Assessing the Turning Ability during Walking in People with Stroke Using L Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Consideration
2.2. Sample Size Calculation
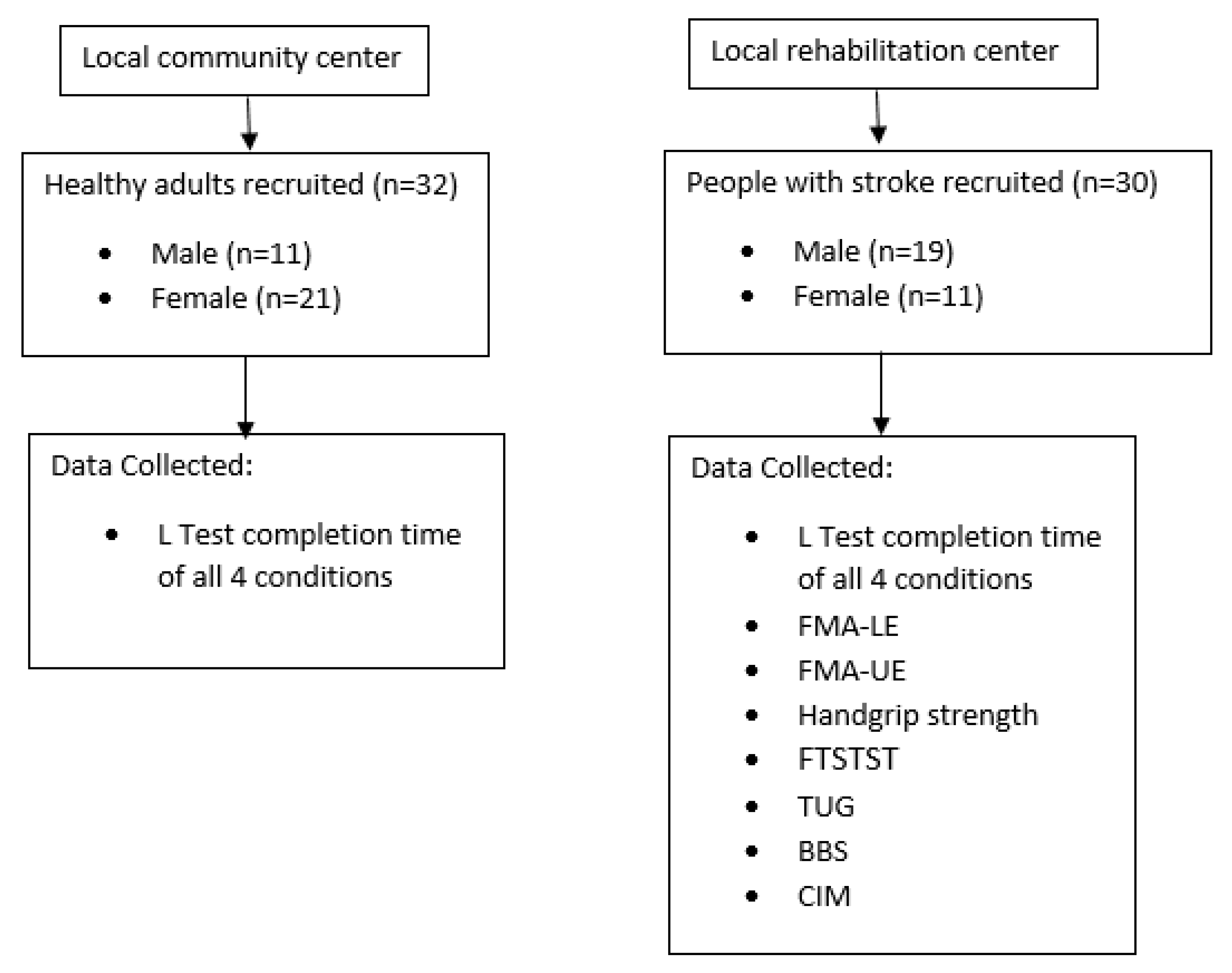
2.3. Participants
2.4. Testing Procedure
2.5. Outcome Measures
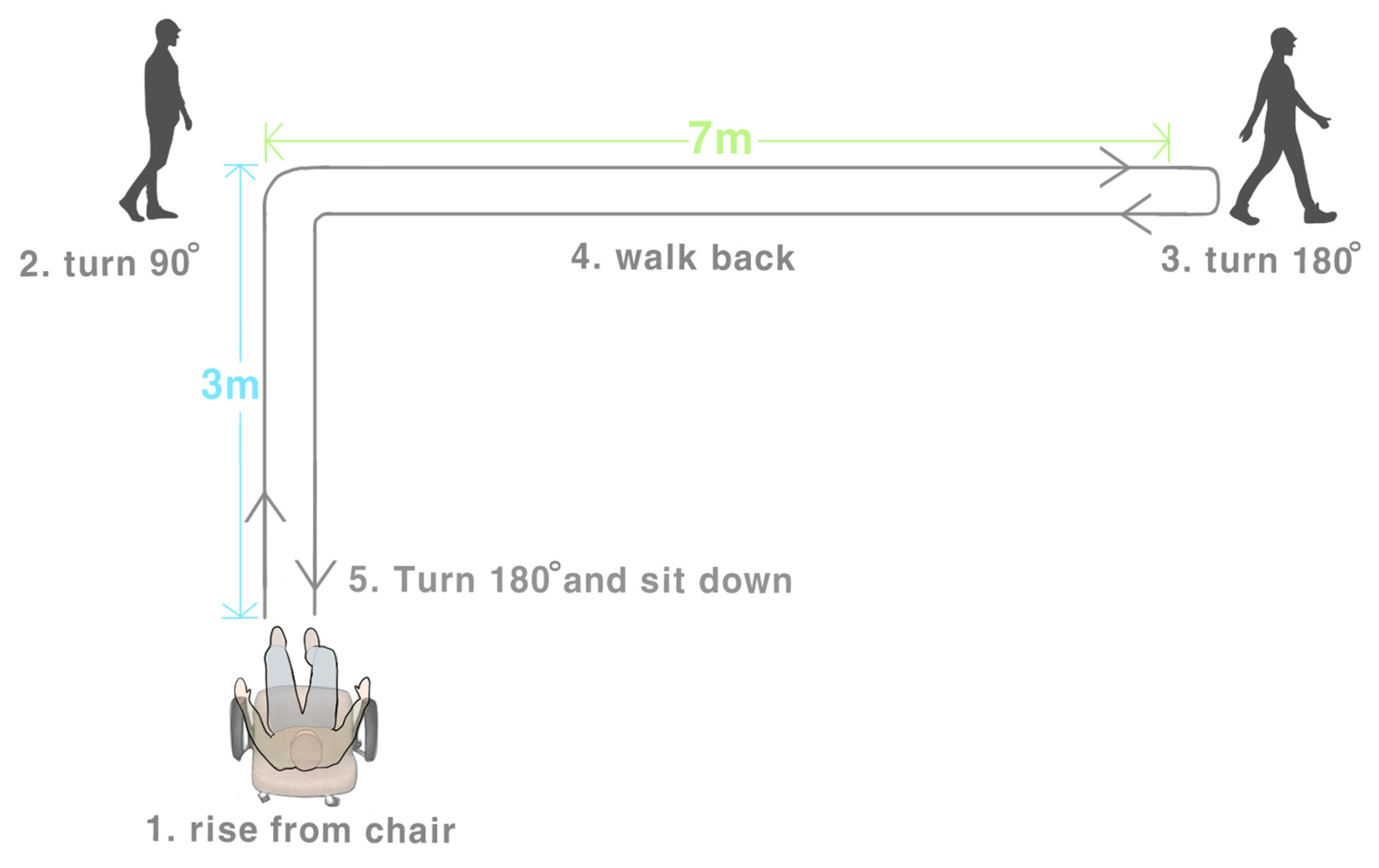
2.5.1. L Test
2.5.2. Fugl–Meyer Assessment
2.5.3. Handgrip Strength Test
2.5.4. FTSTST
2.5.5. TUG Test
2.5.6. BBS
2.5.7. SF-36
2.5.8. CIM
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Participants
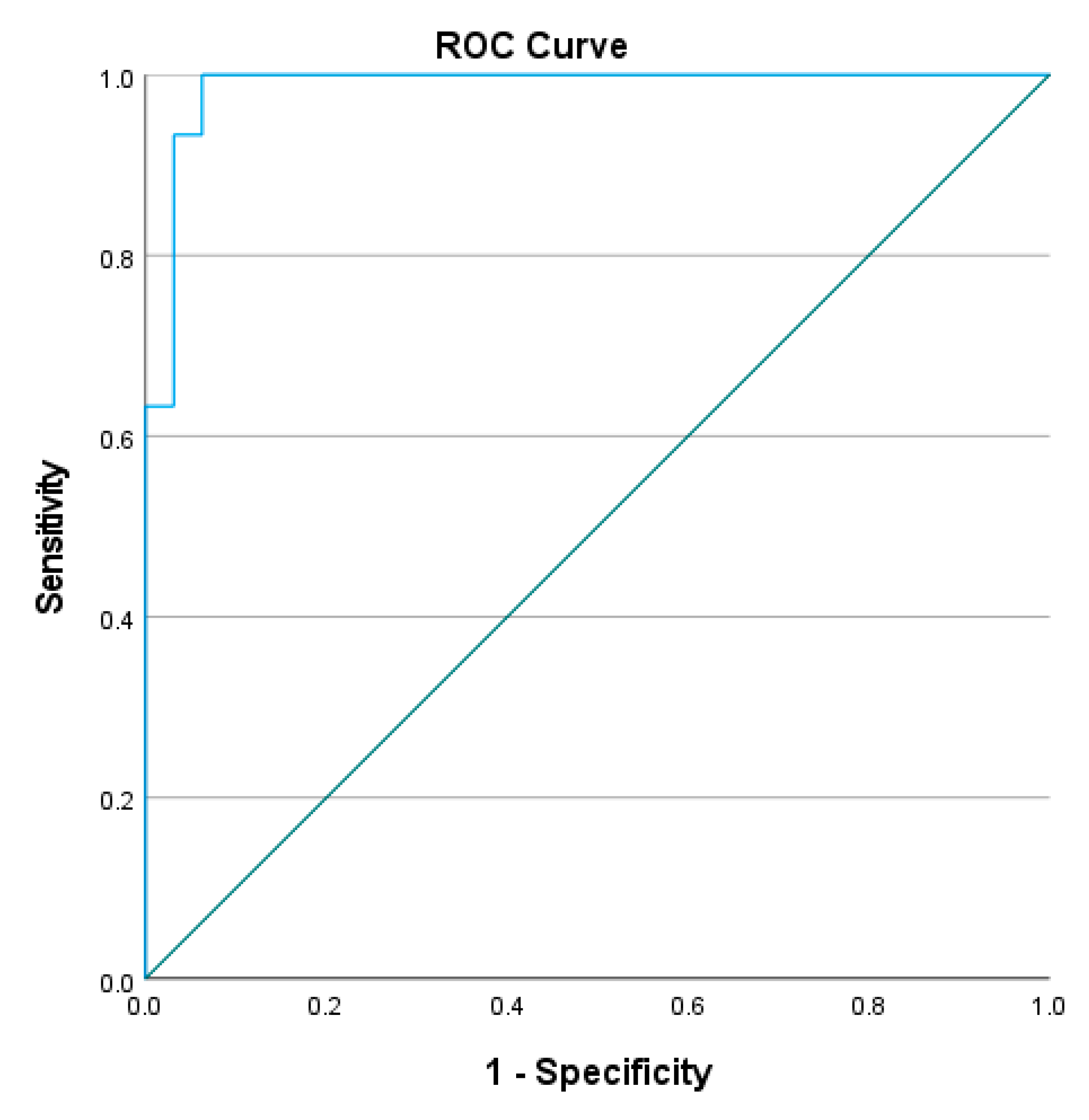
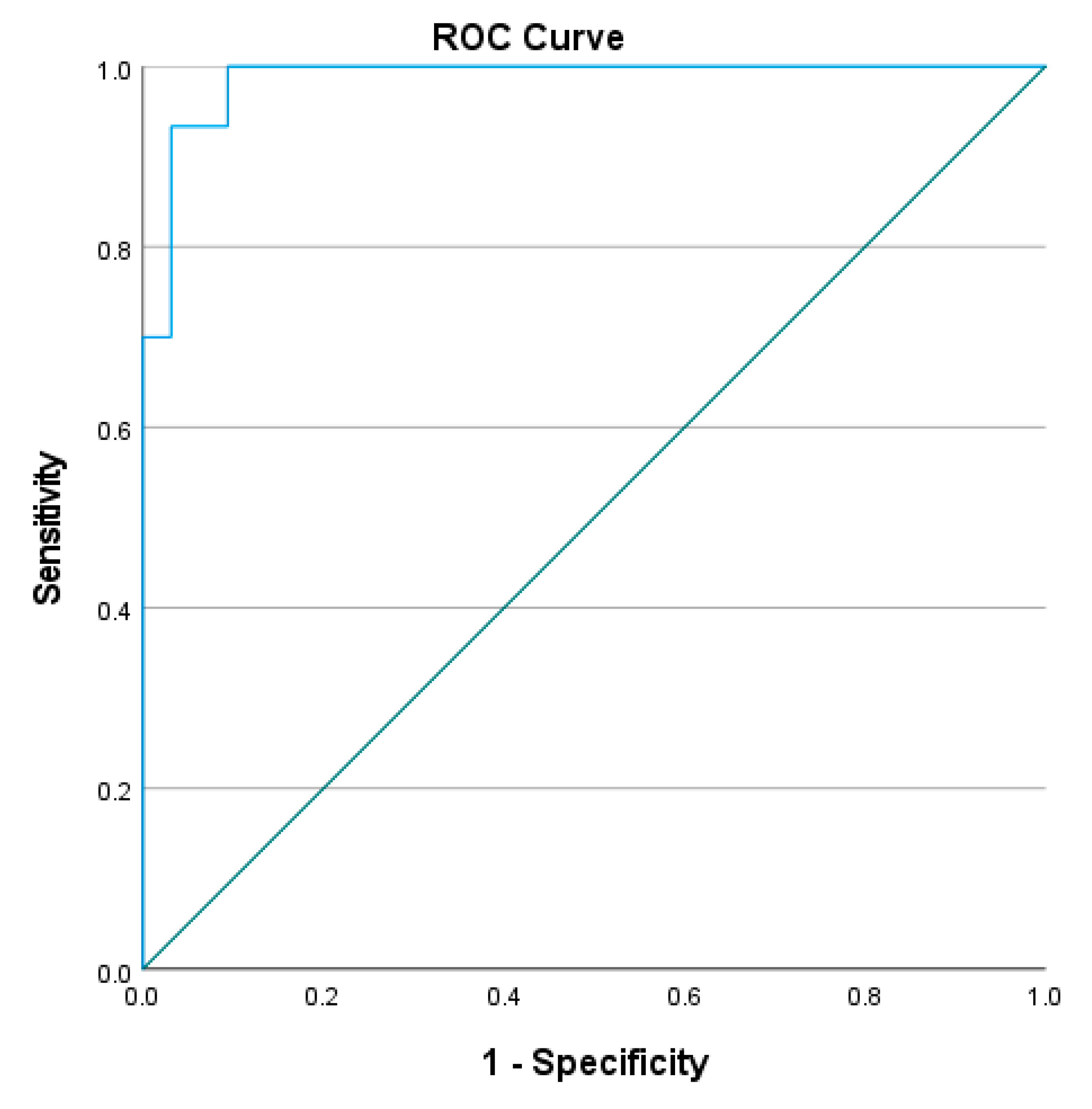
3.2. Reliability of L Test
3.3. Correlation of L Test with Other Stroke-Specific Outcomes
4. Discussion
4.1. Performance of L Test in People with Stroke and Healthy Older Adults
4.2. Reliability of the L Test
4.3. Correlation of the L Test and Other Stroke-Specific Impairments
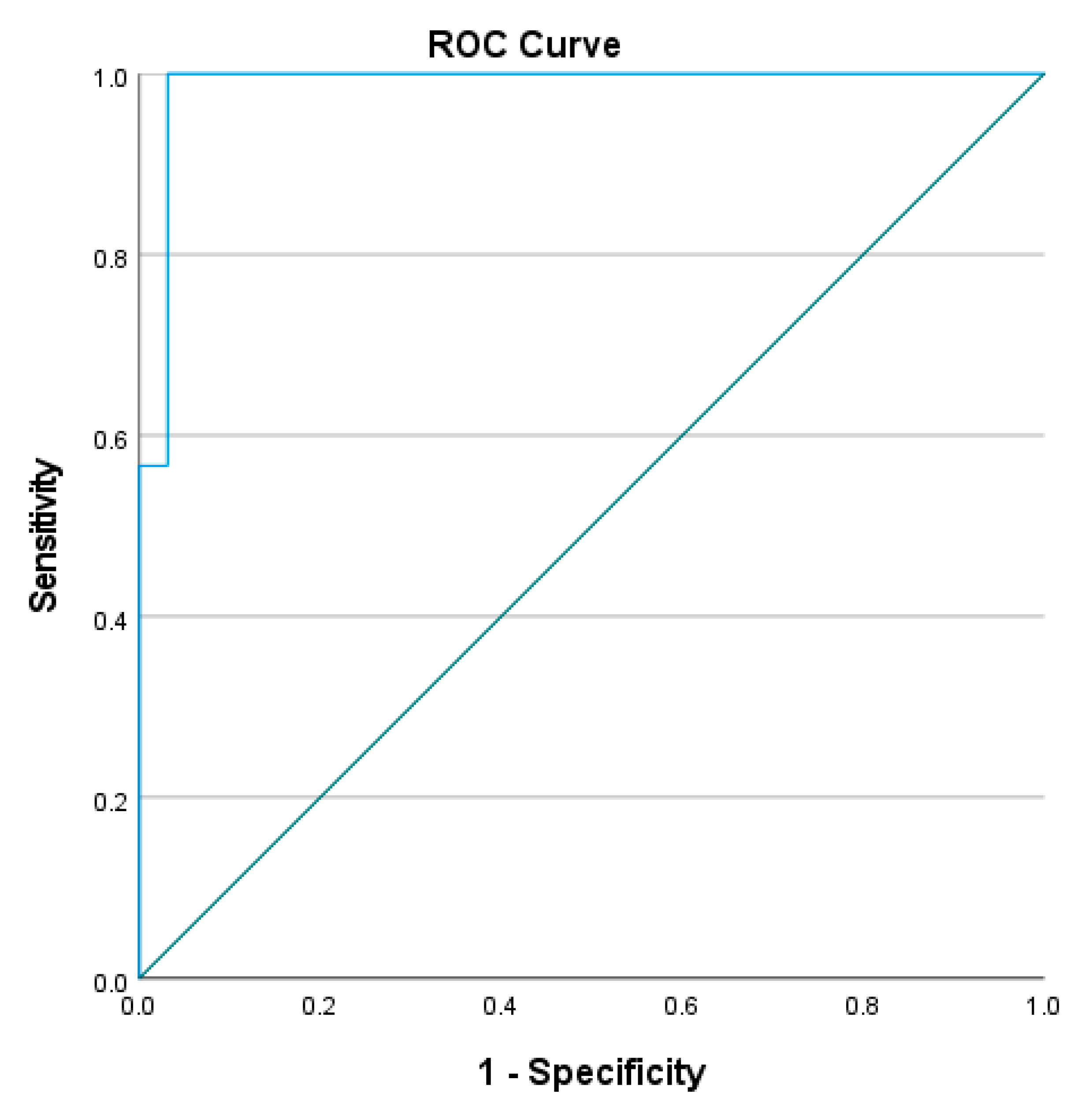
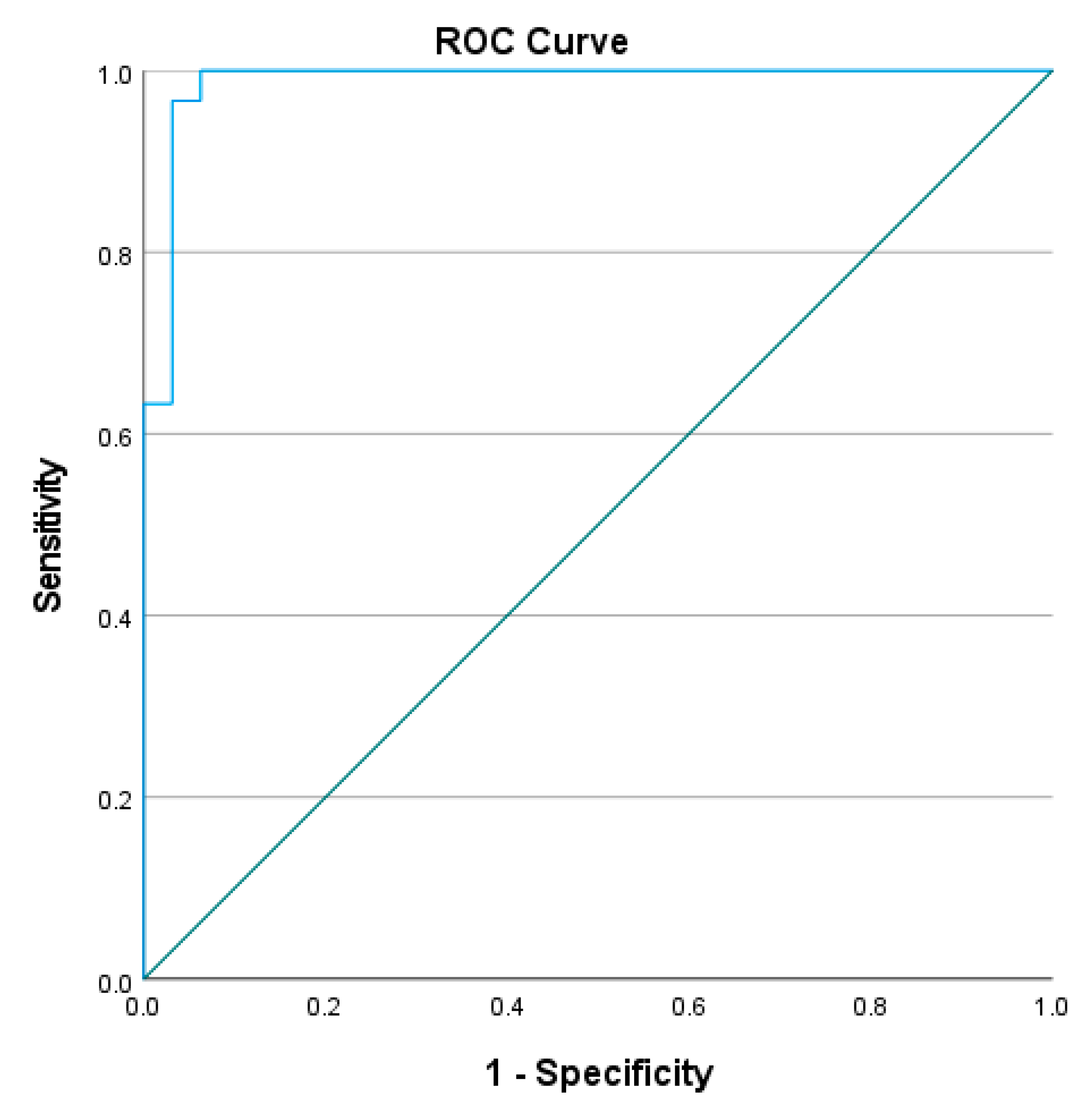
4.4. Optimal Cut-Off for L Test Completion Time
4.5. Limitation of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamontagne, A.; De Serres, S.J.; Fung, J.; Paquet, N. Stroke affects the coordination and stabilization of head, thorax and pelvis during voluntary horizontal head motions performed in walking. Clin. Neurophysiol. 2005, 116, 101–111. [Google Scholar] [CrossRef]
- Shiu, C.H.; Ng, S.S.; Kwong, P.W.; Liu, T.-W.; Tam, E.W.; Fong, S.S. Timed 360 turn test for assessing people with chronic stroke. Arch. Phys. Med. Rehabilit. 2016, 97, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Leddy, A.L.; Crowner, B.E.; Earhart, G.M. Utility of the Mini-BESTest, BESTest, and BESTest sections for balance assessments in individuals with Parkinson disease. J. Neurol. Phys. Ther. JNPT 2011, 35, 90. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.S.; Hui-Chan, C.W. The timed up & go test: Its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch. Phys. Med. Rehabil. 2005, 86, 1641–1647. [Google Scholar] [PubMed]
- Deathe, A.B.; Miller, W.C. The L test of functional mobility: Measurement properties of a modified version of the timed “up & go” test designed for people with lower-limb amputations. Phys. Ther. 2005, 85, 626–635. [Google Scholar] [PubMed]
- Kim, J.; Chu, D.; Jeon, H. Reliability and validity of the L test in participants with chronic stroke. Physiotherapy 2015, 101, 161–165. [Google Scholar] [CrossRef]
- Walter, S.; Eliasziw, M.; Donner, A. Sample size and optimal designs for reliability studies. Stat. Med. 1998, 17, 101–110. [Google Scholar] [CrossRef]
- Lam, S.C.; Wong, Y.Y.; Woo, J. Reliability and validity of the abbreviated mental test (Hong Kong version) in residential care homes. J. Am. Geriatr. Soc. 2010, 58, 2255–2257. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. I. A method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabilit. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef]
- McGrath, R.P.; Kraemer, W.J.; Snih, S.A.; Peterson, M.D. Handgrip strength and health in aging adults. Sport. Med. 2018, 48, 1993–2000. [Google Scholar] [CrossRef]
- Innes, E. Handgrip strength testing: A review of the literature. Aust. Occup. Ther. J. 1999, 46, 120–140. [Google Scholar] [CrossRef]
- Kwong, P.W.; Ng, S.S.; Chung, R.C.; Ng, G.Y. Foot placement and arm position affect the five times sit-to-stand test time of individuals with chronic stroke. BioMed Res. Int. 2014, 2014, 636530. [Google Scholar] [CrossRef]
- Chan, P.P.; Tou, J.I.S.; Mimi, M.T.; Ng, S.S. Reliability and validity of the timed up and go test with a motor task in people with chronic stroke. Arch. Phys. Med. Rehabil. 2017, 98, 2213–2220. [Google Scholar] [CrossRef]
- Berg, K.; Wood-Dauphinee, S.; Williams, J. The Balance Scale: Reliability assessment with elderly residents and patients with an acute stroke. Scand. J. Rehabil. Med. 1995, 27, 27–36. [Google Scholar]
- Blum, L.; Korner-Bitensky, N. Usefulness of the Berg Balance Scale in stroke rehabilitation: A systematic review. Phys. Ther. 2008, 88, 559–566. [Google Scholar] [CrossRef]
- Ware, J.E., Jr. SF-36 health survey update. Spine 2000, 25, 3130–3139. [Google Scholar] [CrossRef]
- Peipert, J.D.; Bentler, P.M.; Klicko, K.; Hays, R.D. Psychometric properties of the kidney disease quality of life 36-item short-form survey (KDQOL-36) in the United States. Am. J. Kidney Dis. 2018, 71, 461–468. [Google Scholar] [CrossRef]
- Liu, T.-W.; Ng, S.S.; Ng, G.Y. Translation and initial validation of the Chinese (Cantonese) version of community integration measure for use in patients with chronic stroke. BioMed Res. Int. 2014, 2014, 623836. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its associated cutoff point. Biom. J. J. Math. Methods Biosci. 2005, 47, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Barrett, K.M.; Meschia, J.F. Stroke; John Wiley & Sons: New York, NY, USA, 2013. [Google Scholar]
- Manaf, H.; Justine, M.; Omar, M.; Md Isa, K.A.; Salleh, Z. Turning ability in stroke survivors: A review of literature. Int. Sch. Res. Not. 2012, 2012, 284924. [Google Scholar] [CrossRef]
- De Quervain, I.A.K.; Simon, S.R.; Leurgans, S.; Pease, W.S.; Mcallister, D. Gait pattern in the early recovery period after stroke. JBJS 1996, 78, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, D.; Gervasoni, E.; Pupillo, E.; Bianchi, E.; Aprile, I.; Imbimbo, I.; Russo, R.; Cruciani, A.; Jonsdottir, J.; Agostini, M. Mobility disorders in stroke, Parkinson disease, and multiple sclerosis: A multicenter cross-sectional study. Am. J. Phys. Med. Rehabil. 2020, 99, 41–47. [Google Scholar] [CrossRef]
- McMorland, A.J.; Runnalls, K.D.; Byblow, W.D. A neuroanatomical framework for upper limb synergies after stroke. Front. Hum. Neurosci. 2015, 9, 82. [Google Scholar] [CrossRef]
- Meyns, P.; Bruijn, S.M.; Duysens, J. The how and why of arm swing during human walking. Gait Posture 2013, 38, 555–562. [Google Scholar] [CrossRef]
- Novak, A.C.; Brouwer, B. Strength and aerobic requirements during stair ambulation in persons with chronic stroke and healthy adults. Arch. Phys. Med. Rehabil. 2012, 93, 683–689. [Google Scholar] [CrossRef]
- Novy, D.M.; Simmonds, M.J.; Olson, S.L.; Lee, C.E.; Jones, S.C. Physical performance: Differences in men and women with and without low back pain. Arch. Phys. Med. Rehabil. 1999, 80, 195–198. [Google Scholar] [CrossRef]
| Stroke (n = 30) | Healthy (n = 32) | ||
|---|---|---|---|
| Age (year, mean ± SD) | 58.0 ± 5.4 | 62.8 ± 6.3 | p < 0.001 |
| Sex (M/F) | 19/11 | 11/21 | p = 0.023 |
| Height (cm, mean ± SD) | 162.4 ± 8.5 | 158.9 ± 8.5 | p = 0.051 |
| Weight (kg, mean ± SD) | 66.9 ± 11.4 | 56.5 ± 9.5 | p < 0.001 |
| BMI (kg/m2) | 25.2 ± 2.9 | 22.3 ± 2.9 | p < 0.001 |
| Paretic side (L/R) | 9/21 | ||
| Stroke nature (ischemia/hemorrhage/other) | 18/9/3 | ||
| Years since stroke (year, mean ± SD) | 7.8 ± 4.8 |
| Condition | Healthy (s, Mean ± SD) | Stroke (s, Mean ± SD) | |
|---|---|---|---|
| 1 | 18.4 ± 3.3 | 32.9 ± 8.0 | p < 0.001 |
| 2 | 18.4 ± 3.4 | 32.6 ± 7.6 | p < 0.001 |
| 3 | 18.6 ± 3.5 | 32.8 ± 7.4 | p < 0.001 |
| 4 | 18.5 ± 3.4 | 32.1 ± 7.4 | p < 0.001 |
| Condition | ICC |
|---|---|
| 1 | 0.945 (0.901–0.971) |
| 2 | 0.978 (0.961–0.989) |
| 3 | 0.965 (0.936–0.982) |
| 4 | 0.977 (0.959–0.988) |
| Test | Condition 1 | p | Condition 2 | p | Condition 3 | p | Condition 4 | p |
|---|---|---|---|---|---|---|---|---|
| FMA | ||||||||
| LE | −0.438 * | 0.020 | −0.344 | 0.073 | −0.348 | 0.070 | −0.310 | 0.109 |
| UE | −0.551 * | 0.002 | −0.534 * | 0.003 | −0.552 * | 0.002 | −0.512 * | 0.005 |
| Handgrip | ||||||||
| Paretic | 0.158 | 0.422 | 0.122 | 0.537 | 0.167 | 0.396 | 0.150 | 0.447 |
| Non-Paretic | 0.075 | 0.703 | 0.022 | 0.912 | −0.014 | 0.943 | 0.024 | 0.902 |
| FTSTST | 0.287 | 0.139 | 0.289 | 0.135 | 0.213 | 0.277 | 0.178 | 0.364 |
| BBS | −0.512 * | 0.005 | −0.494 * | 0.008 | −0.533 * | 0.004 | −0.488 * | 0.008 |
| TUG | 0.761 * | <0.001 | 0.736 * | <0.001 | 0.735 * | <0.001 | 0.724 * | <0.001 |
| SF36 | ||||||||
| Total | −0.038 | 0.849 | −0.064 | 0.745 | 0.001 | 0.995 | 0.043 | 0.829 |
| PCS | −0.150 | 0.445 | −0.177 | 0.369 | −0.135 | 0.495 | −0.067 | 0.733 |
| MCS | 0.089 | 0.651 | 0.072 | 0.715 | 0.136 | 0.490 | 0.136 | 0.491 |
| CIM | −0.356 | 0.063 | −0.350 | 0.068 | −0.302 | 0.119 | −0.255 | 0.190 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, S.S.M.; Tse, M.M.Y.; Chen, P.; Lam, T.P.S.; Yeung, T.H.T.; Liu, T.-W.; So, B.C.L. Assessing the Turning Ability during Walking in People with Stroke Using L Test. Int. J. Environ. Res. Public Health 2023, 20, 3618. https://doi.org/10.3390/ijerph20043618
Ng SSM, Tse MMY, Chen P, Lam TPS, Yeung THT, Liu T-W, So BCL. Assessing the Turning Ability during Walking in People with Stroke Using L Test. International Journal of Environmental Research and Public Health. 2023; 20(4):3618. https://doi.org/10.3390/ijerph20043618
Chicago/Turabian StyleNg, Shamay S. M., Mimi M. Y. Tse, Peiming Chen, Tony P. S. Lam, Tony H. T. Yeung, Tai-Wa Liu, and Billy C. L. So. 2023. "Assessing the Turning Ability during Walking in People with Stroke Using L Test" International Journal of Environmental Research and Public Health 20, no. 4: 3618. https://doi.org/10.3390/ijerph20043618
APA StyleNg, S. S. M., Tse, M. M. Y., Chen, P., Lam, T. P. S., Yeung, T. H. T., Liu, T.-W., & So, B. C. L. (2023). Assessing the Turning Ability during Walking in People with Stroke Using L Test. International Journal of Environmental Research and Public Health, 20(4), 3618. https://doi.org/10.3390/ijerph20043618






