Annual Prevalence of Opioid Receipt by South Carolina Medicaid-Enrolled Children and Adolescents: 2000–2020
Abstract
1. Introduction
2. Materials and Methods
2.1. Data
2.2. Subjects
2.3. Drugs
2.4. Outcomes
3. Results
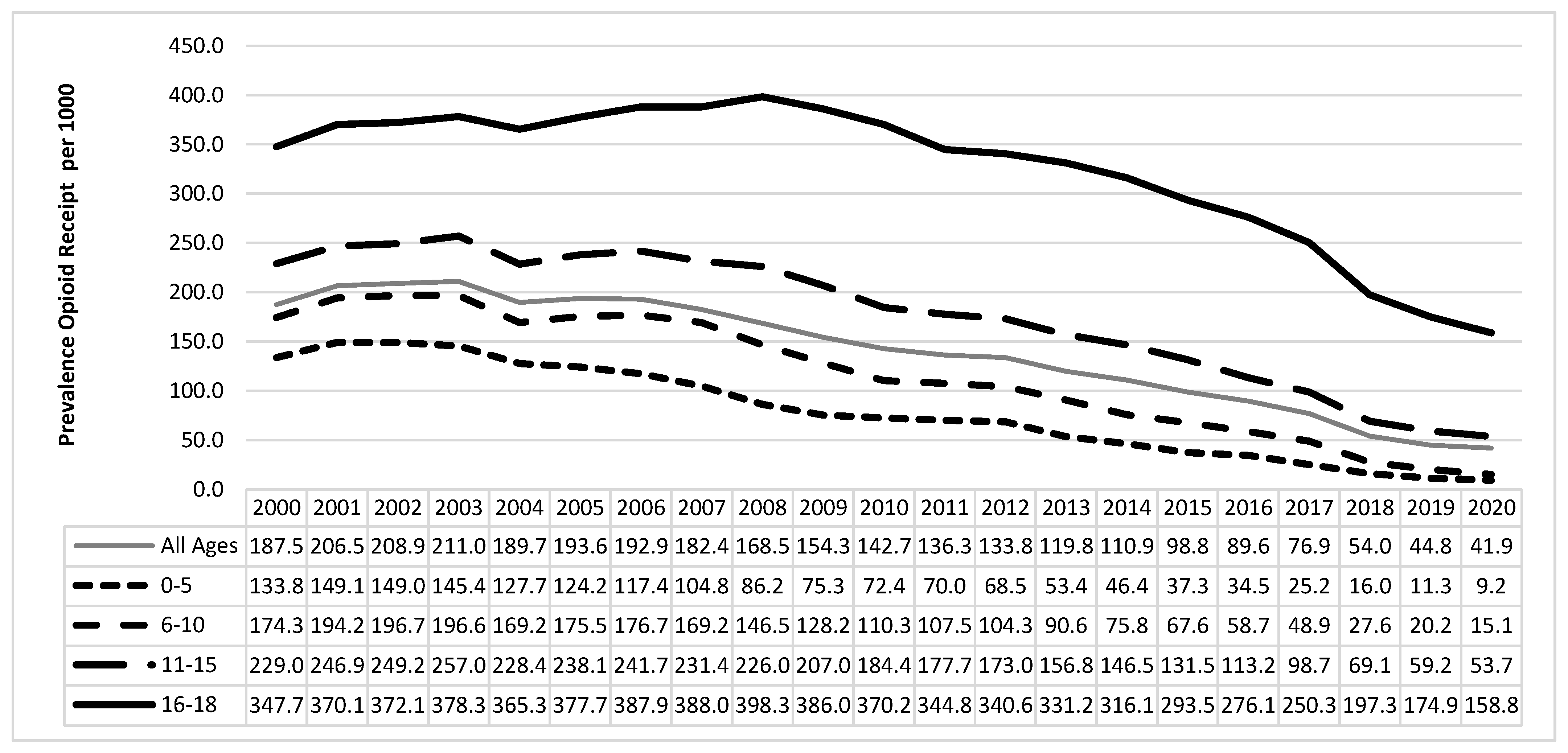
3.1. Descriptive Analyses
3.2. Unadjusted Analyses
3.3. Adjusted Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleming, M.L.; Wanat, M.A. To prescribe codeine or not to prescribe codeine? J. Pain Palliat. Care Pharmacother. 2014, 28, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Florence, C.S.; Zhou, C.; Luo, F.; Xu, L. The Economic Burden of Prescription Opioid Overdose, Abuse, and Dependence in the United States, 2013. Med. Care 2016, 54, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, B.M. FDA: No codeine after tonsillectomy for children. JAMA 2013, 309, 1100. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.D.; Green, T.P.; Coté, C.J. Section on Anesthesiology and Pain Medicine; Committee on Drugs. Codeine: Time to Say "No". Pediatrics 2016, 138, e20162396. [Google Scholar] [CrossRef]
- Woolf, A.D.; Greco, C. Why can’t we retire codeine? Pediatrics 2014, 133, e1354–e1355. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Requires Labeling Changes Forprescription Opioid Cough and Cold Medicines to Limit Their Use to Adults 18 Years and Older; FDA Drug Safety Communication: Washington, DC, USA, 2018. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-requires-labeling-changes-prescription-opioid-cough-and-cold (accessed on 13 March 2023).
- McDonald, E.M.; Kennedy-Hendricks, A.; McGinty, E.E.; Shields, W.C.; Barry, C.L.; Gielen, A.C. Safe Storage of Opioid Pain Relievers among Adults Living in Households with Children. Pediatrics 2017, 139, e20162161. [Google Scholar] [CrossRef]
- Shehab, N.; Lovegrove, M.C.; Geller, A.I.; Rose, K.O.; Weidle, N.J.; Budnitz, D.S. Us emergency department visits for outpatient adverse drug events, 2013–2014. JAMA 2016, 316, 2115–2125. [Google Scholar] [CrossRef]
- Gaw, C.E.; Curry, A.E.; Osterhoudt, K.C.; Wood, J.N.; Corwin, D.J. Characteristics of Fatal Poisonings among Infants and Young Children in the United States. Pediatrics 2023, 151, e2022059016. [Google Scholar] [CrossRef]
- George, J.A.; Kost-Byerly, S.; Monitto, C.L. Opioid therapy in children and adolescents: A physician’s guide to risk assessment, monitoring, and mitigation of abuse. J. Opioid Manag. 2013, 9, 357–368. [Google Scholar] [CrossRef]
- McCabe, S.E.; West, B.T.; Teter, C.J.; Boyd, C.J. Medical and nonmedical use of prescription opioids among high school seniors in the United States. Arch. Pediatr. Adolesc. Med. 2012, 166, 797–802. [Google Scholar] [CrossRef]
- Quinn, P.D.; Fine, K.L.; Rickert, M.E.; Sujan, A.C.; Boersma, K.; Chang, Z.; Franck, J.; Lichtenstein, P.; Larsson, H.; D’onofrio, B.M. Association of Opioid Prescription Initiation during Adolescence and Young Adulthood with Subsequent Substance-Related Morbidity. JAMA Pediatr. 2020, 174, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Miech, R.; Johnston, L.; O’Malley, P.M.; Keyes, K.M.; Heard, K. Prescription Opioids in Adolescence and Future Opioid Misuse. Pediatrics 2015, 136, e1169–e1177. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Mikulich-Gilbertson, S.K.; Sakai, J.T. Prescription Opioid Misuse and Risky Adolescent Behavior. Pediatrics 2020, 145, e20192470. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.M.; Colvin, J.D.; Bartlett, A.H.; Hall, M. Opioid-Related Critical Care Resource Use in US Children’s Hospitals. Pediatrics 2018, 141, e20173335. [Google Scholar] [CrossRef] [PubMed]
- Gaither, J.R.; Leventhal, J.M.; Ryan, S.A.; Camenga, D.R. National Trends in Hospitalizations for Opioid Poisonings among Children and Adolescents, 1997 to 2012. JAMA Pediatr. 2016, 170, 1195–1201. [Google Scholar] [CrossRef]
- Gaither, J.R.; Shabanova, V.; Leventhal, J.M. US National Trends in Pediatric Deaths from Prescription and Illicit Opioids, 1999–2016. JAMA Netw. Open 2018, 1, e186558. [Google Scholar] [CrossRef]
- American Academy of Pediatrics, Committee on Drugs. Use of codeine- and dextromethorphan-containing cough remedies in children. American Academy of Pediatrics. Committee on Drugs. Pediatrics 1997, 99, 918–920. [Google Scholar] [CrossRef]
- Kelly, L.E.; Rieder, M.; Anker, J.V.D.; Malkin, B.; Ross, C.; Neely, M.N.; Carleton, B.; Hayden, M.R.; Madadi, P.; Koren, G. More codeine fatalities after tonsillectomy in North American children. Pediatrics 2012, 129, e1343–e1347. [Google Scholar] [CrossRef]
- Galinkin, J.L. It’s Time to Rethink Use of Codeine in Pediatric Patients. AAP News, 1 September 2011; Volume 32, p. 18. [Google Scholar]
- Haffajee, R.L.; Mello, M.M.; Zhang, F.; Zaslavsky, A.M.; Larochelle, M.R.; Wharam, J.F. Four States with Robust Prescription Drug Monitoring Programs Reduced Opioid Dosages. Health Aff. 2018, 37, 964–974. [Google Scholar] [CrossRef]
- Paulozzi, L.J.; Mack, K.A.; Hockenberry, J.M. Centers for Disease Control and Prevention, Division of Unintentional Injury Prevention. Vital signs: Variation among States in prescribing of opioid pain relievers and benzodiazepines-United States, 2012. MMWR. Morb. Mortal. Wkly. Rep. 2014, 63, 563–568. [Google Scholar]
- Chung, C.P.; Callahan, S.T.; Cooper, W.O.; Dupont, W.D.; Murray, K.T.; Franklin, A.D.; Hall, K.; Dudley, J.A.; Stein, C.M.; Ray, W.A. Outpatient Opioid Prescriptions for Children and Opioid-Related Adverse Events. Pediatrics 2018, 142, e20172156. [Google Scholar] [CrossRef] [PubMed]
- Basco, W.T., Jr.; Ebeling, M.; Garner, S.S.; Hulsey, T.C.; Simpson, K. Opioid Prescribing and Potential Overdose Errors among Children 0 to 36 Months Old. Clin. Pediatr. 2015, 54, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Defalco, F.J.; Ryan, P.B.; Cepeda, M.S. Applying standardized drug terminologies to observational healthcare databases: A case study on opioid exposure. Health Serv. Outcomes Res. Methodol. 2013, 13, 58–67. [Google Scholar] [CrossRef]
- Chua, K.P.; Shrime, M.G.; Conti, R.M. Effect of FDA Investigation on Opioid Prescribing to Children after Tonsillectomy/Adenoidectomy. Pediatrics 2017, 140, e20171765. [Google Scholar] [CrossRef]
- IBM Micromedex RED BOOK [Online]. International Business Machines. Available online: https://www.ibm.com/us-en/marketplace/micromedex-red-book (accessed on 5 July 2019).
- United States Food and Drug Administration. National Drug Code Directory. Available online: http://www.fda.gov/Drugs/InformationOnDrugs/ucm142438.htm (accessed on 7 July 2020).
- Tormoehlen, L.M.; Mowry, J.B.; Bodle, J.D.; Rusyniak, D.E. Increased adolescent opioid use and complications reported to a poison control center following the 2000 JCAHO pain initiative. Clin. Toxicol. 2011, 49, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Feder, K.A.; Letourneau, E.J.; Brook, J. Children in the Opioid Epidemic: Addressing the Next Generation’s Public Health Crisis. Pediatrics 2019, 143, e20181656. [Google Scholar] [CrossRef]
- Coffin, P.; Banta-Green, C. The dueling obligations of opioid stewardship. Ann. Intern. Med. 2014, 160, 207–208. [Google Scholar] [CrossRef]
- Baker, D.W. History of The Joint Commission’s Pain Standards: Lessons for Today’s Prescription Opioid Epidemic. JAMA 2017, 317, 1117–1118. [Google Scholar] [CrossRef]
- Voepel-Lewis, T.; Malviya, S.; Tait, A.R. Inappropriate Opioid Dosing and Prescribing for Children: An Unintended Consequence of the Clinical Pain Score? JAMA Pediatr. 2017, 171, 5–6. [Google Scholar] [CrossRef]
- Tomaszewski, D.M.; Arbuckle, C.; Yang, S.; Linstead, E. Trends in Opioid Use in Pediatric Patients in US Emergency Departments from 2006 to 2015. JAMA Netw. Open 2018, 1, e186161. [Google Scholar] [CrossRef]
- Kaiser, S.V.; Asteria-Penaloza, R.; Vittinghoff, E.; Rosenbluth, G.; Cabana, M.D.; Bardach, N.S. National patterns of codeine prescriptions for children in the emergency department. Pediatrics 2014, 133, e1139–e1147. [Google Scholar] [CrossRef] [PubMed]
- Patrick, S.W.; Fry, C.E.; Jones, T.F.; Buntin, M.B. Implementation Of Prescription Drug Monitoring Programs Associated with Reductions in Opioid-Related Death Rates. Health Aff. 2016, 35, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Toce, M.S.; Michelson, K.; Hudgins, J.; Burns, M.M.; Monuteaux, M.C.; Bourgeois, F.T. Association of State-Level Opioid-Reduction Policies with Pediatric Opioid Poisoning. JAMA Pediatr. 2020, 174, 961–968. [Google Scholar] [CrossRef]
- Gugelmann, H.; Perrone, J.; Nelson, L. Windmills and pill mills: Can PDMPs tilt the prescription drug epidemic? J. Med. Toxicol. 2012, 8, 378–386. [Google Scholar] [CrossRef]
- Paulozzi, L.J.; Strickler, G.K.; Kreiner, P.W.; Koris, C.M. Controlled Substance Prescribing Patterns--Prescription Behavior Surveillance System, Eight States, 2013. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2015, 64, 1–14. [Google Scholar] [CrossRef]
- Rudikoff, A.G.; Tieu, D.D.; Banzali, F.M.; Nguyen, C.V.; Rettig, R.L.; Nashed, M.M.; Mora-Marquez, J.; Chen, Q.; Conte, A.H.; Mason, K.P. Perioperative Acetaminophen and Dexmedetomidine Eliminate Post-Operative Opioid Requirement following Pediatric Tonsillectomy. J. Clin. Med. 2022, 11, 561. [Google Scholar] [CrossRef]
- Kelley-Quon, L.I.; Ourshalimian, S.M.; Lee, J.M.; Russell, K.W.M.; Kling, K.M.; Shew, S.B.M.; Mueller, C.P.; Jensen, A.R.M.; Vu, L.M.; Padilla, B.M.; et al. Multi-Institutional Quality Improvement Project to Minimize Opioid Prescribing in Children after Appendectomy Using NSQIP-Pediatric. J. Am. Coll. Surg. 2022, 234, 290–298. [Google Scholar]
- Feinberg, A.E.; Chesney, T.R.; Srikandarajah, S.; Acuna, S.A.; McLeod, R.S. Opioid Use After Discharge in Postoperative Patients: A Systematic Review. Ann. Surg. 2018, 267, 1056–1062. (In English) [Google Scholar] [CrossRef] [PubMed]
- Poonai, N.; Datoo, N.; Ali, S.; Cashin, M.; Drendel, A.L.; Zhu, R.; Lepore, N.; Greff, M.J.E.; Rieder, M.; Bartley, D. Oral morphine versus ibuprofen administered at home for postoperative orthopedic pain in children: A randomized controlled trial. CMAJ J. 2017, 189, E1252–E1258. (In English) [Google Scholar] [CrossRef]
- Sobieraj, D.M.; Martinez, B.K.; Miao, B.; Cicero, M.X.; Kamin, R.A.; Hernandez, A.V.; Coleman, C.I.; Baker, W.L. Comparative Effectiveness of Analgesics to Reduce Acute Pain in the Prehospital Setting. Prehospital Emerg. Care 2019, 24, 163–174. [Google Scholar] [CrossRef]
- Van Winkle, P.J.; Ghobadi, A.; Chen, Q.; Menchine, M.; Sharp, A.L. Association of age and opioid use for adolescents and young adults in community emergency departments. Am. J. Emerg. Med. 2019, 37, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, M.; Hockenberry, J.M.; Raval, M.V. Opioid Fills in Children Undergoing Surgery from 2011 to 2014: A Retrospective Analysis of Relationships Among Age, Initial Days Supplied, and Refills. Ann. Surg. 2019, 274, e174–e180. [Google Scholar] [CrossRef] [PubMed]
- Basco, W.T., Jr.; Ward, R.C.; Taber, D.J.; Simpson, K.N.; Gebregziabher, M.; Cina, R.A.; McCauley, J.L.; Lockett, M.A.; Moran, W.P.; Mauldin, P.D.; et al. Patterns of dispensed opioids after tonsillectomy in children and adolescents in South Carolina, United States, 2010–2017. Int. J. Pediatr. Otorhinolaryngol. 2021, 143, 110636. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Casavant, M.J.; Spiller, H.A.; Chounthirath, T.; Hodges, N.L.; Smith, G.A. Prescription Opioid Exposures among Children and Adolescents in the United States: 2000–2015. Pediatrics 2017, 139, e20163382. [Google Scholar] [CrossRef]
- Groenewald, C.B.; Rabbitts, J.A.; Hansen, E.E.; Palermo, T.M. Racial differences in opioid prescribing for children in the United States. Pain 2018, 159, 2050–2057. [Google Scholar] [CrossRef]
- Nafiu, O.O.; Chimbira, W.T.; Stewart, M.; Gibbons, K.; Porter, L.K.; Reynolds, P.I. Racial differences in the pain management of children recovering from anesthesia. Paediatr. Anaesth. 2017, 27, 760–767. [Google Scholar] [CrossRef]
- Liao, L.; Reyes, L. Evaluating for Racial Differences in Pain Management of Long-Bone Fractures in a Pediatric Rural Population. Pediatr. Emerg. Care 2018, 37, 348–351. [Google Scholar] [CrossRef]
- Lopes, S.S.; Shi, L.; Sivaraj, L.B.; Truong, K.; Rolke, L.; Heavner, S.F.; Basco, W.T., Jr. Dispensed Opioid Prescription Patterns, by Racial/Ethnic Groups, among South Carolina Medicaid-Funded Children Experiencing Limb Fracture Injuries. Acad. Pediatr. 2022, 22, 631–639. [Google Scholar] [CrossRef]
- Monsivais, D.B.; Engebretson, J.C. “I’m Just Not That Sick”: Pain Medication and Identity in Mexican American Women with Chronic Pain. J. Holist. Nurs. 2012, 30, 188–194. [Google Scholar] [CrossRef]
- Hoffman, K.M.; Trawalter, S.; Axt, J.R.; Oliver, M.N. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc. Natl. Acad. Sci. USA 2016, 113, 4296–4301. [Google Scholar] [CrossRef]
- Hollingshead, N.A.; Meints, S.M.; Miller, M.M.; Robinson, M.E.; Hirsh, A.T. A comparison of race-related pain stereotypes held by White and Black individuals. J. Appl. Soc. Psychol. 2016, 46, 718–723. (In English) [Google Scholar] [CrossRef]
- Mulchan, S.S.; Wakefield, E.O.; Martin, S.R.; Ayr-Volta, L.; Krenicki, K.; Zempsky, W.T. Navigating Ethical Challenges for Pediatric Sickle Cell Pain Management in the Context of the Opioid Epidemic. Clin. J. Pain 2021, 38, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Basco, W.T., Jr.; Roberts, J.R.; Ebeling, M.; Garner, S.S.; Hulsey, T.C.; Simpson, K. Indications for Use of Combination Acetaminophen/Opioid Drugs in Infants <6 Months Old. Clin. Pediatr. 2018, 57, 741–744. [Google Scholar]
- Meckler, G.D.; Sheridan, D.C.; Charlesworth, C.J.; Lupulescu-Mann, N.; Kim, H.; Sun, B.C. Opioid Prescribing Practices for Pediatric Headache. J. Pediatr. 2019, 204, 240–244.e2. [Google Scholar] [CrossRef] [PubMed]
- Phang, K.G.; Roberts, J.R.; Ebeling, M.; Garner, S.S.; Basco, W.T. Opioids or Steroids for Pneumonia or Sinusitis. Pediatrics 2020, 146, e20193690. [Google Scholar] [CrossRef]
- Abou-Karam, M.; Dubé, S.; Kvann, H.S.; Mollica, C.; Racine, D.; Bussières, J.-F.; Lebel, D.; Nguyen, C.; Thibault, M. Parental Report of Morphine Use at Home after Pediatric Surgery. J. Pediatr. 2015, 167, 599–604.e2. [Google Scholar] [CrossRef]
- Monitto, C.L.; Hsu, A.; Gao, S.; Vozzo, P.T.; Park, P.S.; Roter, D.; Yenokyan, G.; White, E.D.; Kattail, D.; Edgeworth, A.E.; et al. Opioid Prescribing for the Treatment of Acute Pain in Children on Hospital Discharge. Anesth. Analg. 2017, 125, 2113–2122. [Google Scholar] [CrossRef]
- Pruitt, L.C.C.; Swords, D.S.; Russell, K.W.; Rollins, M.D.; Skarda, D.E. Prescription vs. consumption: Opioid overprescription to children after common surgical procedures. J. Pediatr. Surg. 2019, 54, 2195–2199. [Google Scholar] [CrossRef]
- Corona, L.E.; Roth, E.B.; Thao, A.; Lin, M.; Lee, T.; Harbaugh, C.; Gadepalli, S.; Waljee, J.; Streur, C.S. Opioid prescribing is excessive and variable after pediatric ambulatory urologic surgery. J. Pediatr. Urol. 2021, 17, 259.e1–259.e6. [Google Scholar] [CrossRef]
- Neill, L.A.; Kim, H.S.; Cameron, K.A.; Lank, P.M.; Patel, D.A.; Hur, S.I.; Opsasnick, L.A.; Curtis, L.M.; Eifler, M.R.; Courtney, D.M.; et al. Who Is Keeping Their Unused Opioids and Why? Pain Med. 2019, 21, 84–91. [Google Scholar] [CrossRef]
- Kuehn, B.M. CDC: Major disparities in opioid prescribing among states: Some states crack down on excess prescribing. JAMA 2014, 312, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Dowell, D.; Ragan, K.R.; Jones, C.M.; Baldwin, G.T.; Chou, R. CDC Clinical Practice Guideline for Prescribing Opioids for Pain—United States, 2022. MMWR. Recomm. Rep. 2022, 71, 1–95. [Google Scholar] [PubMed]

| Variable | Total N (Person-Years) | Received Opioid N Prevalence (95% CI) c | No Opioid N Prevalence (95% CI) c |
|---|---|---|---|
| Age group (years) b | |||
| 0–5 | 3,261,659 | 250,218 76.7 (76.4, 77.0) | 3,011,441 923.3 (922.2, 924.3) |
| 6–10 | 2,043,264 | 219,082 107.2 (106.8, 107.7) | 1,824,182 892.8 (891.5, 894.1) |
| 11–15 | 1,698,041 | 285,284 168.0 (167.4, 168.6) | 1,412,757 832.0 (830.6, 833.4) |
| 16–18 | 1,028,847 | 330,186 320.9 (319.8, 322.0) | 698,661 679.1 (677.5, 680.7) |
| Sex b | |||
| Female | 4,003,077 | 563,812 140.8 (140.5, 141.2) | 3,439,265 859.2 (858.2, 860.1) |
| Male | 4,028,482 | 520,900 129.3 (129.0, 129.7) | 3,507,582 870.7 (869.8, 871.6) |
| Race/Ethnicity b,d | |||
| White | 3,046,257 | 514,325 168.8 (168.4, 169.3) | 2,531,932 831.2 (830.1, 832.2) |
| Black | 3,376,191 | 428,202 126.8 (126.5, 127.2) | 2,947,989 873.2 (872.2, 874.2) |
| Hispanic | 318,014 | 32,937 103.6 (102.5, 104.7) | 285,077 896.4 (893.1, 899.7) |
| Other | 1,291,349 | 109,306 84.6 (84.1, 85.1) | 1,182,043 915.4 (913.7, 917.0) |
| Variable | Adjusted a Odds Ratio (95% Confidence Intervals) | |
|---|---|---|
| Calendar Year | 0.927 (0.926, 0.927) | |
| Age Category | 0–5 years | reference |
| 6–10 years | 1.525 (1.515, 1.534) | |
| 11–15 years | 2.580 (2.565, 2.595) | |
| 16–18 years | 6.284 (6.246, 6.322) | |
| Race/Ethnicity | White race | reference |
| Black race | 0.649 (0.646, 0.652) | |
| Hispanic ethnicity b | 0.732 (0.723, 0.741) | |
| Other race | 0.736 (0.731, 0.741) | |
| Sex | Female | reference |
| Male | 1.016 (1.011, 1.020) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basco, W.T., Jr.; Bundy, D.G.; Garner, S.S.; Ebeling, M.; Simpson, K.N. Annual Prevalence of Opioid Receipt by South Carolina Medicaid-Enrolled Children and Adolescents: 2000–2020. Int. J. Environ. Res. Public Health 2023, 20, 5681. https://doi.org/10.3390/ijerph20095681
Basco WT Jr., Bundy DG, Garner SS, Ebeling M, Simpson KN. Annual Prevalence of Opioid Receipt by South Carolina Medicaid-Enrolled Children and Adolescents: 2000–2020. International Journal of Environmental Research and Public Health. 2023; 20(9):5681. https://doi.org/10.3390/ijerph20095681
Chicago/Turabian StyleBasco, William T., Jr., David G. Bundy, Sandra S. Garner, Myla Ebeling, and Kit N. Simpson. 2023. "Annual Prevalence of Opioid Receipt by South Carolina Medicaid-Enrolled Children and Adolescents: 2000–2020" International Journal of Environmental Research and Public Health 20, no. 9: 5681. https://doi.org/10.3390/ijerph20095681
APA StyleBasco, W. T., Jr., Bundy, D. G., Garner, S. S., Ebeling, M., & Simpson, K. N. (2023). Annual Prevalence of Opioid Receipt by South Carolina Medicaid-Enrolled Children and Adolescents: 2000–2020. International Journal of Environmental Research and Public Health, 20(9), 5681. https://doi.org/10.3390/ijerph20095681





