Biological, Psychiatric, Psychosocial, and Cognitive Factors of Poststroke Depression
Abstract
1. Introduction
2. Materials & Methods
2.1. Sample
2.2. Inclusion Criteria
- Completed neuropsychological and psychological assessment. Participants who were evaluated by the Neurosurgery department’s Neuropsychology Service of the Houston Methodist Neurological Institute at the Houston Methodist Hospital between 1 July 2009 and 6 September 2019.
- Verified stroke: Location and lateralization of strokes were defined by neuroimaging neuroradiologist clinical reports. Lateralization of strokes included 44.9% left hemisphere, 41.2% right hemisphere, and 13.8% bilateral. Locations of strokes were as follows: 35.7% MCA, 23.1% frontal lobe/ACA stroke, 14.8% cerebellar, 9.9% basal ganglia, 6.5% pontine, 4.6% thalamic, 3.4% PCA, 3.4% occipital, and 1.8% parietal. Stroke types included 65.1% ischemic and 31.9% hemorrhagic.
2.3. Exclusion Criteria
- Under the age of 18-years-old.
- A Mini Mental Status Exam score below 20 or invalid testing. All evaluations were deemed valid or invalid before interpreting the results for clinical purposes by considering comprehension, effort, and language proficiency. Often, it was one neurocognitive test that was deemed invalid rather than the entire evaluation.
- When testing was deemed invalid due to sensory loss. Some patients had reduced hearing, and if their hearing devices were not deemed adequate, an additional hearing device (earphones and an amplifier) was provided. Patients were included with visual field cuts and visual neglect. No patients were excluded because of immobility. If patients could not use their upper extremities to write, non-motor neurocognitive tests were used to define memory or executive impairments.
- A stroke history on multiple occasions.
- Those with other non-stroke-related neurologic diagnoses that would affect PSD (epilepsy, TBI, dementia). Patients with a history of stroke and a diagnosis of Vascular dementia were also excluded.
- Unknown type of stroke (ischemic, hemorrhagic) based on the neuroradiology report and clinical chart review.
2.4. Procedures
2.5. Statistical Analyses
- stroke type (ischemic hemorrhagic);
- gender;
- % employed;
- % married;
- % with sleep difficulties;
- % with fatigue;
- % with history of treatment for depression, anxiety;
- % current anxiety disorder;
- % memory problems;
- % executive difficulties.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chun, H.-Y.Y.; Ford, A.; Kutlubaev, M.; Almeida, O.; Mead, G. Depression, Anxiety, and Suicide After Stroke: A Narrative Review of the Best Available Evidence. Stroke 2022, 53, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, G.C.; Roy, D.; Kontos, N.; Beach, S.R. Post-stroke depression: A 2020 updated review. Gen. Hosp. Psychiatry 2020, 66, 70–80. [Google Scholar] [CrossRef]
- Ayerbe, L.; Ayis, S.; Crichton, S.; Wolfe, C.D.; Rudd, A.G. The natural history of depression up to 15 years after stroke: The South London Stroke Register. Stroke 2013, 44, 1105–1110. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 15 October 2022).
- Xie, J.; Geng, X.; Fan, F.; Fu, X.; He, S.; Li, T. The efficacy of therapies for post-stroke depression in aging: An umbrella review. Front. Aging Neurosci. 2022, 23, 993250. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Sheth, B.; Gill, J.; Yadegarfar, M.; Stubbs, B.; Yadegarfar, M.; Meader, N. Prevalence and predictors of post-stroke mood disorders: A meta-analysis and meta-regression of depression, anxiety and adjustment disorder. Gen. Hosp. Psychiatry 2017, 47, 48–60. [Google Scholar] [CrossRef]
- Barker-Collo, S.; Feigin, V.L. The Impact of Neuropsychological Deficits on Functional Stroke Outcomes. Neuropsychol. Rev. 2006, 16, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.G.; Jorge, R.E. Post-Stroke Depression: A Review. Am. J. Psychiatry 2016, 173, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.G.; Starkstein, S.E. Current research in affective disorders following stroke. J. Neuropsychiatry Clin. Neurosci. 1990, 2, 1–14. [Google Scholar] [CrossRef]
- Kutlubaev, M.A.; Hackett, M.L. Part II: Predictors of Depression after Stroke and Impact of Depression on Stroke Outcome: An Updated Systematic Review of Observational Studies. Int. J. Stroke 2014, 9, 1026–1036. [Google Scholar] [CrossRef]
- Hosking, S.G.; Marsh, N.V. Predictors of Depression at One Year Post-stroke in Older Adults. Brain Impair. 2013, 14, 381–391. [Google Scholar] [CrossRef]
- Santos, M.; Kövari, E.; Gold, G.; Bozikas, V.P.; Hof, P.R.; Bouras, C.; Giannakopoulos, P. The neuroanatomical model of post-stroke depression: Towards a change of focus? J. Neurol. Sci. 2009, 283, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, V.; Gallarda, T.; Calvet, D.; Turc, G.; Oppenheim, C.; Rouillon, F.; Mas, J.-L. Depression predictors within six months of ischemic stroke: The DEPRESS Study. Int. J. Stroke 2016, 11, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, J.-R.; Liang, H.-B.; Lu, W.-J.; Yang, G.-Y.; Liu, J.-R.; Zeng, L.-L. Diabetes mellitus is associated with late-onset post-stroke depression. J. Affect. Disord. 2017, 221, 222–226. [Google Scholar] [CrossRef]
- Chaudhary, D.; Friedenberg, I.; Sharma, V.; Sharma, P.; Abedi, V.; Zand, R.; Li, J. Predictors of Post-Stroke Depression: A Retrospective Cohort Study. Brain Sci. 2022, 12, 993. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, T.S.H.; Wium-Andersen, I.K.; Wium-Andersen, M.K.; Jørgensen, M.B.; Prescott, E.; Maartensson, S.; Kragh-Andersen, P.; Osler, M. Incidence of Depression After Stroke, and Associated Risk Factors and Mortality Outcomes, in a Large Cohort of Danish Patients. JAMA Psychiatry 2016, 73, 1032–1040. [Google Scholar] [CrossRef]
- Terroni, L.D.M.N.; Sobreiro, M.F.; Conforto, A.; Adda, C.C.; Guajardo, V.D.; De Lucia, M.C.S.; Fraguas, R. Association among depression, cognitive impairment and executive dysfunction after stroke. Dement. Neuropsychol. 2012, 6, 152–157. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, D.; Zeng, Y.; Wu, W. Risk Factors for Post-stroke Depression: A Meta-analysis. Front. Aging Neurosci. 2017, 9, 218. [Google Scholar] [CrossRef]
- Mayman, N.; Stein, L.K.; Erdman, J.; Kornspun, A.; Tuhrim, S.; Jette, N.; Dhamoon, M.S. Risk and Predictors of Depression Following Acute Ischemic Stroke in the Elderly. Neurology 2021, 96, e2184–e2191. [Google Scholar] [CrossRef]
- Lezak, M.D.; Howieson, D.B.; Bigler, E.D.; Tranel, D. Neuropsychological Assessment, 5th ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Balker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview. J. Clin. Psychiatry 1998, 59, 22–33. [Google Scholar]
- Tennen, H.; McKee, T.E.; Gernert-Dott, P.; Affleck, G. Depressive symptoms, and history of depression predict rehabilitation efficiency in stroke patients. Arch. Phys. Med. Rehabil. 2001, 82, 1645–1649. [Google Scholar]
- Morrison, V.; Pollard, B.; Johnston, M.; MacWalter, R. Anxiety and depression 3 years following stroke: Demographic, clinical, and psychological predictors. J. Psychosom. Res. 2005, 59, 209–213. [Google Scholar] [CrossRef]
- Sagen, U.; Finset, A.; Moum, T.; Mørland, T.; Vik, T.G.; Nagy, T.; Dammen, T. Early detection of patients at risk for anxiety, depression and apathy after stroke. Gen. Hosp. Psychiatry 2010, 32, 80–85. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, F.; Sznajder, K.K.; Zou, C.; Jia, Y.; Yang, X. Resilience as the Mediating Factor in the Relationship Between Sleep Disturbance and Post-stroke Depression of Stroke Patients in China: A Structural Equation Modeling Analysis. Front. Psychiatry 2021, 12, 625002. [Google Scholar] [CrossRef] [PubMed]
- Brodtmann, A.; van de Port, I.G. Fitness, depression, and poststroke fatigue: Worn out or weary? Neurology 2013, 81, 1566–1567. [Google Scholar] [CrossRef]
- Su, Y.; Asamoto, M.; Yuki, M.; Saito, M.; Hasebe, N.; Hirayama, K.; Otsuki, M.; Iino, C. Predictors and short-term outcomes of post-stroke fatigue in initial phase of transition from hospital to home: A prospective observational study. J. Adv. Nurs. 2020, 77, 1825–1838. [Google Scholar] [CrossRef]
- Hinkle, J.; Becker, K.; Kim, J.; Choi-Kwon, S.; Saban, K.; McNair, N.; Mead, G.; American Heart Association Council on Cardiovascular and Stroke Nursing and Stroke Council. Poststroke Fatigue: Emerging Evidence and Approaches to Management: A Scientific Statement for Healthcare Professionals from the American Heart Association. Stroke 2017, 48, e159–e170. [Google Scholar] [CrossRef] [PubMed]
- Dobielska, M.; Bartosik, N.K.; Zyzik, K.A.; Kowalczyk, E.; Karbownik, M.S. Mechanisms of Cognitive Impairment in Depression. May Probiotics Help? Front. Psychiatry 2022, 13, 904426. [Google Scholar] [CrossRef] [PubMed]
- Williams, O.A.; Demeyere, N. Association of Depression and Anxiety with Cognitive Impairment 6 Months After Stroke. Neurology 2021, 96, e1966–e1974. [Google Scholar] [CrossRef]
- Kang, C. Predictors of Post-stroke Cognition Among Geriatric Patients: The Role of Demographics, Pre-stroke Cognition, and Trajectories of Depression. Front. Psychol. 2021, 12, 717817. [Google Scholar] [CrossRef]
- Jaroonpipatkul, C.; Onwanna, J.; Tunvirachaisakul, C.; Jittapiromsak, N.; Rakvongthai, Y.; Chutinet, A.; Supasitthumrong, T.; Maes, M. Depressive symptoms due to stroke are strongly predicted by the volume and location of the cerebral infarction, white matter hyperintensities, hypertension, and age: A precision nomothetic psychiatry analysis. J. Affect. Disord. 2022, 309, 141–150. [Google Scholar] [CrossRef]
- Kapoor, A.; Si, K.; Yu, A.Y.; Lanctot, K.L.; Herrmann, N.; Murray, B.J.; Swartz, R.H. Younger Age and Depressive Symptoms Predict High Risk of Generalized Anxiety After Stroke and Transient Ischemic Attack. Stroke 2019, 50, 2359–2363. [Google Scholar] [CrossRef] [PubMed]
- Barugh, A.J.; Gray, P.; Shenkin, S.D.; MacLullich, A.M.J.; Mead, G.E. Cortisol levels and the severity and outcomes of acute stroke: A systematic review. J. Neurol. 2014, 261, 533–545. [Google Scholar] [CrossRef]
- Blöchl, M.; Nestler, S. Long-term Changes in Depressive Symptoms Before and After Stroke. Neurology 2022, 99, e720–e729. [Google Scholar] [CrossRef]
- Chun, H.Y.; Whiteley, W.N.; Dennis, M.S.; Mead, G.E.; Carson, A.J. Anxiety After Stroke: The Importance of Subtyping. Stroke 2018, 49, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, J.; Sun, W.; Liu, X. The advances of post-stroke depression: 2021 update. J. Neurol. 2021, 269, 1236–1249. [Google Scholar] [CrossRef]
- Lavu, V.K.; Mohamed, R.A.; Huang, R.; Potla, S.; Bhalla, S.; Al Qabandi, Y.; Nandula, S.A.; Boddepalli, C.S.; Gutlapalli, S.D.; Mohammed, L. Evaluation and Treatment of Depression in Stroke Patients: A Systematic Review. Cureus 2022, 14, e28137. [Google Scholar] [CrossRef] [PubMed]
| % | Mean (SD) | |
|---|---|---|
| Chronological age (years) | 59.3 (13.8) | |
| Education level (years) | 14.6 (3.0) | |
| Time since stroke to neuropsych testing (mo.) | 8.1 (5.2) | |
| Side of stroke | ||
| left-sided | 44.9% | |
| right-sided | 41.2% | |
| bilateral | 13.8% | |
| Stroke type | ||
| ischemic | 67.1% | |
| hemorrhagic | 32.9% | |
| Sex | ||
| women | 49.2% | |
| men | 50.8% | |
| Poststroke depression | 30.8% | |
| Depression treatment before stroke | 38.2% | |
| Poststroke anxiety | 16.9% | |
| Anxiety treatment before stroke | 7.4% | |
| Sleep difficulties | 45.5% | |
| Fatigue | 59.1% | |
| Not employed | 68.9% | |
| Marital status | ||
| Married/Domestic partner | 63.7% | |
| Unmarried/Divorced/Single | 36.3% | |
| % with memory loss | 67.7% | |
| % with executive difficulties | 72.0% |
| No PSA | 83.1% (N = 270) |
| Panic Attacks | 6.2% (N = 20) |
| Generalized Anxiety Disorder (GAD) | 4.9% (N = 16) |
| Obsessive Compulsive Disorder | 0.0% (N = 0) |
| Post-traumatic Stress Disorder (PTSD) | 0.6% (N = 2) |
| Social Anxiety, phobia | 1.5% (N = 5) |
| Anxiety NOS | 0.6% (N = 2) |
| Panic Attacks and GAD | 1.8% (N= 6) |
| GAD and Social Anxiety | 0.9% (N = 3) |
| PTSD and Social Anxiety | 0.3% (N = 1) |
| PSD (N = 100) | No Depression (N = 215) | ||
|---|---|---|---|
| % (N) | % (N) | Statistics | |
| Chronological age (years) | 55.7 (14.4) | 60.8 (13.2) | F(1,323) = 10.0, p = 0.002 * |
| Education level (years) | 14.3 (2.6) | 14.8 (3.2) | F(1,323) = 2.11, p = 0.147 |
| Time since stroke to neuropsych testing (mo.) | 9.7 (5.8) | 7.4 (6.8) | F(1,323) = 1.25, p = 0.261 |
| Side of stroke | |||
| left-sided | 45% of depressed | 45% of depressed | chi-square p = 0.72 |
| right-sided | 42.2% | 39.0% | |
| bilateral | 12.8% | 16.0% | |
| Stroke type | |||
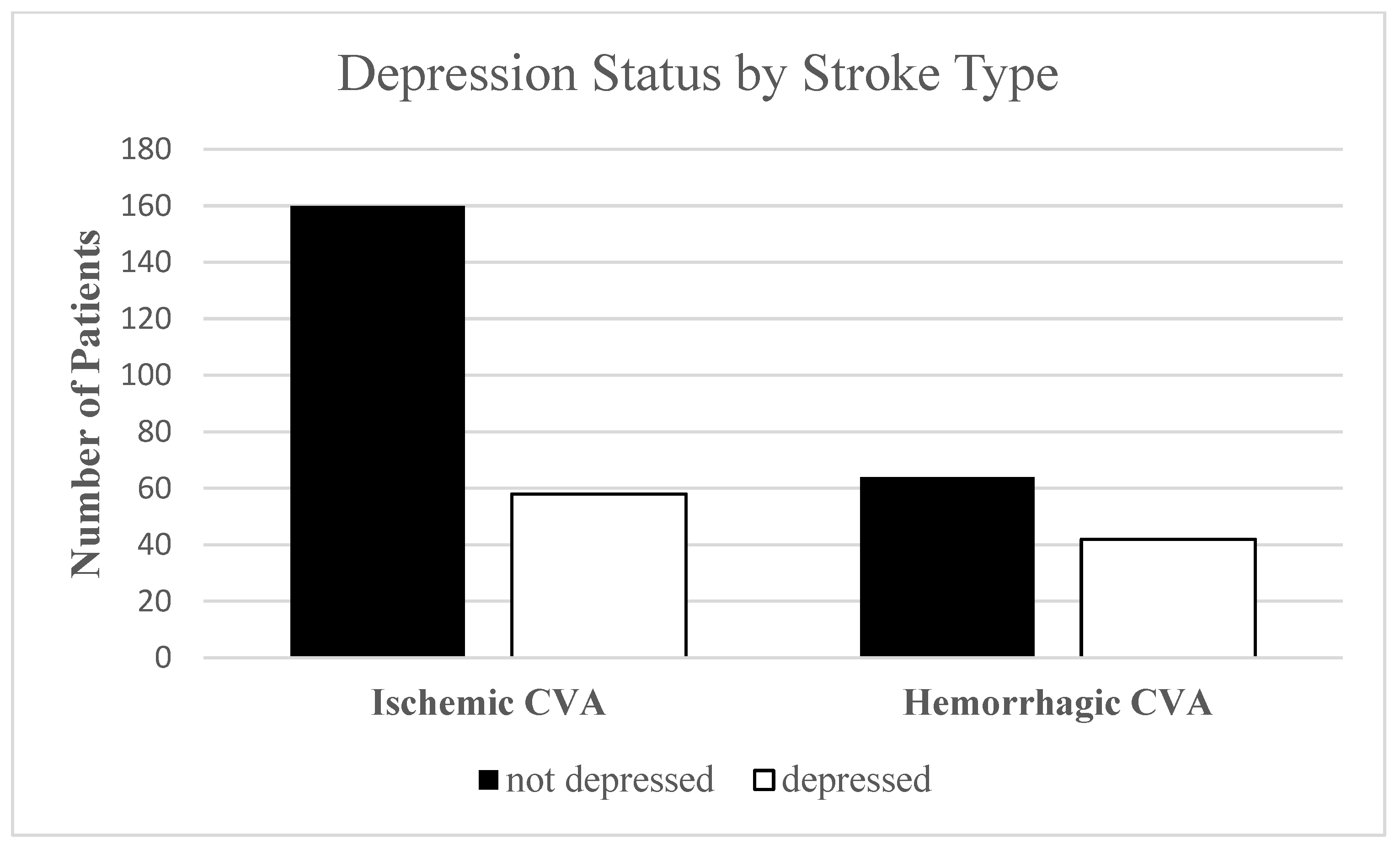
| ischemic | 58.0% | 71.1% | p = 0.015 * |
| hemorrhagic | 42.0% | 28.9% | |
| Sex | |||
| women | 55.0% | 46.7% | p = 0.103 |
| men | 45.0% | 53.3% | |
| Depression treatment before stroke | 61.0% | 28.0% | p < 0.001 * |
| Poststroke anxiety | 40.0% | 9.3% | p < 0.001 * |
| Anxiety treatment before stroke | 20.0% | 2.0% | p < 0.001 * |
| Sleep difficulties | 63.0% | 37.0% | p < 0.001 * |
| Fatigue | 79.0% | 50.2% | p < 0.001 * |
| Not employed | 62.0% | 65.3% | p = 0.32 |
| Married/Domestic partner | 62.0% | 64.4% | p = 0.46 |
| % with memory loss | 79.0% | 62.7% | p = 0.02 * |
| % with executive difficulties | 79.0% | 68.8% | p = 0.39 * |
| Significant Factors | OR | p-Value | 95% CI | |
|---|---|---|---|---|
| Poststroke anxiety | 5.95 | 0.00 | 2.42 | 14.61 |
| Depression treatment before stroke | 3.00 | 0.00 | 1.62 | 5.55 |
| Fatigue | 2.75 | 0.00 | 1.36 | 5.58 |
| Memory impairment | 2.36 | 0.02 | 1.16 | 4.80 |
| Chronological age (years) | 0.97 | 0.01 | 0.94 | 0.99 |
| Non-significant factors | ||||
| Time since stroke to neuropsych testing (mo.) | 1.00 | 0.69 | 0.99 | 1.02 |
| Chronological age (years) | 0.97 | 0.01 | 0.94 | 0.99 |
| Location of the stroke | 1.30 | 0.43 | 0.68 | 2.47 |
| Side of stroke | 0.93 | 0.72 | 0.61 | 1.41 |
| Stroke type | 1.65 | 0.11 | 0.90 | 3.03 |
| Sleep difficulties | 1.45 | 0.25 | 0.77 | 2.76 |
| Employment status | 0.65 | 0.24 | 0.32 | 1.32 |
| Marital status | 1.01 | 0.93 | 0.80 | 1.27 |
| Anxiety treatment before stroke | 1.43 | 0.63 | 0.34 | 6.04 |
| Executive impairment | 1.30 | 0.47 | 0.63 | 2.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dulay, M.F.; Criswell, A.; Hodics, T.M. Biological, Psychiatric, Psychosocial, and Cognitive Factors of Poststroke Depression. Int. J. Environ. Res. Public Health 2023, 20, 5328. https://doi.org/10.3390/ijerph20075328
Dulay MF, Criswell A, Hodics TM. Biological, Psychiatric, Psychosocial, and Cognitive Factors of Poststroke Depression. International Journal of Environmental Research and Public Health. 2023; 20(7):5328. https://doi.org/10.3390/ijerph20075328
Chicago/Turabian StyleDulay, Mario F., Amber Criswell, and Timea M. Hodics. 2023. "Biological, Psychiatric, Psychosocial, and Cognitive Factors of Poststroke Depression" International Journal of Environmental Research and Public Health 20, no. 7: 5328. https://doi.org/10.3390/ijerph20075328
APA StyleDulay, M. F., Criswell, A., & Hodics, T. M. (2023). Biological, Psychiatric, Psychosocial, and Cognitive Factors of Poststroke Depression. International Journal of Environmental Research and Public Health, 20(7), 5328. https://doi.org/10.3390/ijerph20075328






