Normative data, and gender and age effects. Int. J. Audiol. 2008, 47, 67–75. [Google Scholar] [CrossRef]
- Thompson, M.; Medley, A. Performance of Community Dwelling Elderly on the Timed Up and Go Test. Phys. Occup. Ther. Geriatr. 1995, 13, 17–30. [Google Scholar] [CrossRef]
- Samson, M.M.; Meeuwsen, I.B.; Crowe, A.; Dessens, J.A.; Duursma, S.A.; Verhaar, H.J. Relationships between physical performance measures, age, height and body weight in healthy adults. Age Ageing 2000, 29, 235–242. [Google Scholar] [CrossRef]
- Ode, J.J.; Pivarnik, J.M.; Reeves, M.J.; Knous, J.L. Body mass index as a predictor of percent fat in college athletes and nonathletes. Med. Sci. Sport. Exerc. 2007, 39, 403–409. [Google Scholar] [CrossRef]
- Martinez, B.P.; Gomes, I.B.; Oliveira, C.S.D.; Ramos, I.R.; Rocha, M.D.M.; Forgiarini Júnior, L.A.; Camelier, F.W.R.; Camelier, A.A. Accuracy of the Timed Up and Go test for predicting sarcopenia in elderly hospitalised patients. Clinics 2015, 70, 369–372. [Google Scholar] [CrossRef]
- Etman, A.; Wijlhuizen, G.J.; van Heuvelen, M.J.; Chorus, A.; Hopman-Rock, M. Falls incidence underestimates the risk of fall-related injuries in older age groups: A comparison with the FARE (Falls risk by Exposure). Age Ageing 2012, 41, 190–195. [Google Scholar] [CrossRef]
| ICC (SD) | 95% CI | SEM | |
|---|---|---|---|
| Test-retest | 0.879 (0.34) | 0.838 to 0.911 | 0.12 |
| Inter-rater | 0.953 (1.03) | 0.919 to 0.975 | 0.22 |
| Intra-rater | |||
| Rater 1 | 0.804 (0.68) | 0.681 to 0.891 | 0.30 |
| Rater 2 | 0.858 (0.66) | 0.772 to 0.921 | 0.25 |
| Rater 3 | 0.821 (0.69) | 0.701 to 0.901 | 0.29 |
| Age (Years) | Height (m) | Weight (kg) | BMI (kg/m2) | Normative Reference Values | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Decades | Gender | n | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Median | Interquartile Range | Min | Max | 95% CI Lower Bound | 95% CI Upper Bound | 10th Percentile | 90th Percentile | Tukey’s HSD Range Test Compares Group Differences | Mean Difference in TUG Time | 95% CI (Mean Difference) | p-Value |
| 21–85 | Total | 838 | 56.0 | 19.1 | 1.59 | 0.08 | 61.00 | 12.45 | 23.90 | 4.03 | 9.16 | 2.12 | 8.91 | 7.70–10.31 | 4.52 | 21.20 | 9.01 | 9.30 | 11.91 | 6.81 | ||||
| 21–39 | Total | 197 | 24.4 | 3.4 | 1.67 | 0.08 | 66.40 | 14.32 | 23.72 | 4.30 | 7.20 | 1.24 | 7.19 | 6.27–8.02 | 4.52 | 10.35 | 7.02 | 7.37 | 8.84 | 5.52 | ||||
| 40–59 | −1.54 | −2.02 to −1.07 | <0.001 | |||||||||||||||||||||
| 60–69 | −2.29 | −2.70 to −1.88 | <0.001 | |||||||||||||||||||||
| 70–85 | −3.49 | −3.91 to −3.07 | <0.001 | |||||||||||||||||||||
| Females | 79 | 24.6 | 4.4 | 1.60 | 0.06 | 59.30 | 14.33 | 22.93 | 4.90 | 7.13 | 1.20 | 7.17 | 6.24–7.84 | 4.95 | 10.35 | 6.86 | 7.40 | 8.55 | 5.57 | |||||
| Males | 118 | 24.2 | 2.6 | 1.71 | 0.06 | 71.20 | 12.21 | 24.25 | 3.77 | 7.24 | 1.27 | 7.26 | 6.32–8.22 | 4.52 | 9.94 | 7.01 | 7.47 | 8.87 | 5.48 | |||||
| 40–59 | Total | 143 | 53.2 | 5.3 | 1.59 | 0.08 | 61.60 | 11.60 | 24.38 | 4.10 | 8.74 | 1.33 | 8.60 | 7.81–9.50 | 5.66 | 14.48 | 8.52 | 8.96 | 10.32 | 7.33 | ||||
| 60–69 | −0.74 | −1.20 to −0.29 | <0.001 | |||||||||||||||||||||
| 70–85 | −1.95 | −2.41 to −1.48 | <0.001 | |||||||||||||||||||||
| Females | 108 | 53.0 | 5.4 | 1.56 | 0.05 | 59.50 | 10.08 | 24.50 | 3.85 | 8.73 | 1.36 | 8.26 | 7.62–8.70 | 5.66 | 9.56 | 8.46 | 8.98 | 9.09 | 7.19 | |||||
| Males | 35 | 53.8 | 4.7 | 1.69 | 0.07 | 68.20 | 13.51 | 24.00 | 4.56 | 8.78 | 1.25 | 10.05 | 9.83–10.94 | 9.56 | 14.48 | 8.35 | 9.21 | 11.88 | 9.70 | |||||
| 60–69 | Total | 261 | 65.2 | 2.8 | 1.57 | 0.07 | 59.60 | 11.79 | 24.20 | 4.15 | 9.48 | 1.66 | 9.33 | 8.42–10.36 | 6.53 | 15.93 | 9.29 | 9.68 | 11.46 | 7.51 | ||||
| 70–85 | −1.2 | −1.59 to −0.81 | <0.001 | |||||||||||||||||||||
| Females | 194 | 65.2 | 2.8 | 1.54 | 0.05 | 57.20 | 10.75 | 24.10 | 4.16 | 8.42 | 1.59 | 9.22 | 8.39–10.33 | 6.53 | 15.93 | 9.19 | 9.64 | 11.21 | 7.45 | |||||
| Males | 67 | 65.1 | 3.0 | 1.65 | 0.06 | 66.40 | 12.09 | 24.50 | 4.15 | 9.68 | 1.69 | 9.68 | 8.53–10.43 | 6.84 | 14.71 | 9.27 | 10.09 | 11.71 | 7.58 | |||||
| ≥70 | Total | 237 | 73.7 | 3.2 | 1.56 | 0.08 | 57.60 | 10.43 | 23.60 | 3.60 | 10.68 | 2.12 | 8.91 | 7.70–10.30 | 6.99 | 21.20 | 9.01 | 9.30 | 11.91 | 6.81 | ||||
| Females | 161 | 73.6 | 3.1 | 1.52 | 0.05 | 55.00 | 9.39 | 23.70 | 3.82 | 10.85 | 2.31 | 10.94 | 9.30–11.98 | 6.99 | 21.20 | 10.49 | 11.21 | 13.46 | 8.40 | |||||
| Males | 76 | 74.1 | 3.3 | 1.64 | 0.07 | 63.20 | 10.07 | 23.30 | 3.09 | 10.33 | 1.97 | 10.04 | 8.83–11.83 | 7.03 | 17.02 | 9.89 | 10.78 | 12.72 | 8.05 | |||||
| Independent Variable | Pearson’s r | p-Value |
|---|---|---|
| Age | 0.597 | <0.001 * |
| Gender | −0.120 | 0.331 |
| BMI | 0.133 | <0.001 * |
| Underweight | −0.114 | 0.445 |
| Normal to Obese | 0.155 | <0.001 * |
| Height | −0.327 | <0.001 * |
| Weight | −0.074 | 0.0325 |
| HGS | 0.023 | 0.744 |
| Coefficient | Standard Error | p-Value | 95% Confidence Interval | |
|---|---|---|---|---|
| R2 = 0.374 | 9.11 | 1.366 | <0.001 | 6.43 to 11.79 |
| Age | 0.063 | 0.003 | <0.001 | 0.057 to 0.070 |
| Height | −3.19 | 0.881 | <0.001 | −4.92 to −1.46 |
| Weight | 0.026 | 0.006 | <0.001 | 0.015 to 0.037 |
| BMI | 0.142 | 0.130 | =0.273 | −0.112 to 0.396 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, T.C.; Guo, Y.Y.; Ho, D.J.; Sanwari, N.A.B.; Quek, P.H.; Tan, R.S.; Yap, F.S.; Yang, M.; Yeung, M.T. Reference Values, Determinants and Regression Equation for the Timed-Up and Go Test (TUG) in Healthy Asian Population Aged 21 to 85 Years. Int. J. Environ. Res. Public Health 2023, 20, 5712. https://doi.org/10.3390/ijerph20095712
Tan TC, Guo YY, Ho DJ, Sanwari NAB, Quek PH, Tan RS, Yap FS, Yang M, Yeung MT. Reference Values, Determinants and Regression Equation for the Timed-Up and Go Test (TUG) in Healthy Asian Population Aged 21 to 85 Years. International Journal of Environmental Research and Public Health. 2023; 20(9):5712. https://doi.org/10.3390/ijerph20095712
Chicago/Turabian StyleTan, Teck Chye, Yan Y. Guo, Dilys J. Ho, Nur Aidah Binti Sanwari, Patricia H. Quek, Rachel S. Tan, Felicia S. Yap, Mingxing Yang, and Meredith T. Yeung. 2023. "Reference Values, Determinants and Regression Equation for the Timed-Up and Go Test (TUG) in Healthy Asian Population Aged 21 to 85 Years" International Journal of Environmental Research and Public Health 20, no. 9: 5712. https://doi.org/10.3390/ijerph20095712
APA StyleTan, T. C., Guo, Y. Y., Ho, D. J., Sanwari, N. A. B., Quek, P. H., Tan, R. S., Yap, F. S., Yang, M., & Yeung, M. T. (2023). Reference Values, Determinants and Regression Equation for the Timed-Up and Go Test (TUG) in Healthy Asian Population Aged 21 to 85 Years. International Journal of Environmental Research and Public Health, 20(9), 5712. https://doi.org/10.3390/ijerph20095712







