Quality-Adjusted Life Years in Erythropoietic Protoporphyria and Other Rare Diseases: A Patient-Initiated EQ-5D Feasibility Study
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Cohort
2.2. Recruitment of Study Participants
2.3. The EQ-5D Instrument
2.4. Calculation of Quality-Adjusted Life Years
2.5. The EPP-QoL Instrument
2.6. Reliability of the Retrospective Data
2.7. Appropriateness of the Questionnaires
2.8. Statistical Analysis
2.9. EQ-5D and QALY Gains in Highly Specialised Technologies Previously Assessed at NICE
2.10. Researcher Characteristics
3. Results
3.1. Patient Characteristics Data
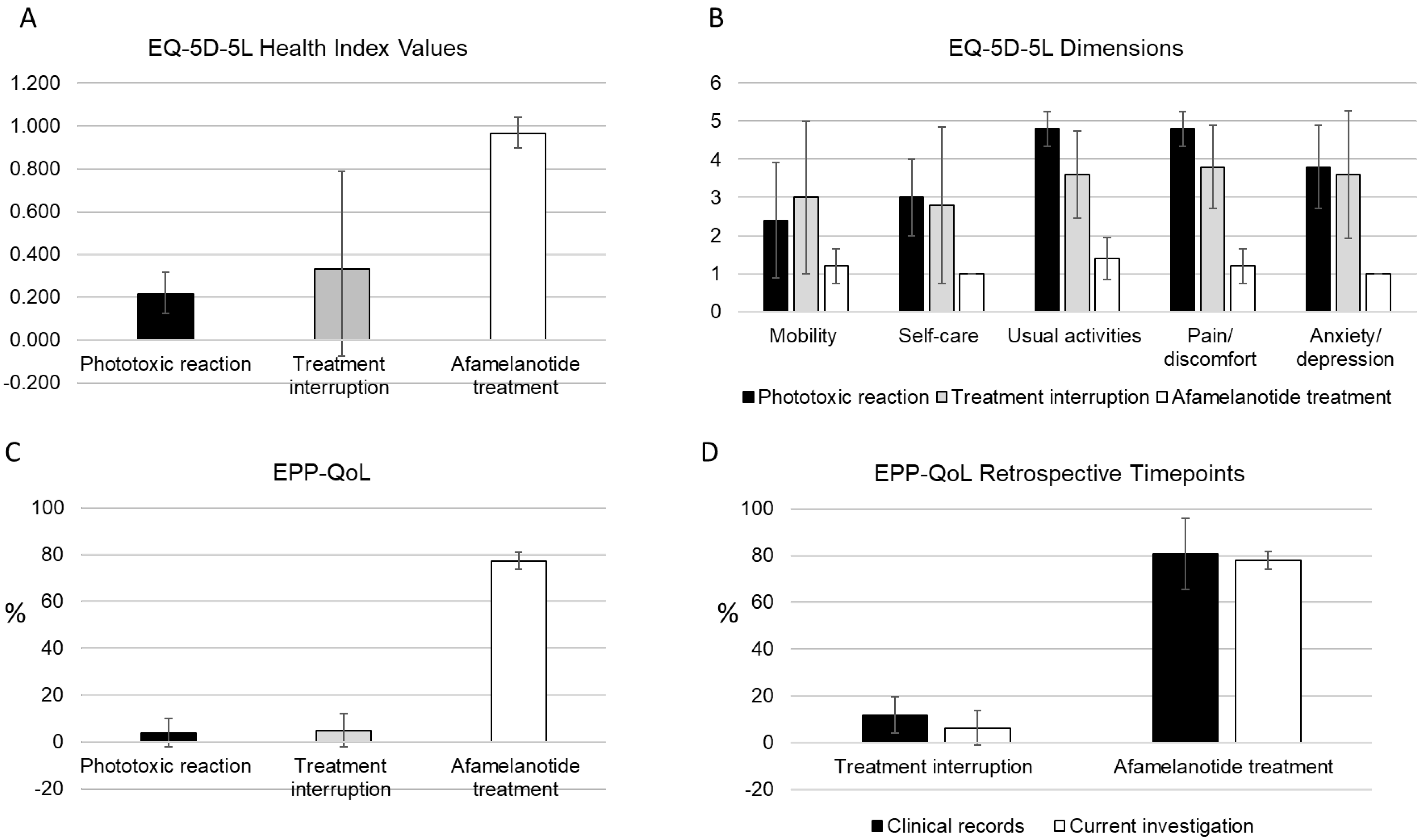
3.2. QoL in EPP as Determined with the EQ-5D Instrument
3.3. QoL in EPP as Determined with the EPP-QoL Questionnaire
3.4. Reliability of the Retrospective Data
3.5. Correlation between the QoL Instruments and the EQ-VAS Scale
3.6. Appropriateness of the Questionnaires
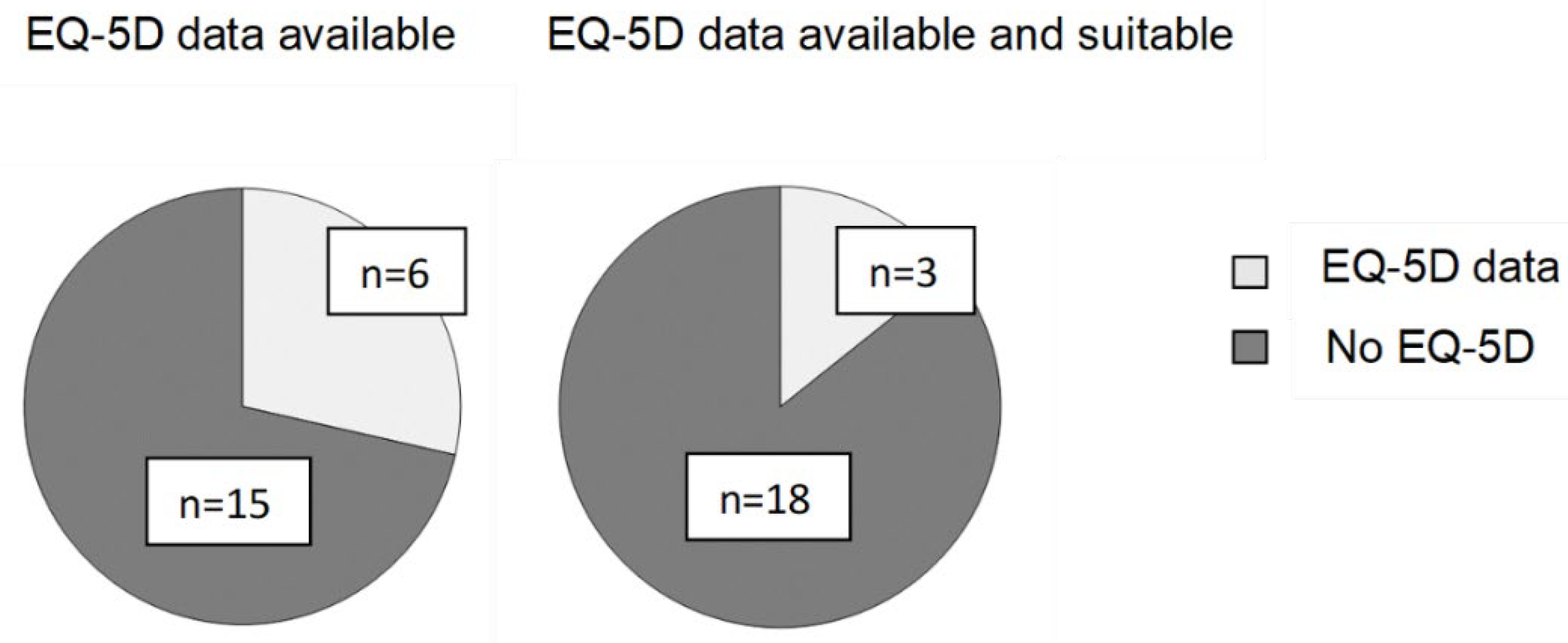
3.7. EQ-5D Data in Previously Assessed Highly Specialised Technologies
3.8. Time Horizons in Previously Assessed Highly Specialised Technologies
3.9. QALY Gains and Discount Rates in Previously Assessed Highly Specialised Technologies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magnus, I.A.; Jarrett, A.; Prankerd, T.A.J.; Rimington, C. Erythropoietic Protoporphyria a New Porphyria Syndrome with Solar Urticaria Due to Protoporphyrinaemia. Lancet 1961, 278, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Whatley, S.D.; Ducamp, S.; Gouya, L.; Grandchamp, B.; Beaumont, C.; Badminton, M.N.; Elder, G.H.; Holme, S.A.; Anstey, A.V.; Parker, M.; et al. C-Terminal Deletions in the ALAS2 Gene Lead to Gain of Function and Cause X-Linked Dominant Protoporphyria without Anemia or Iron Overload. Am. J. Hum. Genet. 2008, 83, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Yien, Y.Y.; Ducamp, S.; van der Vorm, L.N.; Kardon, J.R.; Manceau, H.; Kannengiesser, C.; Bergonia, H.A.; Kafina, M.D.; Karim, Z.; Gouya, L.; et al. Mutation in Human CLPX Elevates Levels of δ-Aminolevulinate Synthase and Protoporphyrin IX to Promote Erythropoietic Protoporphyria. Proc. Natl. Acad. Sci. USA 2017, 114, E8045. [Google Scholar] [CrossRef] [PubMed]
- Schnait, F.G.; Wolff, K.; Konrad, K. Erythropoietic Protoporphyria—Submicroscopic Events during the Acute Photosensitivity Flare. Br. J. Dermatol. 1975, 92, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Holme, S.A.; Anstey, A.V.; Finlay, A.Y.; Elder, G.H.; Badminton, M.N. Erythropoietic Protoporphyria in the U.K.: Clinical Features and Effect on Quality of Life. Br. J. Dermatol. 2006, 155, 574–581. [Google Scholar] [CrossRef]
- Granata, F.; Duca, L.; Graziadei, G.; Brancaleoni, V.; Missineo, P.; De Luca, G.; Fustinoni, S.; Di Pierro, E. Inflammatory Involvement into Phototoxic Reaction in Erythropoietic Protoporphyria (EPP) Patients. Immunol. Res. 2019, 67, 382–389. [Google Scholar] [CrossRef]
- Wensink, D.; Wagenmakers, M.A.E.M.; Wilson, J.H.P.; Langendonk, J.G. Erythropoietic Protoporphyria in the Netherlands: Clinical Features, Psychosocial Impact and the Effect of Afamelanotide. J. Dermatol. 2022, in press. [Google Scholar] [CrossRef]
- Rufener, E.A. Erythropoietic Protoporphyria: A Study of Its Psychosocial Aspects. Br. J. Dermatol. 1987, 116, 703–708. [Google Scholar] [CrossRef]
- Lecluse, A.L.Y.; Kuck-Koot, V.C.M.; van Weelden, H.; Sigurdsson, V.; Russel, I.M.; Frank, J.; Pasmans, S.G.M.A. Erythropoietic Protoporphyria without Skin Symptoms-You Do Not Always See What They Feel. Eur. J. Pediatr. 2008, 167, 703–706. [Google Scholar] [CrossRef]
- Naik, H.; Shenbagam, S.; Go, A.M.; Balwani, M. Psychosocial Issues in Erythropoietic Protoporphyria—The Perspective of Parents, Children, and Young Adults: A Qualitative Study. Mol. Genet. Metab. 2019, 128, 314–319. [Google Scholar] [CrossRef]
- Dickey, A. Pitfalls and Proposed Solutions for Patient Communication about Erythropoietic Protoporphyria: A Survey of Parents and Adult Patients. J. Am. Acad. Dermatol. 2019, 81, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Falchetto, R. The Patient Perspective: A Matter of Minutes. Patient-Cent. Outcomes Res. 2020, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rutter, K.J.; Ashraf, I.; Cordingley, L.; Rhodes, L.E. Quality of Life and Psychological Impact in the Photodermatoses: A Systematic Review. Br. J. Dermatol. 2020, 182, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Barman-Aksözen, J.; Granata, F.; Aksözen, M.H.; Dechant, C.; Falchetto, R. ‘… They Had Interpreted “Disability” as Referring to a Patently Visible Disability’: Experience of a Patient Group with NICE. Disabil. Soc. 2022, 37, 1239–1245. [Google Scholar] [CrossRef]
- Heerfordt, I.M.; Lerche, C.M.; Philipsen, P.A.; Wulf, H.C. Experimental and Approved Treatments for Skin Photosensitivity in Individuals with Erythropoietic Protoporphyria or X-Linked Protoporphyria: A Systematic Review. Biomed. Pharmacother. 2023, 158, 114132. [Google Scholar] [CrossRef] [PubMed]
- Biolcati, G.; Marchesini, E.; Sorge, F.; Barbieri, L.; Schneider-Yin, X.; Minder, E.I. Long-Term Observational Study of Afamelanotide in 115 Patients with Erythropoietic Protoporphyria. Br. J. Dermatol. 2015, 172, 1601–1612. [Google Scholar] [CrossRef]
- Balwani, M. Erythropoietic Protoporphyria and X-Linked Protoporphyria: Pathophysiology, Genetics, Clinical Manifestations, and Management. Mol. Genet. Metab. 2019, 128, 298–303. [Google Scholar] [CrossRef]
- Barman-Aksözen, J.; Nydegger, M.; Schneider-Yin, X.; Minder, A.-E. Increased Phototoxic Burn Tolerance Time and Quality of Life in Patients with Erythropoietic Protoporphyria Treated with Afamelanotide—A Three Years Observational Study. Orphanet J. Rare Dis. 2020, 15, 213. [Google Scholar] [CrossRef]
- Wensink, D.; Wagenmakers, M.A.E.M.; Barman-Aksözen, J.; Friesema, E.C.H.; Wilson, J.H.P.; van Rosmalen, J.; Langendonk, J.G. Association of Afamelanotide With Improved Outcomes in Patients With Erythropoietic Protoporphyria in Clinical Practice. JAMA Dermatol. 2020, 156, 570–575. [Google Scholar] [CrossRef]
- Leaf, R.K.; Elmariah, S.; Jiang, P.Y.; Mead, J.; Hodges, P.; Valiante, S.E.; Mernick, F.; Anderson, K.E.; Dickey, A. Clinical Experience with Afamelanotide for the Protoporphyrias in the United States. Blood 2022, 140, 2201–2202. [Google Scholar] [CrossRef]
- Langendonk, J.G.; Balwani, M.; Anderson, K.E.; Bonkovsky, H.L.; Anstey, A.V.; Bissell, D.M.; Bloomer, J.; Edwards, C.; Neumann, N.J.; Parker, C.; et al. Afamelanotide for Erythropoietic Protoporphyria. N. Engl. J. Med. 2015, 373, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Naik, H.; Overbey, J.R.; Desnick, R.J.; Anderson, K.E.; Bissell, D.M.; Bloomer, J.; Bonkovsky, H.L.; Phillips, J.D.; Wang, B.; Singal, A.; et al. Evaluating Quality of Life Tools in North American Patients with Erythropoietic Protoporphyria and X-Linked Protoporphyria. JIMD Rep. 2019, 50, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Biolcati, G.; Hanneken, S.; Minder, E.I.; Neumann, N.J.; Wilson, J.H.P.; Wolgen, P.J.; Wright, D.J.; Lloyd, A.J. Validation of a Novel Patient Reported Tool to Assess the Impact of Treatment in Erythropoietic Protoporphyria: The EPP-QoL. J. Patient-Rep. Outcomes 2021, 5, 65. [Google Scholar] [CrossRef]
- European Medicines Agency Annex 1, Summary of Product Characteristics. Scenesse: EPAR-Product Information. Last Updated 14 January 2020. Available online: https://www.ema.europa.eu/en/documents/product-information/scenesse-epar-product-information_en.pdf (accessed on 5 January 2023).
- Ghislandi, S.; Apolone, G.; Garattini, L.; Ghislandi, I. Is EQ-5D a Valid Measure of HRQoL in Patients with Movement Disorders? Eur. J. Health Econ. 2002, 3, 125–130. [Google Scholar] [CrossRef]
- de Ridder, D.; Geenen, R.; Kuijer, R.; van Middendorp, H. Psychological Adjustment to Chronic Disease. Lancet 2008, 372, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Cubí-Mollá, P.; Jofre-Bonet, M.; Serra-Sastre, V. Adaptation to Health States: Sick yet Better Off? Health Econ. 2017, 26, 1826–1843. [Google Scholar] [CrossRef]
- Efthymiadou, O.; Mossman, J.; Kanavos, P. Health Related Quality of Life Aspects Not Captured by EQ-5D-5L: Results from an International Survey of Patients. Health Policy 2019, 123, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Priedane, E.; Phillips, G.A.; Brazier, J.E.; Johnson, C.; Gomes-Faria, R.; Kumar, A. PSY197—Limitations of the EQ5D Instrument in the Assessment of Quality of Life in Chronic Rare Diseases—A Case Study from FCS. Value Health 2018, 21, S469–S470. [Google Scholar] [CrossRef]
- Devlin, N.; Brooks, R. EQ-5D and the EuroQol Group: Past, Present and Future. Appl. Health Econ. Health Policy 2017, 15, 127–137. [Google Scholar] [CrossRef]
- Rowen, D.; Brazier, J.; Wong, R.; Wailoo, A. Measuring and Valuing Health-Related Quality of Life When Sufficient EQ-5D Data Is Not Available. NICE DSU Report. Available online: https://www.sheffield.ac.uk/media/34046/download?attachment (accessed on 24 January 2023).
- Norlin, J.M.; Steen Carlsson, K.; Persson, U.; Schmitt-Egenolf, M. Analysis of Three Outcome Measures in Moderate to Severe Psoriasis: A Registry-based Study of 2450 Patients. Br. J. Dermatol. 2012, 166, 797–802. [Google Scholar] [CrossRef]
- Currie, C.; Conway, P. PSK11 Evaluation of the Association between EQ5D Utility and Dermatology Life Quality Index (DLQI) Score in Patients with Psoriasis. Value Health 2007, 10, A470–A471. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Afamelanotide for Treating Erythropoietic Protoporphyria. Final Evaluation Document. London. Available online: https://www.nice.org.uk/guidance/gid-hst10009/documents/final-evaluation-determination-document (accessed on 23 January 2023).
- EuroQol Research Foundation EQ-5D-5L User Guide. Version 3.0. Available online: https://euroqol.org/publications/user-guides (accessed on 23 January 2023).
- van Hout, B.; Janssen, M.F.; Feng, Y.-S.; Kohlmann, T.; Busschbach, J.; Golicki, D.; Lloyd, A.; Scalone, L.; Kind, P.; Pickard, A.S. Interim Scoring for the EQ-5D-5L: Mapping the EQ-5D-5L to EQ-5D-3L Value Sets. Value Health 2012, 15, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Szende, A.; Janssen, B.; Cabases, J. Self-Reported Population Health: An International Perspective Based on EQ-5D; Springer Nature: New York, NY, USA; London, UK, 2014; ISBN 3-319-51664-7. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). Interim Process and Methods of the Highly Specialised Technologies Programme. Updated to Reflect 2017 Changes. London. Available online: https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-highly-specialised-technologies-guidance/HST-interim-methods-process-guide-may-17.pdf (accessed on 23 January 2023).
- Öster, C.; Willebrand, M.; Dyster-Aas, J.; Kildal, M.; Ekselius, L. Validation of the EQ-5D Questionnaire in Burn Injured Adults. Burns 2009, 35, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Spronk, I.; Van Loey, N.E.E.; Sewalt, C.; Nieboer, D.; Renneberg, B.; Moi, A.L.; Oster, C.; Orwelius, L.; van Baar, M.E.; Polinder, S.; et al. Recovery of Health-Related Quality of Life after Burn Injuries: An Individual Participant Data Meta-Analysis. PLoS ONE 2020, 15, e0226653. [Google Scholar] [CrossRef]
- Synodinou, D.; Savoie-White, F.H.; Sangone, A.; Chang, S.-L.; Beaudoin Cloutier, C.; Bergeron, F.; Guertin, J.R. Health Utilities in Burn Injury Survivors: A Systematic Review. Burns 2022, 48, 13–22. [Google Scholar] [CrossRef]
- Torrance, N.; Lawson, K.D.; Afolabi, E.; Bennett, M.I.; Serpell, M.G.; Dunn, K.M.; Smith, B.H. Estimating the Burden of Disease in Chronic Pain with and without Neuropathic Characteristics: Does the Choice between the EQ-5D and SF-6D Matter? Pain 2014, 155, 1996–2004. [Google Scholar] [CrossRef]
- Boersma-van Dam, E.; van de Schoot, R.; Hofland, H.W.C.; Engelhard, I.M.; Van Loey, N.E.E. Individual Recovery of Health-Related Quality of Life during 18 Months Post-Burn Using a Retrospective Pre-Burn Measurement: An Exploratory Study. Qual. Life Res. 2021, 30, 737–749. [Google Scholar] [CrossRef]
- de Graaf, M.W.; Reininga, I.H.F.; Wendt, K.W.; Heineman, E.; El Moumni, M. Pre-Injury Health Status of Injured Patients: A Prospective Comparison with the Dutch Population. Qual. Life Res. 2019, 28, 649–662. [Google Scholar] [CrossRef]
- Stull, D.E.; Leidy, N.K.; Parasuraman, B.; Chassany, O. Optimal Recall Periods for Patient-Reported Outcomes: Challenges and Potential Solutions. Curr. Med. Res. Opin. 2009, 25, 929–942. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Evaluation Consultation Document 2—Afamelanotide for Treating Erythropoietic Protoporphyria. Final Evaluation Document. Available online: https://www.nice.org.uk/guidance/gid-hst10009/documents/evaluation-consultation-document-3 (accessed on 23 January 2023).
- Schlander, M.; Garattini, S.; Holm, S.; Kolominsky-Rabas, P.; Nord, E.; Persson, U.; Postma, M.; Richardson, J.; Simoens, S.; de Solà Morales, O.; et al. Incremental Cost per Quality-Adjusted Life Year Gained? The Need for Alternative Methods to Evaluate Medical Interventions for Ultra-Rare Disorders. J. Comp. Eff. Res. 2014, 3, 399–422. [Google Scholar] [CrossRef]
- Brouwer, W.; van Baal, P.; van Exel, J.; Versteegh, M. When Is It Too Expensive? Cost-Effectiveness Thresholds and Health Care Decision-Making. Eur. J. Health Econ. 2019, 20, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Norwegian Medicines Agency Afamelanotide (Scenesse) for the Treatment of Erythropoietic Protoporphyria (EPP). Available online: https://nyemetoder.no/Documents/Rapporter/Afamelanotide%20(Scenesse)%20-%20hurtig%20metodevurdering.pdf (accessed on 27 February 2023).
| Patient Characteristics | Results |
|---|---|
| Age [years] | 41.2 (±6.9) |
| Age at first symptoms [years] | 1.9 (±0.8) |
| Age at diagnosis [years] | 13.6 (±9.2) |
| Erythrocyte-free protoporphyrin [µmol/L; ULN = 0.2] | 19.5 (±5.0) |
| Patients with phototoxic reactions caused by artificial light [n] | 4 |
| Patients with phototoxic reactions in winter [n] | 5 |
| Patients with an EPP-related liver condition [n] | 1 |
| Patients with a lifetime diagnosis of depression [n] | 3 |
| Treatment with afamelanotide [years] | 9.1 (±4.0) |
| Duration of treatment interruption [months] | 9.1 (±8.3) |
| Time since interruption [months] | 35 (±18.0) |
| Number and severity of phototoxic reactions VAS 0 (no pain)—VAS 10 (worst imaginable pain) | |
| Number of phototoxic reactions per year, before treatment with afamelanotide [n] | 21.2 (±8.8) |
| Number of phototoxic reactions per year, under treatment with afamelanotide [n] | 4.6 (±3.3) |
| Maximum pain intensity of a phototoxic reaction experienced before treatment with afamelanotide [VAS] | 10 (±0) |
| Maximum pain intensity of a phototoxic reaction experienced under treatment with afamelanotide [VAS] | 5.6 (±3.3) |
| Phototoxic burn tolerance time | |
| Maximum time in sunlight without experiencing symptoms before treatment with afamelanotide [minutes] | 4.4 (±3) |
| Maximum time in sunlight without experiencing symptoms under treatment with afamelanotide [minutes] | 252 (±78) |
| Phototoxic burn protection factor | 87.2 (±55) |
| Self-assessed effectiveness of treatments VAS 0 (no effect)—VAS 10 (best possible effect) | |
| Afamelanotide [VAS] | 8.8 (±0.7) |
| Best alternative [VAS] | 0 (±0) |
| Afamelanotide (ID927) | Accepted Method for QALY Quantification | Utilities | Assumed Time Horizon | QALY Gain, Undiscounted | QALY Gain, Discounted |
|---|---|---|---|---|---|
| Initial assessment (2018) | DLQI data as collected in the pivotal RCT | 0.016 | 35 years | 0.56 | 0.33 |
| Current assessment (2022) | EQ-5D data as collected by the IPPN, modified by NICE | 0.397 | 60 years | 23.796 | 9.995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barman-Aksözen, J.; Minder, A.-E.; Granata, F.; Pettersson, M.; Dechant, C.; Aksözen, M.H.; Falchetto, R. Quality-Adjusted Life Years in Erythropoietic Protoporphyria and Other Rare Diseases: A Patient-Initiated EQ-5D Feasibility Study. Int. J. Environ. Res. Public Health 2023, 20, 5296. https://doi.org/10.3390/ijerph20075296
Barman-Aksözen J, Minder A-E, Granata F, Pettersson M, Dechant C, Aksözen MH, Falchetto R. Quality-Adjusted Life Years in Erythropoietic Protoporphyria and Other Rare Diseases: A Patient-Initiated EQ-5D Feasibility Study. International Journal of Environmental Research and Public Health. 2023; 20(7):5296. https://doi.org/10.3390/ijerph20075296
Chicago/Turabian StyleBarman-Aksözen, Jasmin, Anna-Elisabeth Minder, Francesca Granata, Mårten Pettersson, Cornelia Dechant, Mehmet Hakan Aksözen, and Rocco Falchetto. 2023. "Quality-Adjusted Life Years in Erythropoietic Protoporphyria and Other Rare Diseases: A Patient-Initiated EQ-5D Feasibility Study" International Journal of Environmental Research and Public Health 20, no. 7: 5296. https://doi.org/10.3390/ijerph20075296
APA StyleBarman-Aksözen, J., Minder, A.-E., Granata, F., Pettersson, M., Dechant, C., Aksözen, M. H., & Falchetto, R. (2023). Quality-Adjusted Life Years in Erythropoietic Protoporphyria and Other Rare Diseases: A Patient-Initiated EQ-5D Feasibility Study. International Journal of Environmental Research and Public Health, 20(7), 5296. https://doi.org/10.3390/ijerph20075296







