Nutritional Status of Coronary Artery Disease Patients—Preliminary Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Ethical Concerns
3. Results
3.1. Nutritional Status of CAD Patients
3.2. Nutritional Status and Clinical Performance
3.3. Nutritional Status and CAD Parameters
3.4. Women and Men Subgroup Analysis
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN Guidelines on Definitions and Terminology of Clinical Nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, R.; Kanda, D.; Ikeda, Y.; Tokushige, A.; Sonoda, T.; Anzaki, K.; Ohishi, M. Prognostic Impact of Malnutrition on Cardiovascular Events in Coronary Artery Disease Patients with Myocardial Damage. BMC Cardiovasc. Disord. 2021, 21, 479. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.R.; Lee, Y.K.; Jin Cho, A.; Park, H.C.; Han, C.H.; Choi, M.J.; Koo, J.R.; Yoon, J.W.; Noh, J.W. Malnutrition, Inflammation, Progression of Vascular Calcification and Survival: Interrelationships in Hemodialysis Patients. PLoS ONE 2019, 14, e0216415. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Yang, Y.; Chen, J.; Jiang, Y.; Li, A.; Huang, M.; Dong, Y.; Wang, S.; Ding, S. Bioelectrical Impedance Analysis versus Quantitative Computer Tomography and Anthropometry for the Assessment of Body Composition Parameters in China. Sci. Rep. 2021, 11, 11076. [Google Scholar] [CrossRef]
- Kuriyan, R. Body Composition Techniques. Indian J. Med. Res. 2018, 148, 648–658. [Google Scholar] [CrossRef]
- World Health Organization. BMI Classification. Global Database on Body Mass Index. Available online: http://www.who.int/bmi/index.jsp?introPage=intro_3.html (accessed on 1 April 2019).
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis—Part I: Review of Principles and Methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Ringaitiene, D.; Gineityte, D.; Vicka, V.; Sabestinaite, A.; Klimasauskas, A.; Gaveliene, E.; Rucinskas, K.; Ivaska, J.; Sipylaite, J. Concordance of the New ESPEN Criteria with Low Phase Angle in Defining Early Stages of Malnutrition in Cardiac Surgery. Clin. Nutr. 2018, 37, 1596–1601. [Google Scholar] [CrossRef]
- Popiołek, J.; Teter, M.; Kozak, G.; Powrózek, T.; Mlak, R.; Karakuła-Juchnowicz, H.; Małecka-Massalska, T. Anthropometrical and Bioelectrical Impedance Analysis Parameters in Anorexia Nervosa Patients’ Nutritional Status Assessment. Medicina 2019, 55, 671. [Google Scholar] [CrossRef]
- Popiołek-Kalisz, J.; Teter, M.; Kozak, G.; Powrózek, T.; Mlak, R.; Sobieszek, G.; Karakuła-Juchnowicz, H.; Małecka-Massalska, T. Potential Bioelectrical Impedance Analysis (BIA) Parameters in Prediction Muscle Strength in Women with Anorexia Nervosa. World J. Biol. Psychiatry 2020, 22, 203–213. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis—Part II: Utilization in Clinical Practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Byambasukh, O.; Eisenga, M.F.; Gansevoort, R.T.; Bakker, S.J.L.; Corpeleijn, E. Body Fat Estimates from Bioelectrical Impedance Equations in Cardiovascular Risk Assessment: The PREVEND Cohort Study. Eur. J. Prev. Cardiol. 2019, 26, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Diemer, F.S.; Brewster, L.M.; Haan, Y.C.; Oehlers, G.P.; van Montfrans, G.A.; Nahar-van Venrooij, L.M.W. Body Composition Measures and Cardiovascular Risk in High-Risk Ethnic Groups. Clin. Nutr. 2019, 38, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Kalisz, J.; Fornal, E. The Impact of Flavonols on Cardiovascular Risk. Nutrients 2022, 14, 1973. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Kalisz, J. The Impact of Dietary Flavonols on Central Obesity Parameters in Polish Adults. Nutrients 2022, 14, 5051. [Google Scholar] [CrossRef]
- Popiolek-Kalisz, J. The Relationship between Dietary Flavonols Intake and Metabolic Syndrome in Polish Adults. Nutrients 2023, 15, 854. [Google Scholar] [CrossRef]
- Kondrup, J.; Ramussen, H.H.; Hamberg, O.; Stanga, Z.; Camilo, M.; Richardson, R.; Elia, M.; Allison, S.; Meier, R.; Plauth, M. Nutritional Risk Screening (NRS 2002): A New Method Based on an Analysis of Controlled Clinical Trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Kang, M.C.; Kim, J.H.; Ryu, S.W.; Moon, J.Y.; Park, J.H.; Park, J.K.; Park, J.H.; Baik, H.W.; Seo, J.M.; Son, M.W.; et al. Prevalence of Malnutrition in Hospitalized Patients: A Multicenter Cross-Sectional Study. J. Korean Med. Sci. 2018, 33, e10. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Fang, W.H.; Wang, C.C.; Kao, T.W.; Yang, H.F.; Wu, C.J.; Sun, Y.S.; Wang, Y.C.; Chen, W.L. Fat-to-Muscle Ratio Is a Useful Index for Cardiometabolic Risks: A Population-Based Observational Study. PLoS ONE 2019, 14, e0214994. [Google Scholar] [CrossRef]
- Eun, Y.; Lee, S.N.; Song, S.W.; Kim, H.N.; Kim, S.H.; Lee, Y.A.; Kang, S.G.; Rho, J.S.; Yoo, K.D. Fat-to-Muscle Ratio: A New Indicator for Coronary Artery Disease in Healthy Adults. Int. J. Med. Sci. 2021, 18, 3738–3743. [Google Scholar] [CrossRef] [PubMed]
- Correa-Rodríguez, M.; González-Ruíz, K.; Rincón-Pabón, D.; Izquierdo, M.; García-Hermoso, A.; Agostinis-Sobrinho, C.; Sánchez-Capacho, N.; Roa-Cubaque, M.A.; Ramírez-Vélez, R. Normal-Weight Obesity Is Associated with Increased Cardiometabolic Risk in Young Adults. Nutrients 2020, 12, 1106. [Google Scholar] [CrossRef] [PubMed]
- Stegger, J.G.; Schmidt, E.B.; Obel, T.; Berentzen, T.L.; Tjønneland, A.; Sørensen, T.I.A.; Overvad, K. Body Composition and Body Fat Distribution in Relation to Later Risk of Acute Myocardial Infarction: A Danish Follow-up Study. Int. J. Obes. 2011, 35, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Wleklik, M.; Denfeld, Q.; Lisiak, M.; Czapla, M.; Kałużna-Oleksy, M.; Uchmanowicz, I. Frailty Syndrome in Older Adults with Cardiovascular Diseases–What Do We Know and What Requires Further Research? Int. J. Environ. Res. Public Health 2022, 19, 2234. [Google Scholar] [CrossRef]
- Czapla, M.; Uchmanowicz, I.; Juárez-Vela, R.; Durante, A.; Kałużna-Oleksy, M.; Łokieć, K.; Baeza-Trinidad, R.; Smereka, J. Relationship between Nutritional Status and Length of Hospital Stay among Patients with Atrial Fibrillation—A Result of the Nutritional Status Heart Study. Front. Nutr. 2022, 9, 1086715. [Google Scholar] [CrossRef]
- Czapla, M.; Juárez-Vela, R.; Łokieć, K.; Wleklik, M.; Karniej, P.; Smereka, J. The Association between Nutritional Status and Length of Hospital Stay among Patients with Hypertension. Int. J. Environ. Res. Public Health 2022, 19, 5827. [Google Scholar] [CrossRef]
- Czapla, M.; Karniej, P.; Juárez-Vela, R.; Łokieć, K. The Association between Nutritional Status and In-Hospital Mortality among Patients with Acute Coronary Syndrome—A Result of the Retrospective Nutritional Status Heart Study (Nshs). Nutrients 2020, 12, 3091. [Google Scholar] [CrossRef]
- Czapla, M.; Juárez-Vela, R.; Łokieć, K.; Karniej, P. The Association between Nutritional Status and In-Hospital Mortality among Patients with Heart Failure—A Result of the Retrospective Nutritional Status Heart Study 2 (NSHS2). Nutrients 2021, 13, 1669. [Google Scholar] [CrossRef]
- Garlini, L.M.; Alves, F.D.; Ceretta, L.B.; Perry, I.S.; Souza, G.C.; Clausell, N.O. Phase Angle and Mortality: A Systematic Review. Eur. J. Clin. Nutr. 2019, 73, 495–508. [Google Scholar] [CrossRef]
- Huang, L.; He, R.; Sun, X.; Lv, J.; Chen, S. Association of Controlling Nutritional Status Score With Adverse Outcomes in Patients With Coronary Artery Disease: A Systematic Review and Meta-Analysis. Angiology 2023, 74, 149–158. [Google Scholar] [CrossRef]
- Tonet, E.; Campo, G.; Maietti, E.; Formiga, F.; Martinez-Sellés, M.; Pavasini, R.; Biscaglia, S.; Serenelli, M.; Sanchis, J.; Diez-Villanueva, P.; et al. Nutritional Status and All-Cause Mortality in Older Adults with Acute Coronary Syndrome. Clin. Nutr. 2020, 39, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- de Borba, E.L.; Ceolin, J.; Ziegelmann, P.K.; Bodanese, L.C.; Gonçalves, M.R.; Cañon-Montañez, W.; Mattiello, R. Phase Angle of Bioimpedance at 50 KHz Is Associated with Cardiovascular Diseases: Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2022, 76, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yu, Y.; Jiang, H.; Yang, Q.; Liao, R.; Wang, L.; Zhang, Z.; Fu, C.; Su, B. Visceral Fat Area Is a Better Predictor Than Coronary Artery Calcification Score for Cardiovascular Outcomes and All-Cause Death in Patients on Hemodialysis. J. Ren. Nutr. 2021, 31, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, C.J.; Jhee, J.H.; Yun, H.; Kim, H.; Jung, S.; Kee, Y.K.; Yoon, C.; Park, J.T.; Kim, H.C.; et al. Extracellular Fluid Excess Is Significantly Associated With Coronary Artery Calcification in Patients With Chronic Kidney Disease. J. Am. Heart Assoc. 2018, 7, e008935. [Google Scholar] [CrossRef] [PubMed]
- Kurmus, O.; Aslan, T.; Eren, M.; Akbuga, K.; Erkan, A.F.; Ekici, B.; Akgul Ercan, E.; Kervancioglu, C. Nutritional Status and Severity of Coronary Artery Disease. Coron. Artery Dis. 2021, 32, 644–649. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, N.N.; Kjærulff, T.M.; Ward, L.C.; Sæbye, D.; Holst, C.; Heitmann, B.L. Body Water Distribution and Risk of Cardiovascular Morbidity and Mortality in a Healthy Population: A Prospective Cohort Study. PLoS ONE 2014, 9, e87466. [Google Scholar] [CrossRef]
| R | 95% CI | p | |
|---|---|---|---|
| Parameter | NRS 2002 | ||
| BMI [kg/m2] | 0.002 | −0.288; 0.292 | 0.99 |
| PA 5 kHz [°] | −0.21 | −0.460; 0.074 | 0.15 |
| PA 50 kHz [°] | −0.31 | −0.544; −0.038 | 0.03 * |
| PA 100 kHz [°] | −0.27 | −0.510; 0.009 | 0.06 |
| PA 200 kHz [°] | −0.11 | −0.376; 0.175 | 0.45 |
| Z200/5 parameter | 0.34 | 0.065; 0.563 | 0.02 * |
| Cm [nF] | −0.14 | −0.407; 0.139 | 0.32 |
| BMI | |||
| NRS 2002 [points] | 0.002 | −0.288; 0.292 | 0.99 |
| PA 5 kHz [°] | 0.31 | 0.018; 0.548 | 0.04 * |
| PA 50 kHz [°] | 0.16 | −0.139; 0.429 | 0.29 |
| PA 100 kHz [°] | 0.15 | −0.144; 0.424 | 0.31 |
| PA 200 kHz [°] | 0.15 | −0.151; 0.418 | 0.33 |
| Z200/5 parameter | −0.21 | −0.468; 0.090 | 0.17 |
| Cm [nF] | 0.19 | −0.105; 0.456 | 0.20 |
| CCS ≤ 2 N = 32 | SD | CCS ≥ 3 N = 18 | SD | p | |
|---|---|---|---|---|---|
| NRS 2002 [points] | 0.28 | 0.52 | 0.67 | 0.59 | 0.02 * |
| BMI [kg/m2] | 29.28 | 3.86 | 28.79 | 3.33 | 0.76 |
| PA 5 kHz [°] | 2.61 | 0.63 | 2.87 | 1.08 | 0.61 |
| PA 50 kHz [°] | 5.40 | 0.90 | 5.26 | 1.31 | 0.54 |
| PA 100 kHz [°] | 5.49 | 0.83 | 5.27 | 1.16 | 0.27 |
| PA 200 kHz [°] | 4.46 | 0.88 | 4.24 | 0.84 | 0.30 |
| Z200/5 | 0.79 | 0.03 | 0.80 | 0.05 | 0.85 |
| Cm [nF] | 1.65 | 0.59 | 1.98 | 1.02 | 0.52 |
| FFM [%] | 70.37 | 7.70 | 72.93 | 7.81 | 0.15 |
| FM [%] | 24.46 | 8.21 | 27.02 | 7.79 | 0.13 |
| TBW [%] | 51.51 | 5.63 | 53.42 | 5.70 | 0.13 |
| ECF [%] | 44.49 | 2.74 | 55.89 | 4.28 | 0.87 |
| ICF [%] | 55.54 | 2.70 | 44.11 | 4.28 | 0.87 |
| LVEF [%] | 51.08 | 16.76 | 54.79 | 15.10 | 0.68 |
| Number of significantly lesioned coronary arteries | 1.13 | 1.10 | 1.39 | 1.33 | 0.63 |
| Parameter | R | 95% CI | p |
|---|---|---|---|
| CCS class | |||
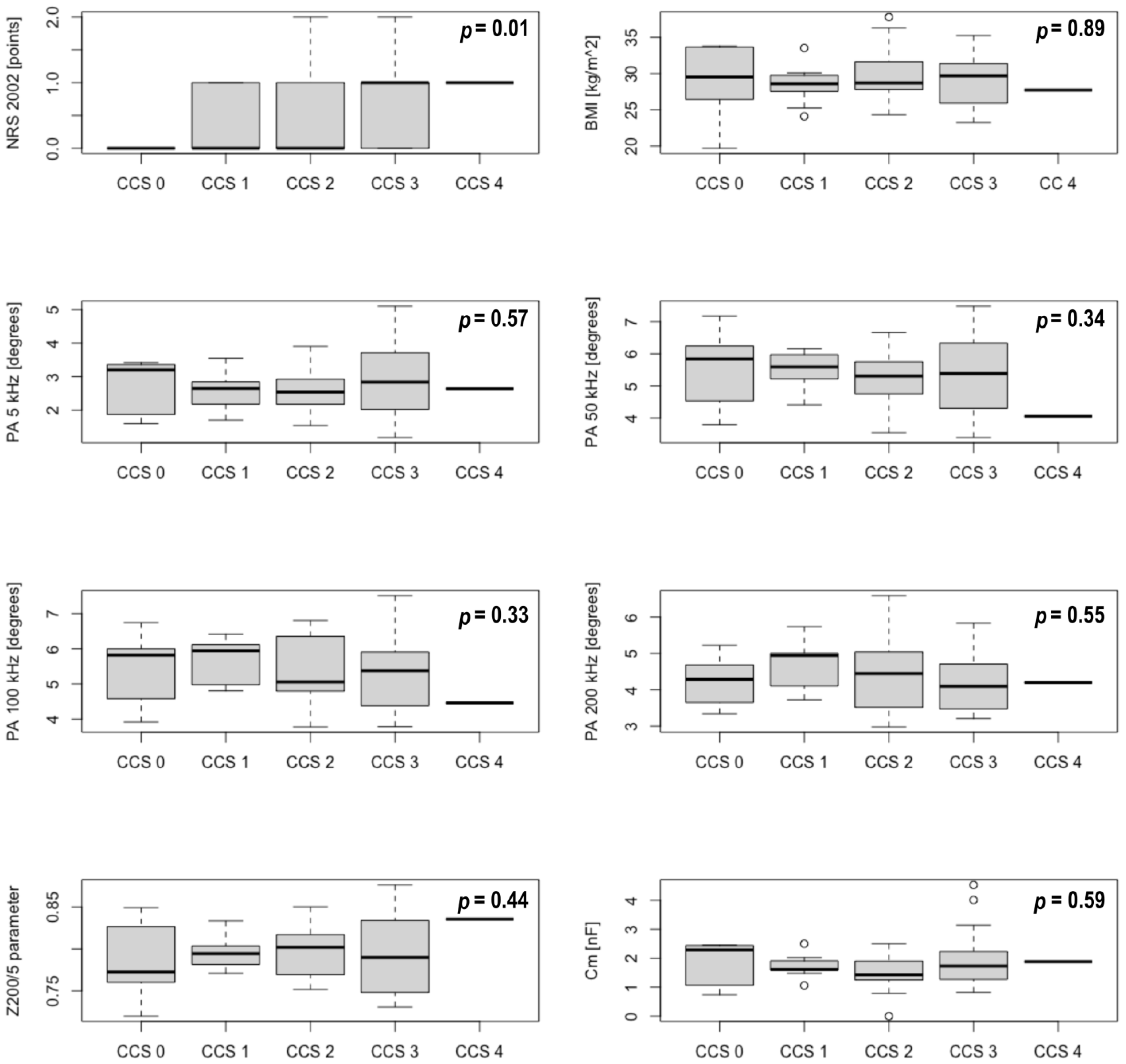
| NRS 2002 [points] | 0.37 | 0.099; 0.586 | 0.01 * |
| BMI [kg/m2] | 0.02 | −0.271; 0.309 | 0.89 |
| PA 5 kHz [°] | 0.08 | −0.201; 0.352 | 0.57 |
| PA 50 kHz [°] | −0.14 | −0.400; 0.147 | 0.34 |
| PA 100 kHz [°] | −0.14 | −0.403; 0.143 | 0.33 |
| PA 200 kHz [°] | −0.09 | −0.357; 0.196 | 0.55 |
| Z200/5 | −0.26 | −0.530; 0.063 | 0.11 |
| Cm [nF] | 0.08 | −0.206; 0.348 | 0.59 |
| FFM [%] | −0.001 | −0.280; 0.277 | 0.99 |
| FM [%] | −0.002 | −0.279; 0.277 | 0.99 |
| TBW [%] | 0.002 | −0.278; 0.280 | 0.99 |
| ECF [%] | 0.05 | −0.232; 0.323 | 0.73 |
| ICF [%] | −0.06 | −0.328; 0.227 | 0.70 |
| Significantly lesioned coronary arteries | |||
| NRS 2002 [points] | 0.01 | −0.268; 0.289 | 0.94 |
| BMI [kg/m2] | −0.13 | −0.409; 0.162 | 0.37 |
| PA 5 kHz [°] | 0.04 | −0.244; 0.312 | 0.80 |
| PA 50 kHz [°] | 0.002 | −0.277; 0.280 | 0.99 |
| PA 100 kHz [°] | 0.02 | −0.260; 0.297 | 0.90 |
| PA 200 kHz [°] | 0.09 | −0.191; 0.361 | 0.53 |
| Z200/5 | 0.02 | −0.255; 0.301 | 0.87 |
| Cm [nF] | −0.04 | −0.317; 0.239 | 0.77 |
| FFM [%] | 0.02 | −0.260; 0.297 | 0.89 |
| FM [%] | −0.02 | −0.294; 0.262 | 0.91 |
| TBW [%] | 0.02 | −0.262; 0.294 | 0.91 |
| ECF [%] | 0.07 | −0.216; 0.338 | 0.64 |
| ICF [%] | −0.07 | −0.340; 0.215 | 0.64 |
| LVEF | |||
| NRS [points] | 0.19 | −0.131; 0.479 | 0.24 |
| BMI [kg/m2] | 0.38 | 0.057; 0.635 | 0.02 * |
| PA 5 kHz [°] | 0.12 | −0.208; 0.416 | 0.48 |
| PA 50 kHz [°] | 0.28 | −0.042; 0.545 | 0.09 |
| PA 100 kHz [°] | 0.28 | −0.042; 0.545 | 0.09 |
| PA 200 kHz [°] | 0.23 | −0.096; 0.506 | 0.17 |
| Z200/5 | −0.26 | −0.530; 0.063 | 0.11 |
| Cm [nF] | −0.01 | −0.320; 0.311 | 0.98 |
| FFM [%] | −0.09 | −0.392; 0.235 | 0.60 |
| FM [%] | 0.08 | −0.238; 0.389 | 0.61 |
| TBW [%] | −0.08 | −0.389; 0.238 | 0.61 |
| ECF [%] | −0.39 | −0.625; −0.080 | 0.02 * |
| ICF [%] | 0.38 | 0.073; 0.621 | 0.02 * |
| Women | Men | |||||
|---|---|---|---|---|---|---|
| R | 95% CI | p | R | 95% CI | p | |
| NRS 2002 | ||||||
| BMI [kg/m2] | −0.05 | −0.462 0.379 | 0.82 | −0.05 | −0.357; 0.448 | 0.80 |
| PA 5 kHz [°] | 0.05 | −0.355 0.434 | 0.82 | −0.42 | −0.698; −0.028 | 0.04 * |
| PA 50 kHz [°] | −0.22 | −0.566 −0.192 | 0.29 | −0.44 | −0.712; −0.056 | 0.03 * |
| PA 100 kHz [°] | −0.24 | −0.580 0.171 | 0.25 | −0.34 | −0.645; 0.069 | 0.10 |
| PA 200 kHz [°] | −0.19 | −0.544 0.221 | 0.36 | −0.05 | −0.436; 0.352 | 0.81 |
| Z200/5 parameter | 0.18 | −0.228 0.540 | 0.38 | 0.53 | 0.169; 0.764 | 0.01 * |
| Cm [nF] | 0.09 | −0.319 0.466 | 0.68 | −0.37 | −0.665; 0.034 | 0.07 |
| BMI | ||||||
| NRS 2002 [points] | −0.05 | −0.462 0.379 | 0.82 | 0.05 | −0.357; 0.448 | 0.80 |
| PA 5 kHz [°] | 0.32 | −0.121 0.651 | 0.15 | 0.43 | 0.027; 0.708 | 0.04 * |
| PA 50 kHz [°] | 0.22 | −0.222 0.587 | 0.32 | 0.27 | −0.153; 0.605 | 0.21 |
| PA 100 kHz [°] | 0.15 | −0.292 0.536 | 0.51 | 0.34 | −0.070; 0.655 | 0.10 |
| PA 200 kHz [°] | −0.03 | −0.448 0.394 | 0.88 | 0.47 | 0.081; 0.734 | 0.02 * |
| Z200/5 | −0.30 | −0.642 0.137 | 0.17 | −0.22 | −0.575; 0.198 | 0.29 |
| Cm [nF] | 0.34 | −0.092 0.668 | 0.12 | 0.16 | −0.256; 0.533 | 0.44 |
| Women | Men | |||
|---|---|---|---|---|
| R | p | R | p | |
| CCS class | ||||
| NRS 2002 [points] | 0.58 | 0.003 * | 0.19 | 0.35 |
| BMI [kg/m2] | 0.11 | 0.63 | −0.11 | 0.61 |
| PA 5 kHz [°] | 0.21 | 0.31 | −0.05 | 0.82 |
| PA 50 kHz [°] | −0.02 | 0.94 | −0.30 | 0.15 |
| PA 100 kHz [°] | −0.02 | 0.94 | −0.31 | 0.13 |
| PA 200 kHz [°] | −0.001 | 0.997 | −0.20 | 0.35 |
| Z200/5 | −0.34 | 0.14 | −0.27 | 0.26 |
| Cm [nF] | 0.12 | 0.58 | 0.07 | 0.75 |
| FFM [%] | −0.14 | 0.50 | 0.18 | 0.40 |
| FM [%] | 0.13 | 0.52 | −0.18 | 0.40 |
| TBW [%] | −0.13 | 0.52 | 0.18 | 0.40 |
| ECF [%] | 0.05 | 0.81 | 0.10 | 0.64 |
| ICF [%] | −0.05 | 0.81 | −0.11 | 0.60 |
| Significantly lesioned coronary arteries | ||||
| NRS 2002 [points] | 0.27 | 0.20 | −0.17 | 0.43 |
| BMI [kg/m2] | −0.19 | 0.41 | −0.03 | 0.88 |
| PA 5 kHz [°] | −0.26 | 0.22 | 0.21 | 0.32 |
| PA 50 kHz [°] | −0.35 | 0.09 | 0.25 | 0.23 |
| PA 100 | −0.28 | 0.18 | 0.23 | 0.28 |
| PA 200 | 0.001 | 0.995 | 0.14 | 0.50 |
| Z200/5 | 0.34 | 0.09 | −0.22 | 0.30 |
| Cm | −0.39 | 0.06 | 0.12 | 0.58 |
| FFM | −0.31 | 0.14 | 0.06 | 0.77 |
| FM | 0.31 | 0.13 | −0.06 | 0.77 |
| TBW | −0.31 | 0.13 | 0.06 | 0.77 |
| ECF | 0.25 | 0.23 | −0.25 | 0.22 |
| ICF | −0.25 | 0.23 | 0.25 | 0.23 |
| LVEF | ||||
| NRS 2002 [points] | 0.15 | 0.52 | 0.23 | 0.34 |
| BMI [kg/m2] | 0.51 | 0.04 * | 0.23 | 0.36 |
| PA 5 kHz [°] | 0.27 | 0.25 | −0.01 | 0.97 |
| PA 50 kHz [°] | 0.35 | 0.13 | 0.35 | 0.14 |
| PA 100 kHz [°] | 0.33 | 0.15 | 0.37 | 0.12 |
| PA 200 kHz [°] | 0.22 | 0.36 | 0.31 | 0.20 |
| Z200/5 | −0.34 | 0.14 | −0.27 | 0.26 |
| Cm [nF] | 0.21 | 0.37 | −0.25 | 0.31 |
| FFM [%] | −0.05 | 0.85 | 0.07 | 0.77 |
| FM [%] | 0.04 | 0.87 | −0.07 | 0.77 |
| TBW [%] | −0.04 | 0.87 | 0.07 | 0.77 |
| ECF [%] | −0.19 | 0.42 | −0.37 | 0.12 |
| ICF [%] | 0.19 | 0.42 | 0.36 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popiolek-Kalisz, J.; Blaszczak, P. Nutritional Status of Coronary Artery Disease Patients—Preliminary Results. Int. J. Environ. Res. Public Health 2023, 20, 3464. https://doi.org/10.3390/ijerph20043464
Popiolek-Kalisz J, Blaszczak P. Nutritional Status of Coronary Artery Disease Patients—Preliminary Results. International Journal of Environmental Research and Public Health. 2023; 20(4):3464. https://doi.org/10.3390/ijerph20043464
Chicago/Turabian StylePopiolek-Kalisz, Joanna, and Piotr Blaszczak. 2023. "Nutritional Status of Coronary Artery Disease Patients—Preliminary Results" International Journal of Environmental Research and Public Health 20, no. 4: 3464. https://doi.org/10.3390/ijerph20043464
APA StylePopiolek-Kalisz, J., & Blaszczak, P. (2023). Nutritional Status of Coronary Artery Disease Patients—Preliminary Results. International Journal of Environmental Research and Public Health, 20(4), 3464. https://doi.org/10.3390/ijerph20043464







