Inter-Examiner and Intra-Examiner Reliability of Quantitative and Qualitative Ultrasonography Assessment of Peripheral and Respiratory Muscles in Critically Ill Patients
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design, Setting, and Ethical Considerations
2.2. Population, Sample, Inclusion, and Exclusion Criteria
2.3. Data Collection
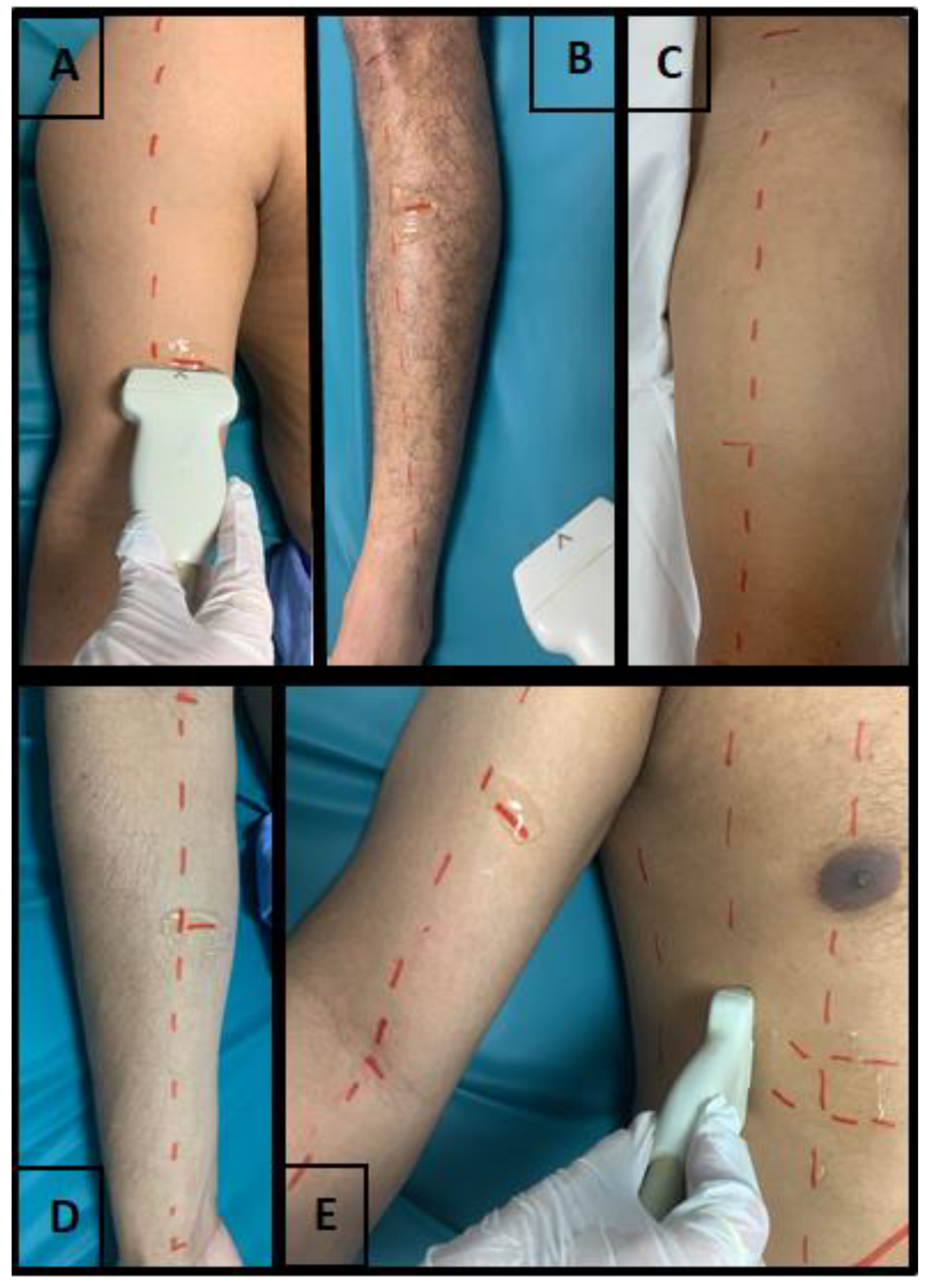
2.4. Ultrasonography Assessment Protocol
2.4.1. Diaphragmatic Assessment
2.4.2. Peripheral Muscles Assessment
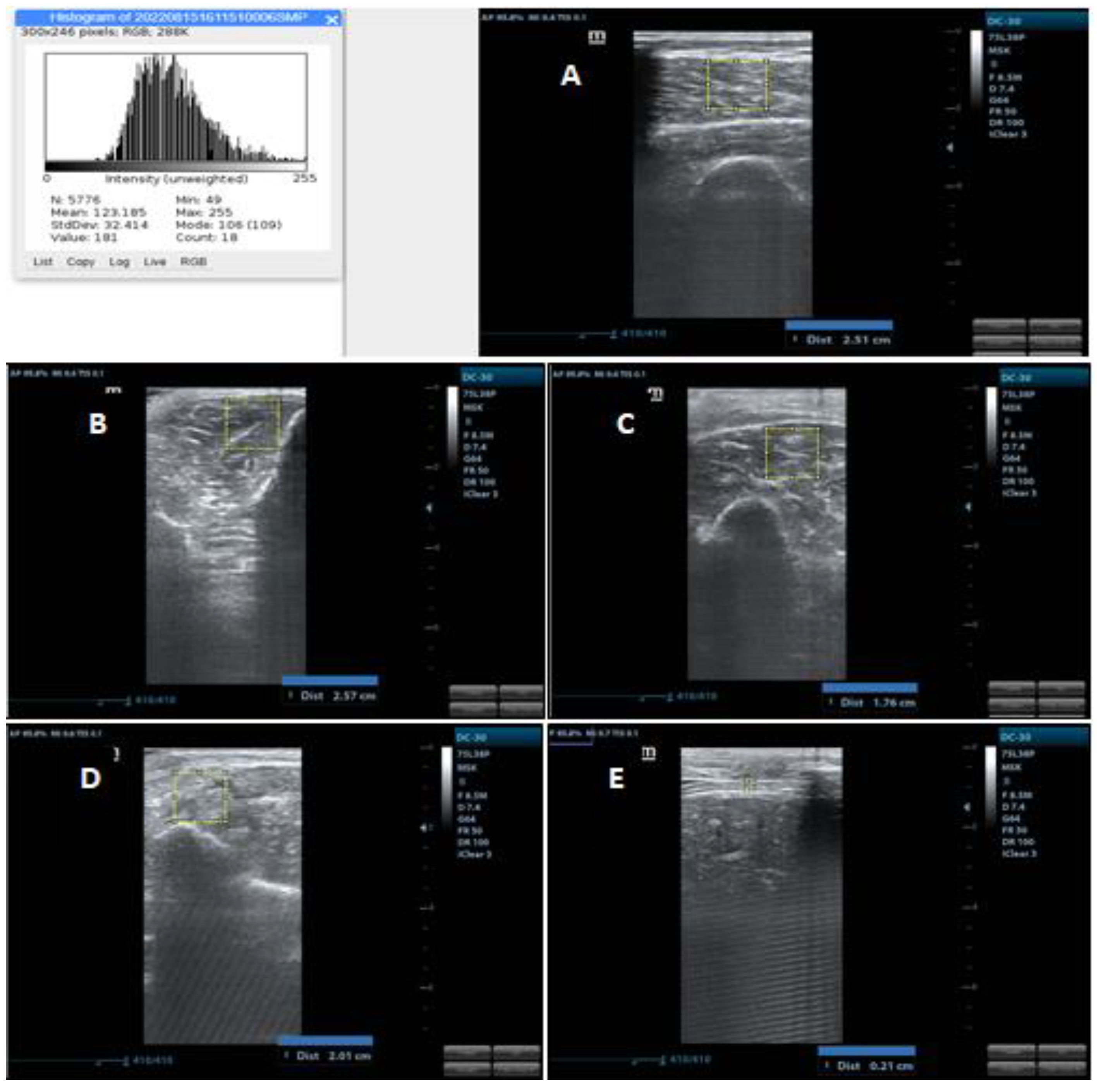
2.4.3. Muscle Thickness
2.4.4. Muscle Echogenicity
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez, B.; Alves, G.A. Muscle Assessment in Intensive Care Unit. PROFISIO 2017, 3, 51–79. [Google Scholar]
- Wischmeyer, P.E.; San-Millan, I. Winning the War against ICU-Acquired Weakness: New Innovations in Nutrition and Exercise Physiology. Crit. Care 2015, 19, S6. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, C.L.; Stiller, K.; Needham, D.M.; Tipping, C.J.; Harrold, M.; Baldwin, C.E.; Bradley, S.; Berney, S.; Caruana, L.R.; Elliott, D.; et al. Expert Consensus and Recommendations on Safety Criteria for Active Mobilization of Mechanically Ventilated Critically Ill Adults. Crit. Care 2014, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moisey, L.L.; Mourtzakis, M.; Cotton, B.A.; Premji, T.; Heyland, D.K.; Wade, C.E.; Bulger, E.; Kozar, R.A. Skeletal Muscle Predicts Ventilator-Free Days, ICU-Free Days, and Mortality in Elderly ICU Patients. Crit. Care 2013, 17, 1. [Google Scholar] [CrossRef]
- Tillquist, M.; Kutsogiannis, D.J.; Wischmeyer, P.E.; Kummerlen, C.; Leung, R.; Stollery, D.; Karvellas, C.J.; Preiser, J.C.; Bird, N.; Kozar, R.; et al. Bedside Ultrasound Is a Practical and Reliable Measurement Tool for Assessing Quadriceps Muscle Layer Thickness. J. Parenter. Enter. Nutr. 2014, 38, 886–890. [Google Scholar] [CrossRef]
- Paris, M.T.; Mourtzakis, M.; Day, A.; Leung, R.; Watharkar, S.; Kozar, R.; Earthman, C.; Kuchnia, A.; Dhaliwal, R.; Moisey, L.; et al. Validation of Bedside Ultrasound of Muscle Layer Thickness of the Quadriceps in the Critically Ill Patient (VALIDUM Study). J. Parenter. Enter. Nutr. 2017, 41, 171–180. [Google Scholar] [CrossRef]
- Perkisas, S.; Bastijns, S.; Baudry, S.; Bauer, J.; Beaudart, C.; Beckwée, D.; Cruz-Jentoft, A.; Gasowski, J.; Hobbelen, H.; Jager-Wittenaar, H.; et al. Application of Ultrasound for Muscle Assessment in Sarcopenia: 2020 SARCUS Update. Eur. Geriatr. Med. 2021, 12, 45–59. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, J.; Gu, Q.; Gu, Y.; Zhao, Y.; Ge, X.; Sun, X.; Lian, J.; Zeng, Q. Changes in Muscle Ultrasound for the Diagnosis of Intensive Care Unit Acquired Weakness in Critically Ill Patients. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Phadke, R.; Rawal, J.; McPhail, M.J.W.; Sidhu, P.S.; Rowlerson, A.; Moxham, J.; Harridge, S.; Hart, N.; Montgomery, H.E. Qualitative Ultrasound in Acute Critical Illness Muscle Wasting. Crit. Care Med. 2015, 43, 1603–1611. [Google Scholar] [CrossRef]
- Parry, S.M.; El-Ansary, D.; Cartwright, M.S.; Sarwal, A.; Berney, S.; Koopman, R.; Annoni, R.; Puthucheary, Z.; Gordon, I.R.; Morris, P.E.; et al. Ultrasonography in the Intensive Care Setting Can Be Used to Detect Changes in the Quality and Quantity of Muscle and Is Related to Muscle Strength and Function. J. Crit. Care 2015, 30, 1151.e9–1151.e14. [Google Scholar] [CrossRef]
- Hayes, K.; Holland, A.E.; Pellegrino, V.A.; Mathur, S.; Hodgson, C.L. Acute Skeletal Muscle Wasting and Relation to Physical Function in Patients Requiring Extracorporeal Membrane Oxygenation (ECMO). J. Crit. Care 2018, 48, 1–8. [Google Scholar] [CrossRef]
- Mayer, K.P.; Thompson Bastin, M.L.; Montgomery-Yates, A.A.; Pastva, A.M.; Dupont-Versteegden, E.E.; Parry, S.M.; Morris, P.E. Acute Skeletal Muscle Wasting and Dysfunction Predict Physical Disability at Hospital Discharge in Patients with Critical Illness. Crit. Care 2020, 24, 1–12. [Google Scholar] [CrossRef]
- Palakshappa, J.A.; Reilly, J.P.; Schweickert, W.D.; Anderson, B.J.; Khoury, V.; Shashaty, M.G.; Fitzgerald, D.; Forker, C.; Butler, K.; Ittner, C.A.; et al. Quantitative Peripheral Muscle Ultrasound in Sepsis: Muscle Area Superior to Thickness. J. Crit. Care 2018, 47, 324–330. [Google Scholar] [CrossRef]
- Jaber, S.; Petrof, B.J.; Jung, B.; Chanques, G.; Berthet, J.P.; Rabuel, C.; Bouyabrine, H.; Courouble, P.; Koechlin-Ramonatxo, C.; Sebbane, M.; et al. Rapidly Progressive Diaphragmatic Weakness and Injury during Mechanical Ventilation in Humans. Am. J. Respir. Crit. Care Med. 2011, 183, 364–371. [Google Scholar] [CrossRef]
- Mateos-angulo, A.; Galán-mercant, A.; Cuesta-vargas, A.I. Muscle Thickness and Echo Intensity by Ultrasonography and Cognitive and Physical Dimensions in Older Adults. Diagnostics 2021, 11, 1471. [Google Scholar] [CrossRef]
- Sarwal, A.; Parry, S.M.; Berry, M.J.; Hsu, F.C.; Lewis, M.T.; Justus, N.W.; Morris, P.E.; Denehy, L.; Berney, S.; Dhar, S.; et al. Interobserver Reliability of Quantitative Muscle Sonographic Analysis in the Critically Ill Population. J. Ultrasound Med. 2015, 34, 1191–1200. [Google Scholar] [CrossRef]
- Mayer, K.P.; Dhar, S.; Cassity, E.; Denham, A.; England, J.; Morris, P.E.; Dupont-versteegden, E.E. Interrater Reliability of Muscle Ultrasonography Image Acquisition by Physical Therapists in Patients Who Have or Who Survived Critical Illness. Phys. Ther. 2020, 100, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Formenti, P.; Umbrello, M.; Coppola, S.; Froio, S.; Chiumello, D. Clinical Review: Peripheral Muscular Ultrasound in the ICU. Ann. Intensive Care 2019, 9. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Parry, S.; Connolly, B.; Puthucheary, Z. Skeletal Muscle Ultrasound in Critical Care: A Tool in Need of Translation. Ann. Am. Thorac. Soc. 2017, 14, 1495–1503. [Google Scholar] [CrossRef]
- Casey, P.; Alasmar, M.; McLaughlin, J.; Ang, Y.; McPhee, J.; Heire, P.; Sultan, J. The Current Use of Ultrasound to Measure Skeletal Muscle and Its Ability to Predict Clinical Outcomes: A Systematic Review. J. Cachexia. Sarcopenia Muscle 2022, 13, 2298–2309. [Google Scholar] [CrossRef] [PubMed]
- Kottner, J.; Audigé, L.; Brorson, S.; Donner, A.; Gajewski, B.J.; Hróbjartsson, A.; Roberts, C.; Shoukri, M.; Streiner, D.L. Guidelines for Reporting Reliability and Agreement Studies (GRRAS) Were Proposed. J. Clin. Epidemiol. 2011, 64, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.D.; Eliasziw, M.; Donner, A. Sample Size and Optimal Designs for Reliability Studies. Stat. Med. 1998, 17, 101–110. [Google Scholar] [CrossRef]
- Schepens, T.; Goligher, E.C. Using Ultrasound to Prevent Diaphragm Dysfunction. ICU Manag. Pract. 2018, 18. [Google Scholar]
- Arts, I.M.P.; Pillen, S.; Schelhaas, H.J.; Overeem, S.; Zwarts, M.J. Normal Values for Quantitative Muscle Ultrasonography in Adults. Muscle Nerve 2010, 41, 32–41. [Google Scholar] [CrossRef]
- Gruther, W.; Benesch, T.; Zorn, C.; Paternostro-Sluga, T.; Quittan, M.; Fialka-Moser, V.; Spiss, C.; Kainberger, F.; Crevenna, R. Muscle Wasting in Intensive Care Patients: Ultrasound Observation of the M. Quadriceps Femoris Muscle Layer. J. Rehabil. Med. 2008, 40, 185–189. [Google Scholar] [CrossRef]
- Cartwright, M.S.; Kwayisi, G.; Griffin, L.P.; Sarwal, A.; Walker, F.O.; Harris, J.M.; Berry, M.J.; Chahal, P.S.; Morris, P.E. Quantitative Neuromuscular Ultrasound in the Intensive Care Unit. Muscle Nerve 2013, 47, 255–259. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, Criteria, and Rules of Thumb for Evaluating Normed and Standardized Assessment Instruments in Psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Nagae, M.; Umegaki, H.; Yoshiko, A.; Fujita, K. Muscle Ultrasound and Its Application to Point-of-Care Ultrasonography: A Narrative Review. Ann. Med. 2023, 55, 190–197. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Jiang, Y.; Su, N.; Li, J.; Kang, L.; Zhang, Y.; Yang, M. Evaluation of Appendicular Muscle Mass in Sarcopenia in Older Adults Using Ultrasonography: A Systematic Review and Meta-Analysis. Gerontology 2022, 68, 1174–1198. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Hadda, V.; Kumar, R.; Hussain, T.; Khan, M.A.; Madan, K.; Mohan, A.; Khilnani, G.C.; Guleria, R. Reliability of Ultrasonographic Arm Muscle Thickness Measurement by Various Levels of Health Care Providers in ICU. Clin. Nutr. ESPEN 2018, 24, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Baston, C.M.; Gellhorn, A.C.; Hough, C.L.; Bunnell, A.E. Interrater Reliability of Quantitative Ultrasound Measures of Muscle in Critically Ill Patients. PMR 2022, 14, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Dhungana, A.; Khilnani, G.; Hadda, V.; Guleria, R. Reproducibility of Diaphragm Thickness Measurements by Ultrasonography in Patients on Mechanical Ventilation. World J. Crit. Care Med. 2017, 6, 185–189. [Google Scholar] [CrossRef]
- Hadda, V.; Kumar, R.; Madan, K.; Khan, M.; Mohan, A.; Khilnani, G.; Guleria, R. Intraobserver Variability and Reliability of Diaphragm Thickness Measurement on Ultrasonography by Critical Care Physician Among Patients with Sepsis. J. Assoc. Chest Physicians 2019, 7, 18. [Google Scholar] [CrossRef]
- Boon, A.J.; Harper, C.J.; Ghahfarokhi, L.S.; Strommen, J.A.; Watson, J.C.; Sorenson, E.J. Two-Dimensional Ultrasound Imaging of the Diaphragm: Quantitative Values in Normal Subjects. Muscle Nerve 2013, 47, 884–889. [Google Scholar] [CrossRef]
- Baldwin, C.E.; Paratz, J.D.; Bersten, A.D. Diaphragm and Peripheral Muscle Thickness on Ultrasound: Intra-Rater Reliability and Variability of a Methodology Using Non-Standard Recumbent Positions. Respirology 2011, 16, 1136–1143. [Google Scholar] [CrossRef]
- Vieira, L.; Rocha, L.P.B.; Mathur, S.; Santana, L.; de Melo, P.F.; da Silva, V.Z.M.; Durigan, J.L.Q.; Cipriano, G. Reliability of Skeletal Muscle Ultrasound in Critically Ill Trauma Patients. Rev. Bras. Ter. Intensiv. 2019, 31, 464–473. [Google Scholar] [CrossRef]
- Abiko, T.; Ohmae, K.; Murata, S.; Shiraiwa, K.; Horie, J. Reliability of Muscle Thickness and Echo Intensity Measurements of the Quadriceps: A Novice Examiner. J. Bodyw. Mov. Ther. 2022, 31, 164–168. [Google Scholar] [CrossRef]
- Zaidman, C.M.; Wu, J.S.; Wilder, S.; Darras, B.T.; Rutkove, S.B. Minimal Training Is Required to Reliably Perform Quantitative Ultrasound of Muscle. Muscle Nerve 2014, 50, 124. [Google Scholar] [CrossRef]
| Characteristics | Results |
|---|---|
| Age in years (Mean ± SD) | 62 (±18.92) |
| Sex | |
| Male (%) | 60% |
| Female (%) | 40% |
| Condition for admission to the ICU | |
| Clinical (%) | 70% |
| Surgical (%) | 30% |
| Days of ICU stay at the time of assessment (Mean ± SD) | 9.10 (±6.14) |
| Use of mechanical ventilation during ICU stay | |
| Yes (%) | 90% |
| No (%) | 10% |
| SAPSII (Mean ± SD) | 68.40 (±16.10) |
| Examiners | Mean (±SD) in cm | |
|---|---|---|
| Biceps brachii | Examiner 1 | 2.47 (±0.70) |
| Examiner 2 | 1.98 (±0.52) | |
| Examiner 3 | 2.46 (±0.52) | |
| Examiner 4 | 2.59 (±0.61) | |
| Forearm flexor group | Examiner 1 | 2.46 (±0.47) |
| Examiner 2 | 2.01 (±0.34) | |
| Examiner 3 | 2.53 (±0.52) | |
| Examiner 4 | 2.32 (±0.40) | |
| Quadriceps femoris | Examiner 1 | 2.64 (±0.64) |
| Examiner 2 | 2.68 (±0.83) | |
| Examiner 3 | 3.06 (±0.87) | |
| Examiner 4 | 2.73 (±0.70) | |
| Tibialis anterior | Examiner 1 | 1.95 (±0.29) |
| Examiner 2 | 1.92 (±0.28) | |
| Examiner 3 | 2.02 (±0.28) | |
| Examiner 4 | 1.96 (±0.24) | |
| Diaphragm | Examiner 1 | 0.20 (±0.06) |
| Examiner 2 | 0.21 (±0.08) | |
| Examiner 3 | 0.22 (±0.07) | |
| Examiner 4 | 0.21 (±0.04) |
| Biceps Brachii | Forearm Flexor Group | Quadriceps Femoris | Tibialis Anterior | Diaphragm | |
|---|---|---|---|---|---|
| Mean (±SD) in cm | 119.23 (±27.15) | 131.30 (±27.56) | 124.65 (±28.37) | 137.17 (±22.71) | 124.43 (±24.83) |
| ICC | 0.952 | 0.973 | 0.970 | 0.954 | 0.867 |
| Correlation | Excellent | Excellent | Excellent | Excellent | Excellent |
| Muscles | Intra-Examiner Reliability | Inter-Examiner Reliability | |||
|---|---|---|---|---|---|
| Examiners | Results | Correlation | Results | Correlation | |
| Biceps brachii | Examiner 1 | 0.982 | Excellent | 0.894 | Excellent |
| Examiner 2 | 0.951 | Excellent | |||
| Examiner 3 | 0.977 | Excellent | |||
| Examiner 4 | 0.988 | Excellent | |||
| Forearm flexor group | Examiner 1 | 0.972 | Excellent | 0.789 | Excellent |
| Examiner 2 | 0.918 | Excellent | |||
| Examiner 3 | 0.942 | Excellent | |||
| Examiner 4 | 0.922 | Excellent | |||
| Quadriceps femoris | Examiner 1 | 0.950 | Excellent | 0.917 | Excellent |
| Examiner 2 | 0.984 | Excellent | |||
| Examiner 3 | 0.972 | Excellent | |||
| Examiner 4 | 0.960 | Excellent | |||
| Tibialis anterior | Examiner 1 | 0.924 | Excellent | 0.942 | Excellent |
| Examiner 2 | 0.913 | Excellent | |||
| Examiner 3 | 0.930 | Excellent | |||
| Examiner 4 | 0.902 | Excellent | |||
| Diaphragm | Examiner 1 | 0.718 | Good | 0.778 | Excellent |
| Examiner 2 | 0.972 | Excellent | |||
| Examiner 3 | 0.931 | Excellent | |||
| Examiner 4 | 0.798 | Excellent | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, F.D.S.; Dos Santos, J.L.; Alves, M.E.D.; Alves, J.d.Á.B.; Cerqueira, T.C.F.; De Santana Filho, V.J. Inter-Examiner and Intra-Examiner Reliability of Quantitative and Qualitative Ultrasonography Assessment of Peripheral and Respiratory Muscles in Critically Ill Patients. Int. J. Environ. Res. Public Health 2023, 20, 5636. https://doi.org/10.3390/ijerph20095636
Barbosa FDS, Dos Santos JL, Alves MED, Alves JdÁB, Cerqueira TCF, De Santana Filho VJ. Inter-Examiner and Intra-Examiner Reliability of Quantitative and Qualitative Ultrasonography Assessment of Peripheral and Respiratory Muscles in Critically Ill Patients. International Journal of Environmental Research and Public Health. 2023; 20(9):5636. https://doi.org/10.3390/ijerph20095636
Chicago/Turabian StyleBarbosa, Felipe Douglas Silva, José Lucas Dos Santos, Maria Emilia Dantas Alves, Juliana de Ávila Barreto Alves, Telma Cristina Fontes Cerqueira, and Valter Joviniano De Santana Filho. 2023. "Inter-Examiner and Intra-Examiner Reliability of Quantitative and Qualitative Ultrasonography Assessment of Peripheral and Respiratory Muscles in Critically Ill Patients" International Journal of Environmental Research and Public Health 20, no. 9: 5636. https://doi.org/10.3390/ijerph20095636
APA StyleBarbosa, F. D. S., Dos Santos, J. L., Alves, M. E. D., Alves, J. d. Á. B., Cerqueira, T. C. F., & De Santana Filho, V. J. (2023). Inter-Examiner and Intra-Examiner Reliability of Quantitative and Qualitative Ultrasonography Assessment of Peripheral and Respiratory Muscles in Critically Ill Patients. International Journal of Environmental Research and Public Health, 20(9), 5636. https://doi.org/10.3390/ijerph20095636






