Changes in Skeletal Muscle Troponin T and Vitamin D Binding Protein (DBP) Concentrations in the Blood of Male Amateur Athletes Participating in a Marathon and 100 km Adventure Race
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Measures
2.4. Statistical Analysis
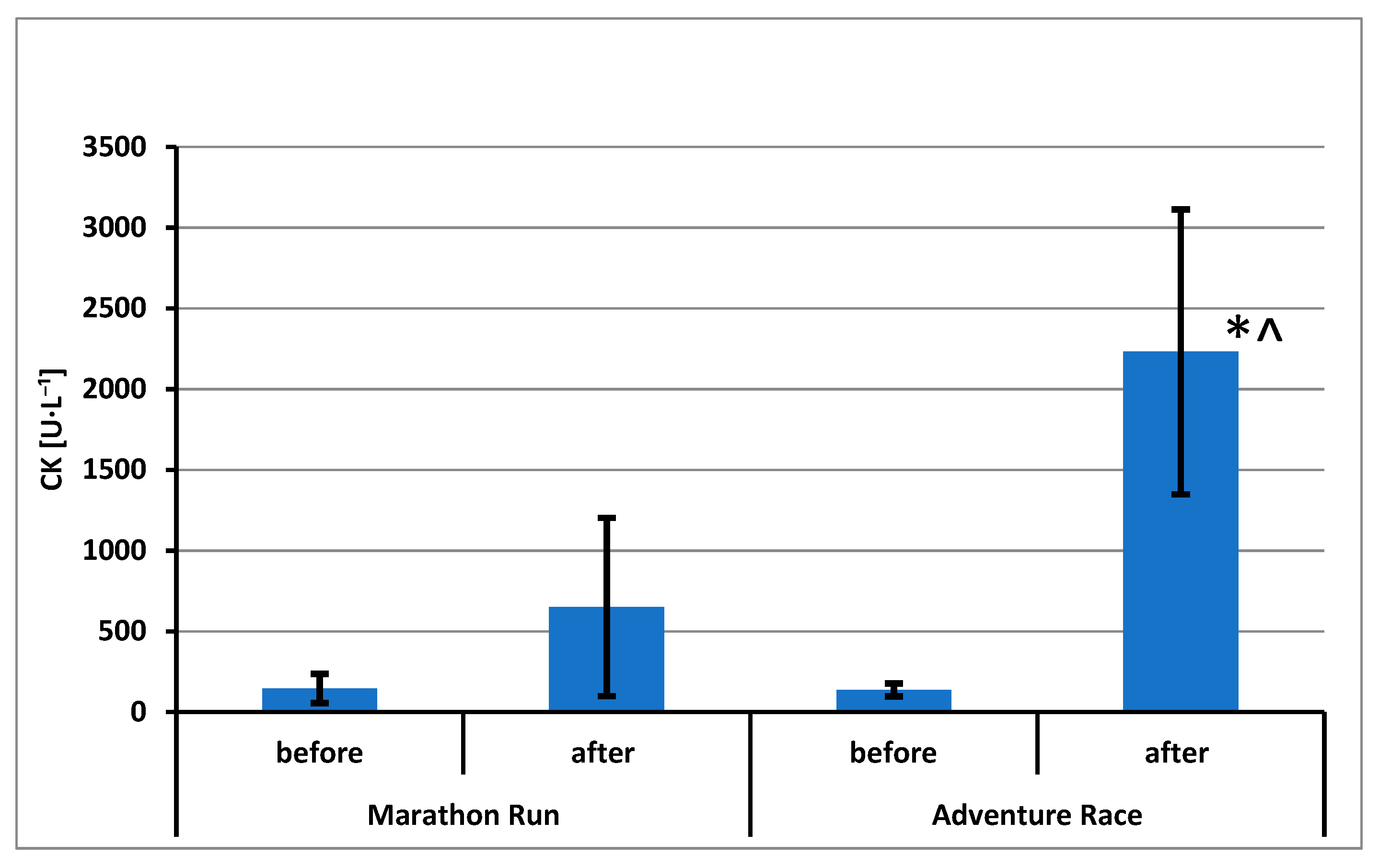
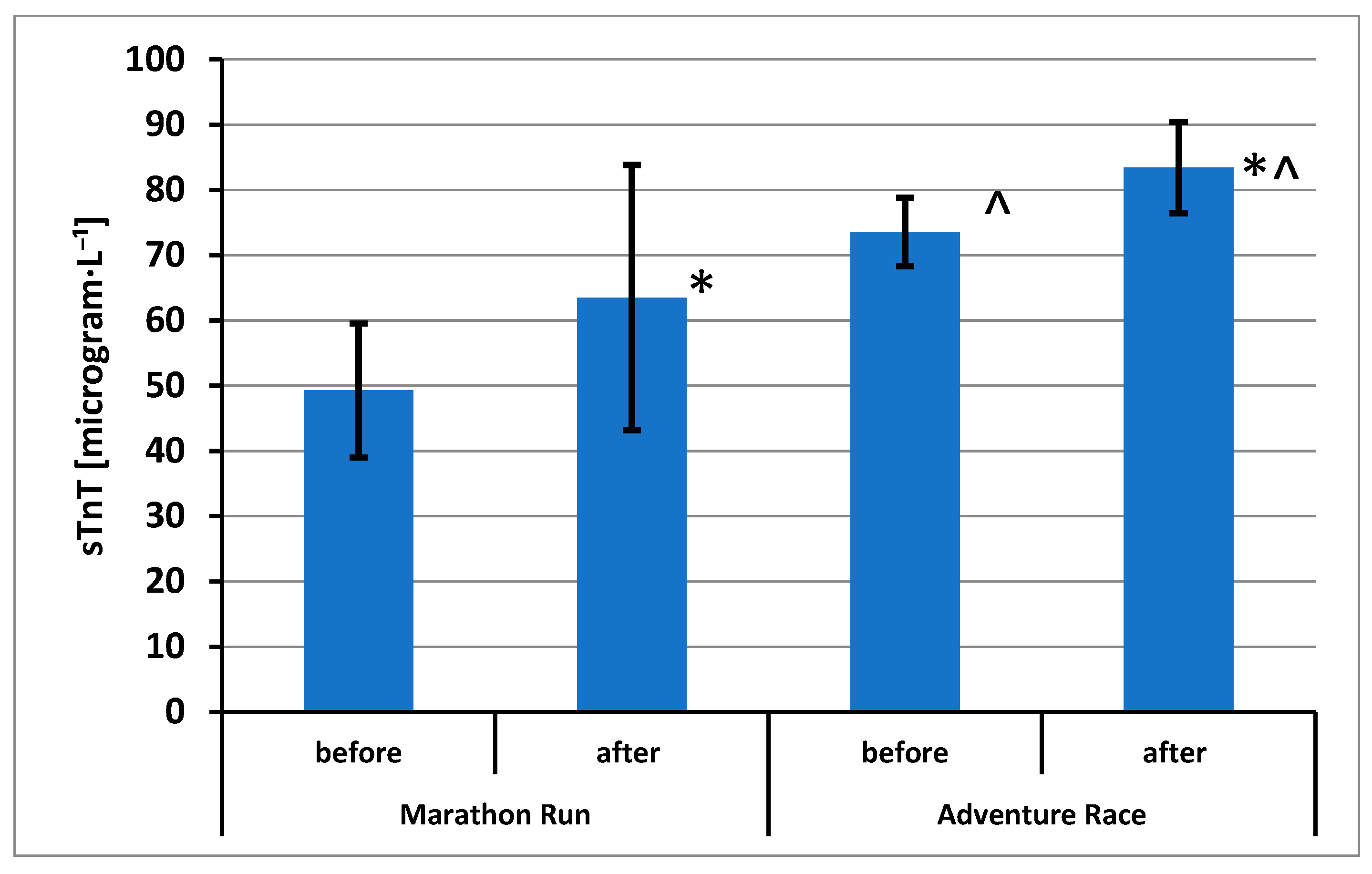
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alison, D. The Unstoppable 21st-Century Marathon Boom. Marathon Beyond 2010, 14, 80–92. [Google Scholar]
- Vitiello, D.; Palacin, F.; Poinsard, L.; Kirsch, M.; Jouini, S.; Billat, V. Marathon-Induced Cardiac Fatigue: A Review over the Last Decade for the Preservation of the Athletes’ Health. Int. J. Environ. Res. Public Health 2021, 18, 8676. [Google Scholar] [CrossRef] [PubMed]
- Van Steirteghem, A.C.; Zweig, M.H.; Alan, N.; Schechter, A.N. Radioimmunoassay of Creatine Kinase Isoenzymes in Human Serum: Isoenzyme MM. Clin. Chem. 1978, 24, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Hebisz, R.; Borkowski, J.; Hebisz, P. Creatine Kinase and Myoglobin Plasma Levels in Mountain Bike and Road Cyclists 1 h after the Race. Int. J. Environ. Res. Public Health 2022, 19, 9456. [Google Scholar] [CrossRef] [PubMed]
- Priest, J.B.; Oei, T.O.; Moorehead, W.R. Exercise-induced changes in common laboratory tests. Am. J. Clin. Pathol. 1982, 77, 285–289. [Google Scholar] [CrossRef]
- Agner, E.; Kelbaek, H.; Fogh-Andersen, N.; Mørck, H.I. Coronary and skeletal muscle enzyme changes during a 14 km run. Acta Med. Scand. 1988, 224, 183–186. [Google Scholar] [CrossRef]
- Sorichter, S.; Mair, J.; Koller, A.; Pelsers, M.; Puschendorf, B.; Glatz, J. Early assesment of exercise induced skeletal muscle injury using plasma fatty acid binding protein. Br. J. Sports Med. 1998, 32, 121–124. [Google Scholar] [CrossRef]
- Vissing, K.; Overgaard, K.; Nedergaard, A.; Fredsted, A.; Schjerling, P. Effects of concentric and repeated eccentric exercise on muscle damage and calpain-calpastatin gene expression in human skeletal muscle. Eur. J. Appl. Physiol. 2008, 103, 323–332. [Google Scholar] [CrossRef]
- Koller, A.; Mair, J.; Schobersberger, W.; Wohlfarter, T.; Haid, C.; Mayr, M.; Villiger, B.; Frey, W.; Puschendorf, B. Effects of prolonged strenuous endurance exercise on plasma myosin heavy chain fragments and other muscular proteins. Cycling vs running. J. Sports Med. Phys. Fit. 1998, 38, 10–17. [Google Scholar]
- Sorichter, S.; Mair, J.; Koller, A.; Müller, E.; Kremser, C.; Judmaier, W.; Haid, C.; Calzolari, C.; Puschendorf, B. Creatine kinase, myosin heavy chains and magnetic resonance imaging after eccentric exercise. J. Sports Sci. 2001, 19, 687–691. [Google Scholar] [CrossRef]
- Sorichter, S.; Mair, J.; Koller, A.; Gebert, W.; Rama, D.; Calzolari, C.; Artner-Dworzak, E.; Puschendorf, B. Skeletal troponin I as a marker of exercise-induced muscle damage. J. Appl. Physiol. 1997, 83, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Amat, A.M.; Corrales, J.A.M.; Serrano, F.R.; Boulaiz, H.; Salazar, J.C.P.; Hita Contreras, F.; Caba Perez, O.; Delgado, E.C.; Martín, I.; Jimenez, A.A. Role of alpha-actin in muscle damage of injured athletes in comparison with traditional markers. Br. J. Sports Med. 2007, 41, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.P. Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc. Immunol. Rev. 2006, 12, 6–33. [Google Scholar]
- Kasapis, C.; Thompson, P.D. The Effects of Physical Activity on Serum C-Reactive Protein and Inflammatory Markers: A Systematic Review. J. Am. Coll. Cardiol. 2005, 45, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Tékus, É.; Váczi, M.; Horváth-Szalai, Z.; Ludány, A.; Kőszegi, T.; Wilhelm, M. Plasma Actin, Gelsolin and Orosomucoid Levels after Eccentric Exercise. J. Hum. Kinet. 2017, 56, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Vascolcellos, C.A.; Lind, S.E. Coordinated Inhibition of Actin-Induced Platelet Aggregation by Plasma Gelsolin and Vitamin D-Binding Protein. Blood 1993, 82, 3648–3657. [Google Scholar] [CrossRef]
- Smit, M.J.; Duursma, A.M.; Bouma, J.M.; Gruber, M. Receptor-mediated endocytosis of lactate dehydrogenase M4 by liver macrophages: A mechanism for elimination of enzymes from plasma. Evidence for competition by creatine kinase MM, adenylate kinase, malate, and alcohol dehydrogenase. J. Biol. Chem. 1987, 262, 13020–13026. [Google Scholar] [CrossRef] [PubMed]
- Radi, Z.A.; Koza-Taylor, P.H.; Bell, R.R.; Obert, L.A.; Runnels, H.A.; Beebe, J.S.; Lawton, M.P.; Sadis, S. Increased serum enzyme levels associated with kupffer cell reduction with no signs of hepatic or skeletal muscle injury. Am. J. Pathol. 2011, 179, 240–247. [Google Scholar] [CrossRef]
- Ohsawa, M.; Kimura, H. Formation of vitamin D-binding protein-actin and binary and ternary plasma gelsolin-actin complexes in human serum. Biochim. Biophys. Acta 1989, 992, 195–200. [Google Scholar] [CrossRef]
- Gressner, O.; Meier, U.; Hillebrandt, S.; Wasmuth, H.E.; Köhl, J.; Sauerbruch, T.; Lammert, F. Gc-globulin concentrations and C5 haplotype-tagging polymorphisms contribute to variations in serum activity of complement factor C5. Clin. Biochem. 2007, 40, 771–775. [Google Scholar] [CrossRef]
- Borkowski, J.; Gmyrek, G.B.; Madej, J.P.; Nowacki, W.; Goluda, M.; Gabrys, M.; Stefaniak, T.; Chelmonska-Soyta, A. Serum and peritoneal evaluation of vitamin D-binding protein in women with endometriosis. Postep. Hig. Med. Dosw. Online 2008, 62, 103–109. [Google Scholar]
- Ebashi, S.; Wakabayashi, T.; Ebashi, F. Troponin and its components. J. Biochem. 1971, 69, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.B.; Horvath, S.M.; Wilkerson, J.E. Acute blood biochemical alterations in response to marathon running. Eur. J. Appl. Physiol. Occup. Physiol. 1975, 34, 173–181. [Google Scholar] [CrossRef]
- Smith, J.E.; Garbutt, G.; Lopes, P.; Pedoe, D.T. Effects of prolonged strenuous exercise (marathon running) on biochemical and haematological markers used in the investigation of patients in the emergency department. Br. J. Sports Med. 2004, 38, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Damas, F.; Nosaka, K.; Libardi, C.A.; Chen, T.C.; Ugrinowitsch, C. Susceptibility to Exercise-Induced Muscle Damage: A Cluster Analysis with a Large Sample. Int. J. Sports Med. 2016, 37, 633–640. [Google Scholar] [CrossRef]
- Jastrzębski, Z.; Żychowska, M.; Radzimiński, Ł.; Konieczna, A.; Kortas, J. Damage to Liver and Skeletal Muscles in Marathon Runners During a 100 km Run with Regard to Age and Running Speed. J. Hum. Kinet. 2015, 45, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W.; Cooke, M.B.; Shelmadine, B.D.; Hudson, G.M.; Redd, L.; Willoughby, D.S. Effects of eccentric treadmill exercise on inflammatory gene expression in human skeletal muscle. Appl. Physiol. Nutr. Metab. 2009, 34, 745–753. [Google Scholar] [CrossRef]
- Avela, J.; Kyröläinen, H.; Komi, P.V.; Rama, D. Reduced reflex sensitivity persists several days after long-lasting stretch-shortening cycle exercise. J. Appl. Physiol. 1999, 86, 1292–1300. [Google Scholar] [CrossRef]
- Rama, D.; Margaritis, I.; Orsetti, A.; Marconnet, P.; Gros, P.; Larue, C.; Trinquier, S.; Pau, B.; Calzolari, C. Troponin I immunoenzymometric assays for detection of muscle damage applied to monitoring a triathlon. Clin. Chem. 1996, 42, 2033–2035. [Google Scholar] [CrossRef]
- Fowler, V.M.; Bennett, V. Tropomyosin: A new component of the erythrocyte membrane skeleton. Prog. Clin. Biol. Res. 1984, 159, 57–71. [Google Scholar]
- Rozmus, D.; Ciesielska, A.; Płomiński, J.; Grzybowski, R.; Fiedorowicz, E.; Kordulewska, N.; Savelkoul, H.; Kostyra, E.; Cieślińska, A. Vitamin D Binding Protein (VDBP) and Its Gene Polymorphisms-The Risk of Malignant Tumors and Other Diseases. Int. J. Mol. Sci. 2020, 21, 7822. [Google Scholar] [CrossRef]
- Dahl, B.; Schiødt, F.V.; Gehrchen, P.M.; Ramlau, J.; Kiær, T.; Ott, P. Gc-globulin is an acute phase reactant and an indicator of muscle injury after spinal surgery. Inflamm. Res. 2001, 50, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; Blum, C.B.; Ramakrishnan, R.; Dell, R.B.; Goodman, D.S. Turnover of the plasma binding protein for vitamin D and its metabolites in normal human subjects. J. Clin. Endocrinol. Metab. 1981, 53, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.G.; Harper, K.D.; Guoth, M.; Pietra, G.G.; Sanger, J.W. Angiopathic consequences of saturating the plasma scavenger system for actin. Proc. Natl. Acad. Sci. USA 1990, 87, 1381–1385. [Google Scholar] [CrossRef]
- Yu, C.-C.; Żendzian-Piotrowska, M.; Charmas, M.; Długołęcka, B.; Baranowski, M.; Górski, J.; Bucki, R. Change in blood gelsolin concentration in response to physical exercise. Biol. Sport 2013, 30, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sánchez, F.J.; Villalón, J.M.; Zamorano-León, J.J.; Rosas, L.F.; Proietti, R.; Mateos-Caceres, P.J.; González-Armengol, J.J.; Villarroel, P.; Macaya, C.; López-Farré, A.J. Functional status and inflammation after preseason training program in professional and recreational soccer players: A proteomic approach. J. Sports Sci. Med. 2011, 10, 45–51. [Google Scholar]
- Wernbom, M.; Paulsen, G.; Nilsen, T.S.; Hisdal, J.; Raastad, T. Contractile function and sarcolemmal permeability after acute low-load resistance exercise with blood flow restriction. Eur. J. Appl. Physiol. 2012, 112, 2051–2063. [Google Scholar] [CrossRef]


| Group | Age [Years] | Body Height [cm] | Body Mass [kg] | Race Result [h] |
|---|---|---|---|---|
| Marathon Run (MR) | 31.7 ± 6.3 | 180.6 ± 4.6 | 70.8 ± 2.6 | 3.5 ± 0.3 |
| Adventure Race (AR) | 31.2 ± 6.4 | 180.4 ± 4.2 | 71.8 ± 3.7 | 30.6 ± 3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borkowski, J.; Stefaniak, T.; Cych, P. Changes in Skeletal Muscle Troponin T and Vitamin D Binding Protein (DBP) Concentrations in the Blood of Male Amateur Athletes Participating in a Marathon and 100 km Adventure Race. Int. J. Environ. Res. Public Health 2023, 20, 5692. https://doi.org/10.3390/ijerph20095692
Borkowski J, Stefaniak T, Cych P. Changes in Skeletal Muscle Troponin T and Vitamin D Binding Protein (DBP) Concentrations in the Blood of Male Amateur Athletes Participating in a Marathon and 100 km Adventure Race. International Journal of Environmental Research and Public Health. 2023; 20(9):5692. https://doi.org/10.3390/ijerph20095692
Chicago/Turabian StyleBorkowski, Jacek, Tadeusz Stefaniak, and Piotr Cych. 2023. "Changes in Skeletal Muscle Troponin T and Vitamin D Binding Protein (DBP) Concentrations in the Blood of Male Amateur Athletes Participating in a Marathon and 100 km Adventure Race" International Journal of Environmental Research and Public Health 20, no. 9: 5692. https://doi.org/10.3390/ijerph20095692
APA StyleBorkowski, J., Stefaniak, T., & Cych, P. (2023). Changes in Skeletal Muscle Troponin T and Vitamin D Binding Protein (DBP) Concentrations in the Blood of Male Amateur Athletes Participating in a Marathon and 100 km Adventure Race. International Journal of Environmental Research and Public Health, 20(9), 5692. https://doi.org/10.3390/ijerph20095692






