Adsorption of Anionic and Cationic Dyes on Activated Carbon Prepared from Oak Cupules: Kinetics and Thermodynamics Studies
Abstract
1. Introduction
2. Experimental
2.1. Preparation of the Adsorbate Solutions
2.2. Preparation of the Activated Charcoal
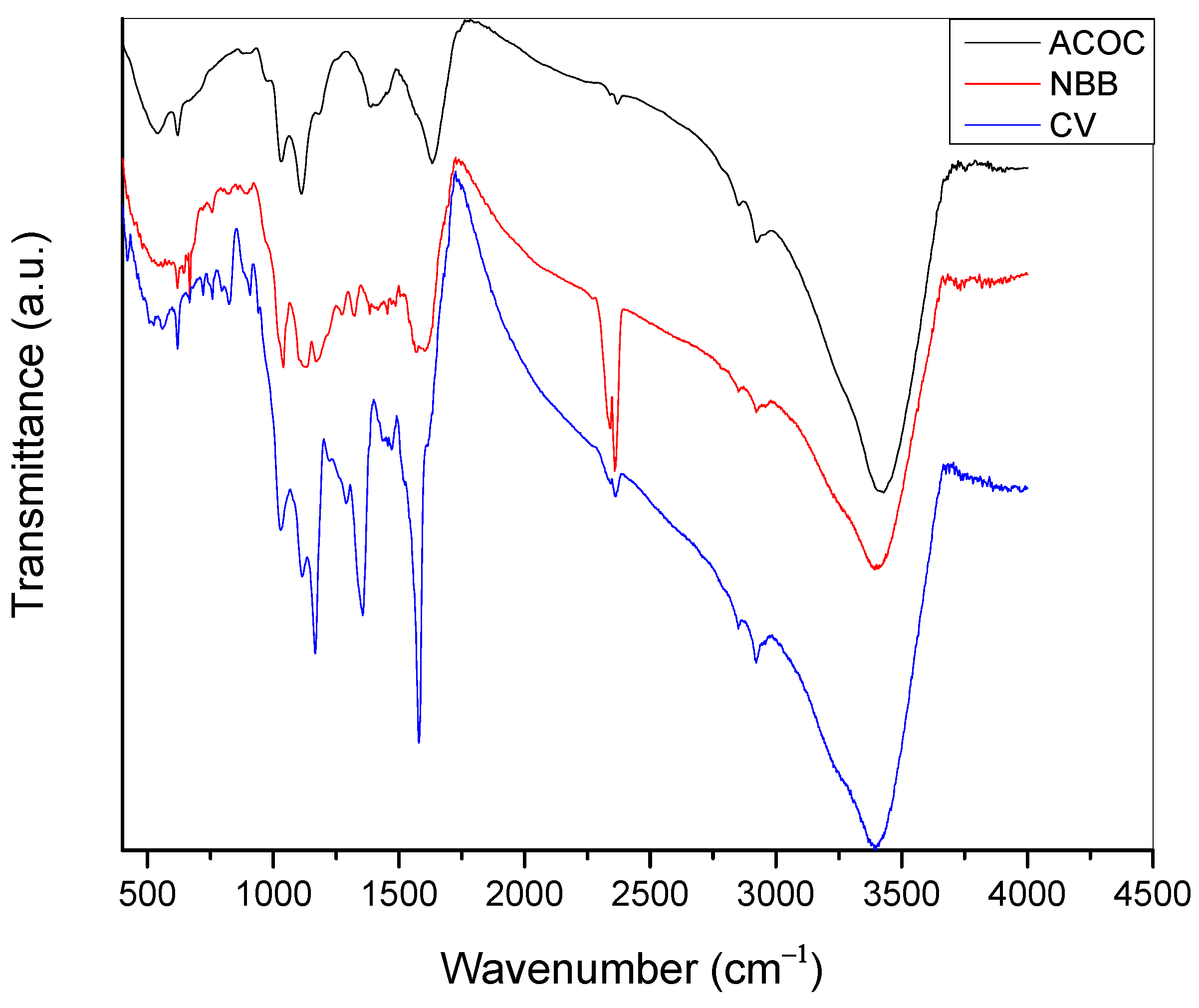
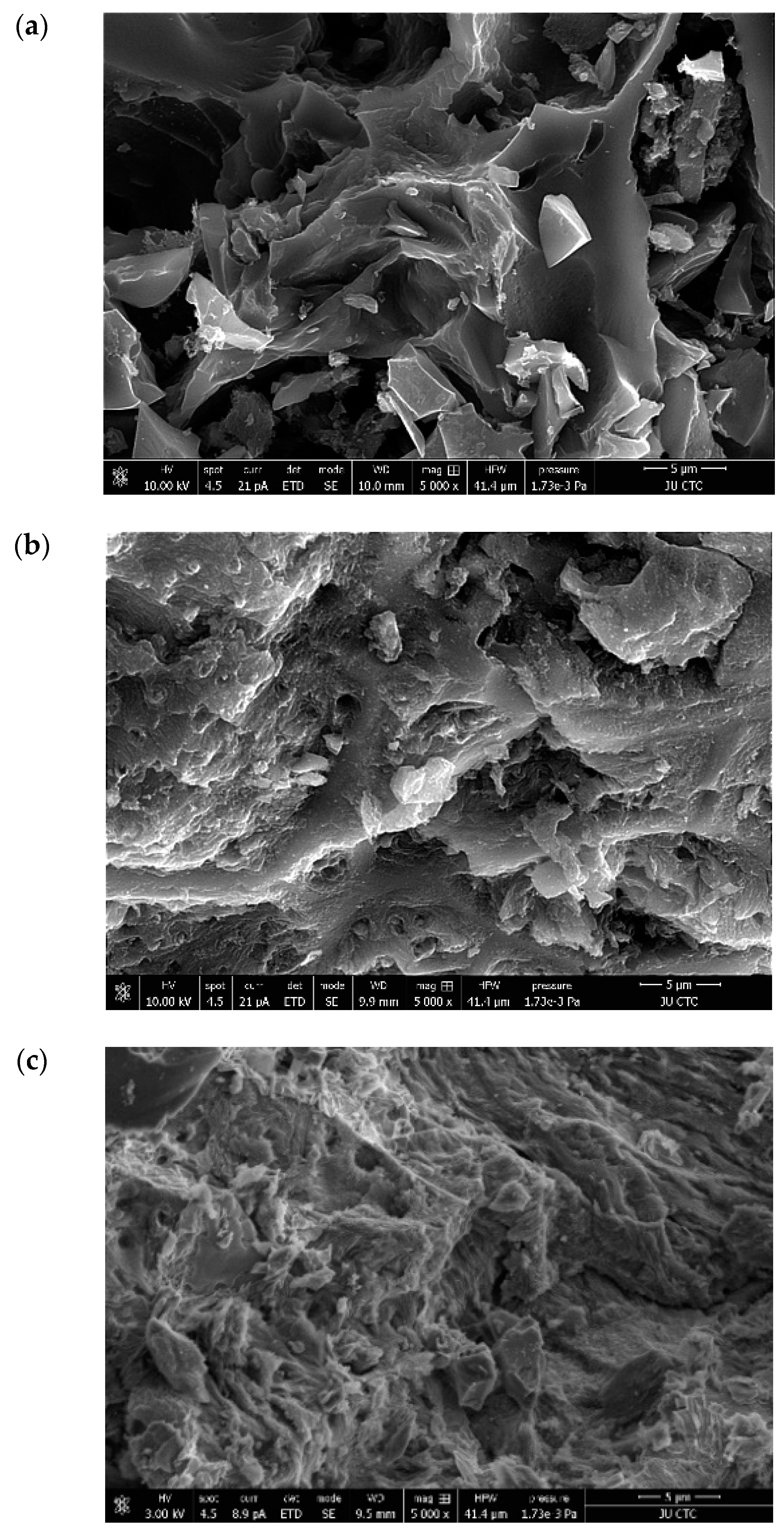
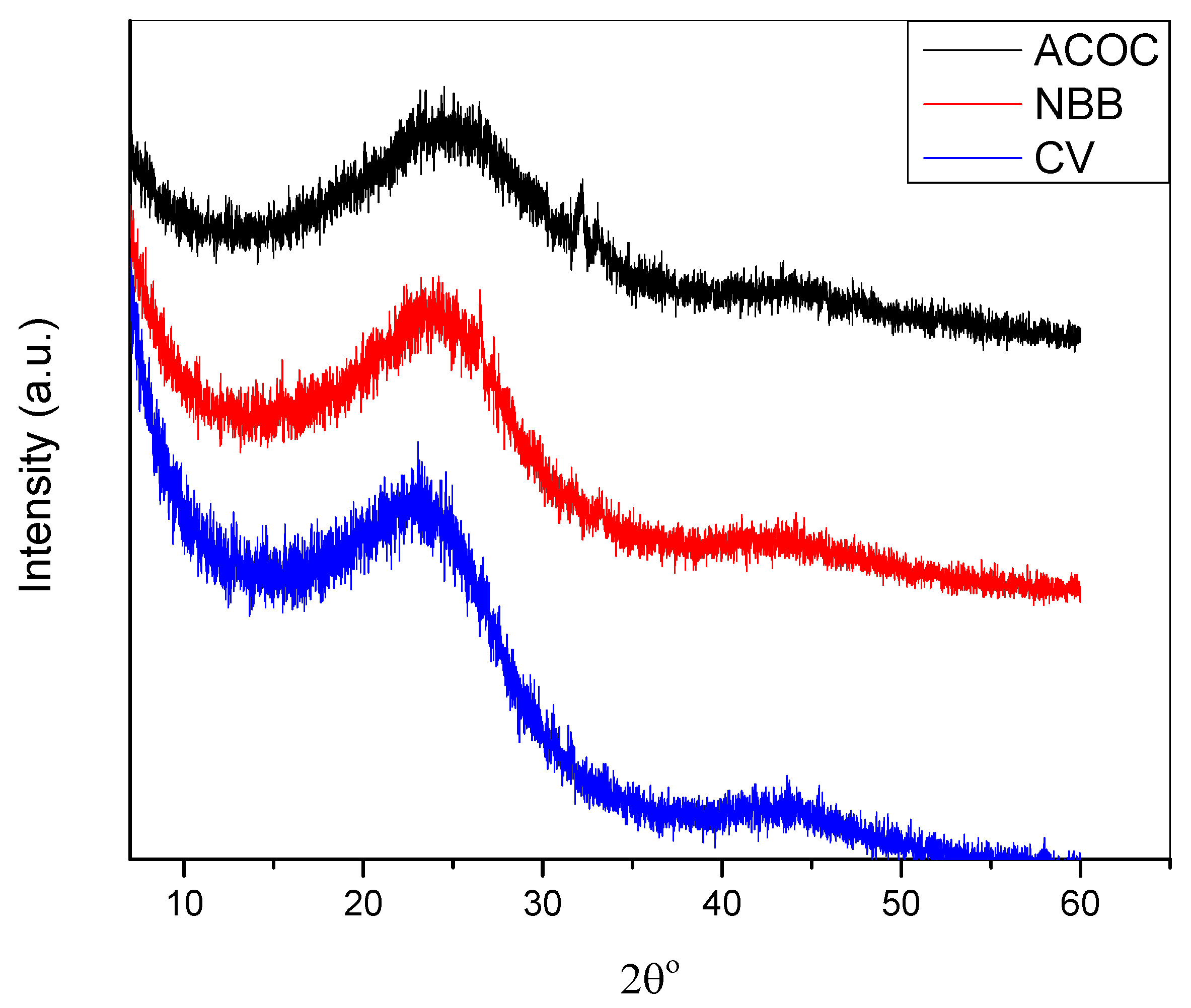
2.3. Characterizations of the Activated Charcoal
2.4. Batch Adsorption Experiments
2.5. Isotherm and Kinetic Studies
2.6. Adsorption Thermodynamic
3. Results and Discussion
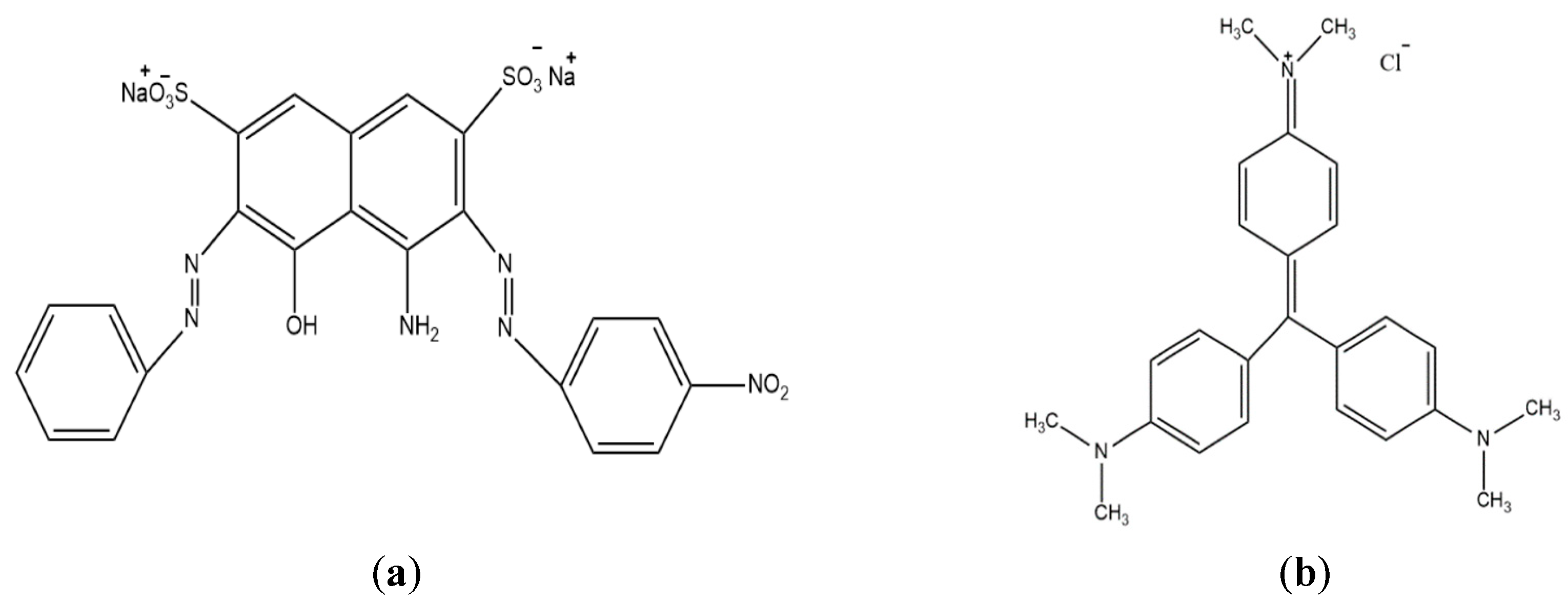
3.1. Characterization of Adsorbate
3.2. Parametric Study of the Adsorption Process
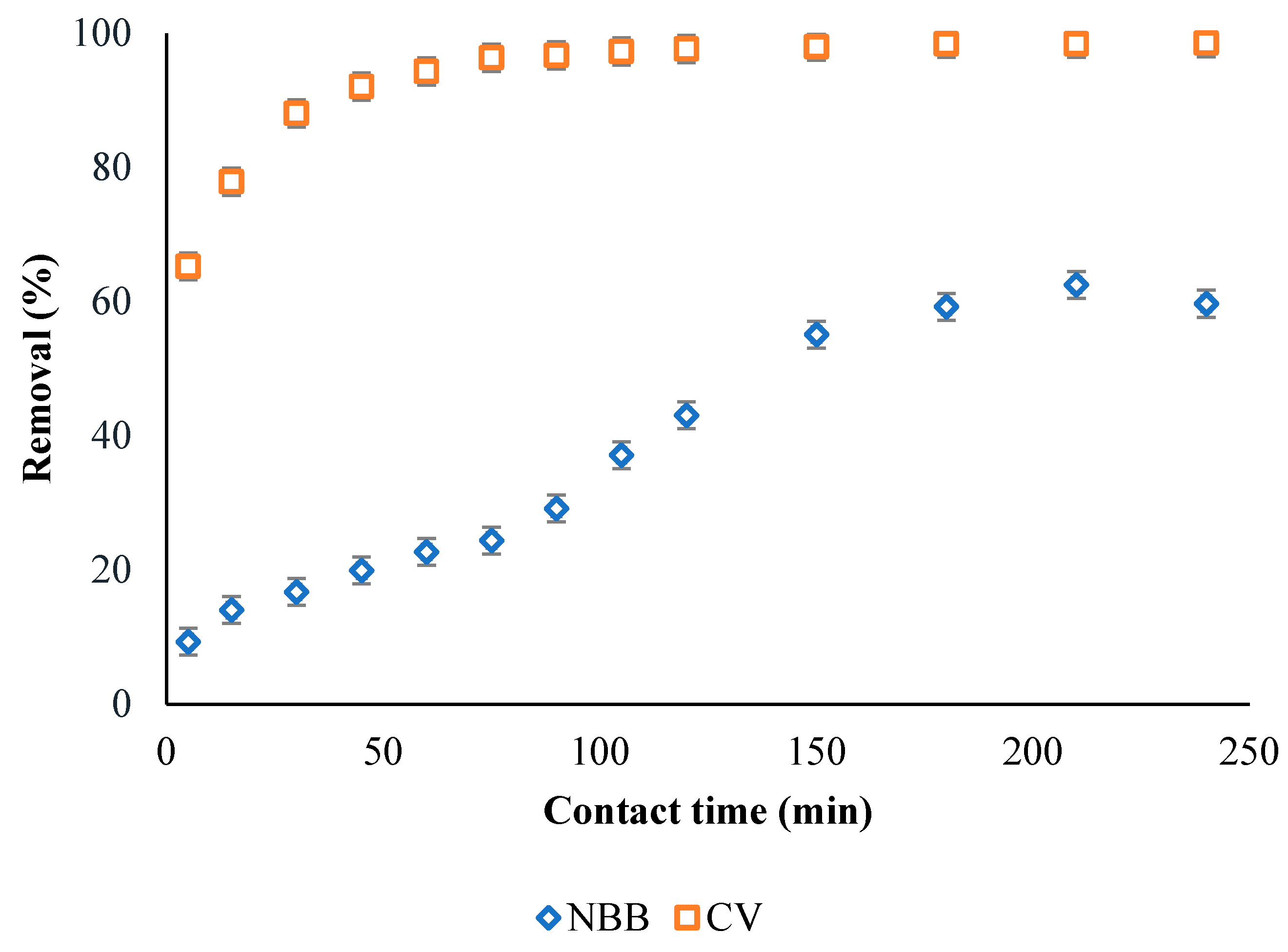
3.2.1. Effect of Contact Time
3.2.2. Effect of ACOC Dose
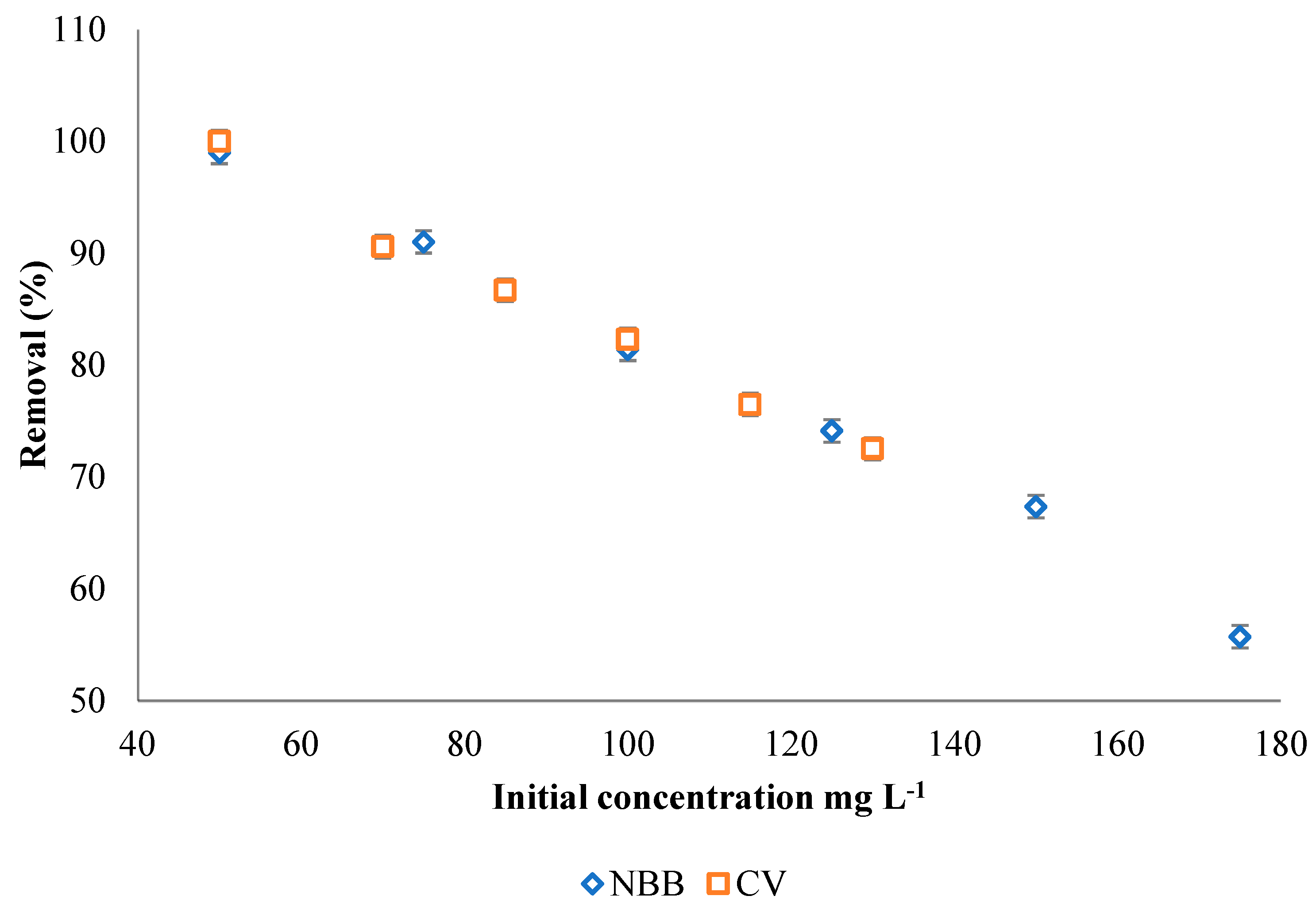
3.2.3. Effect of Dye Concentration
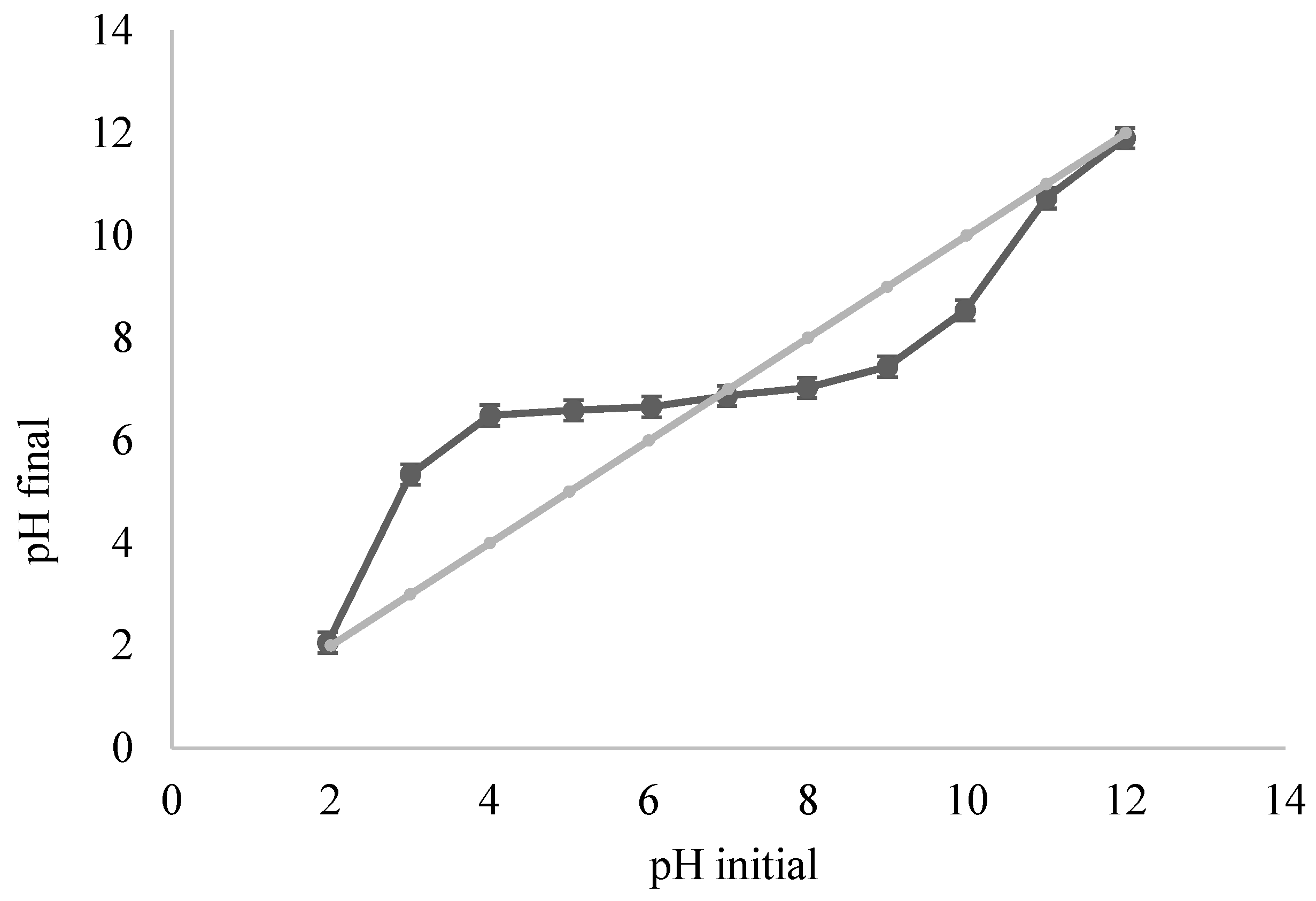
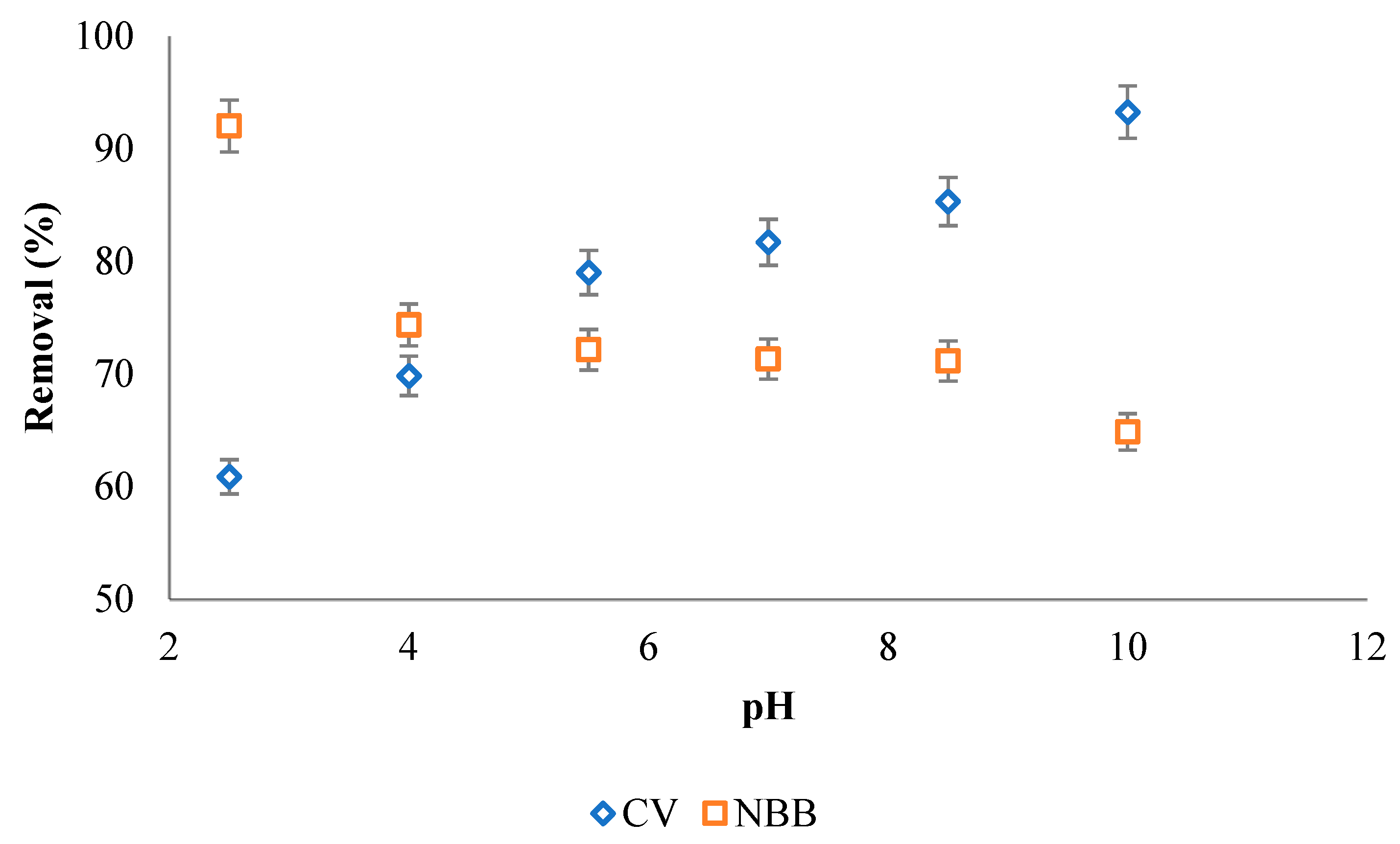
3.2.4. Effect of pH
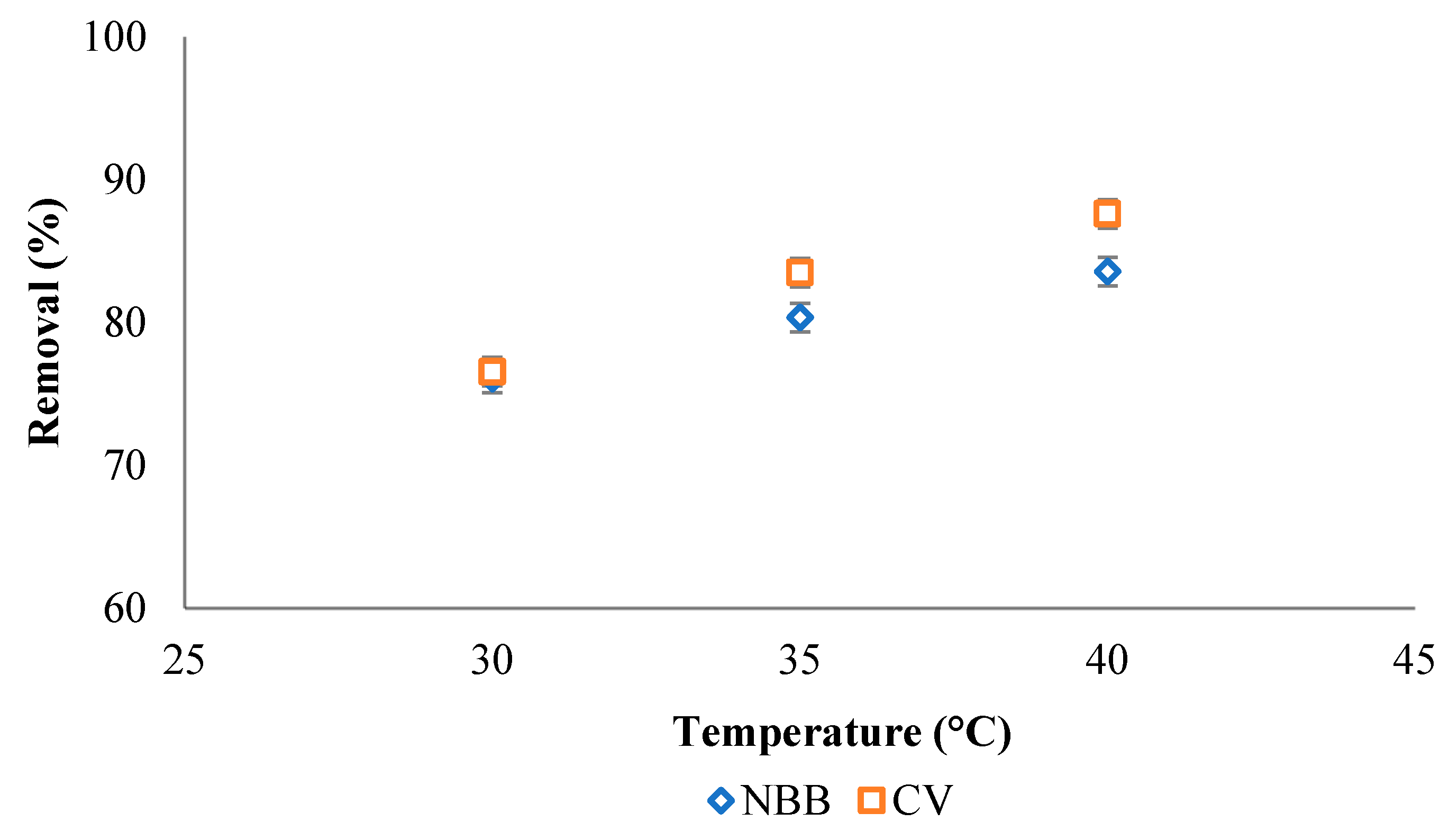
3.2.5. Effect of Temperature
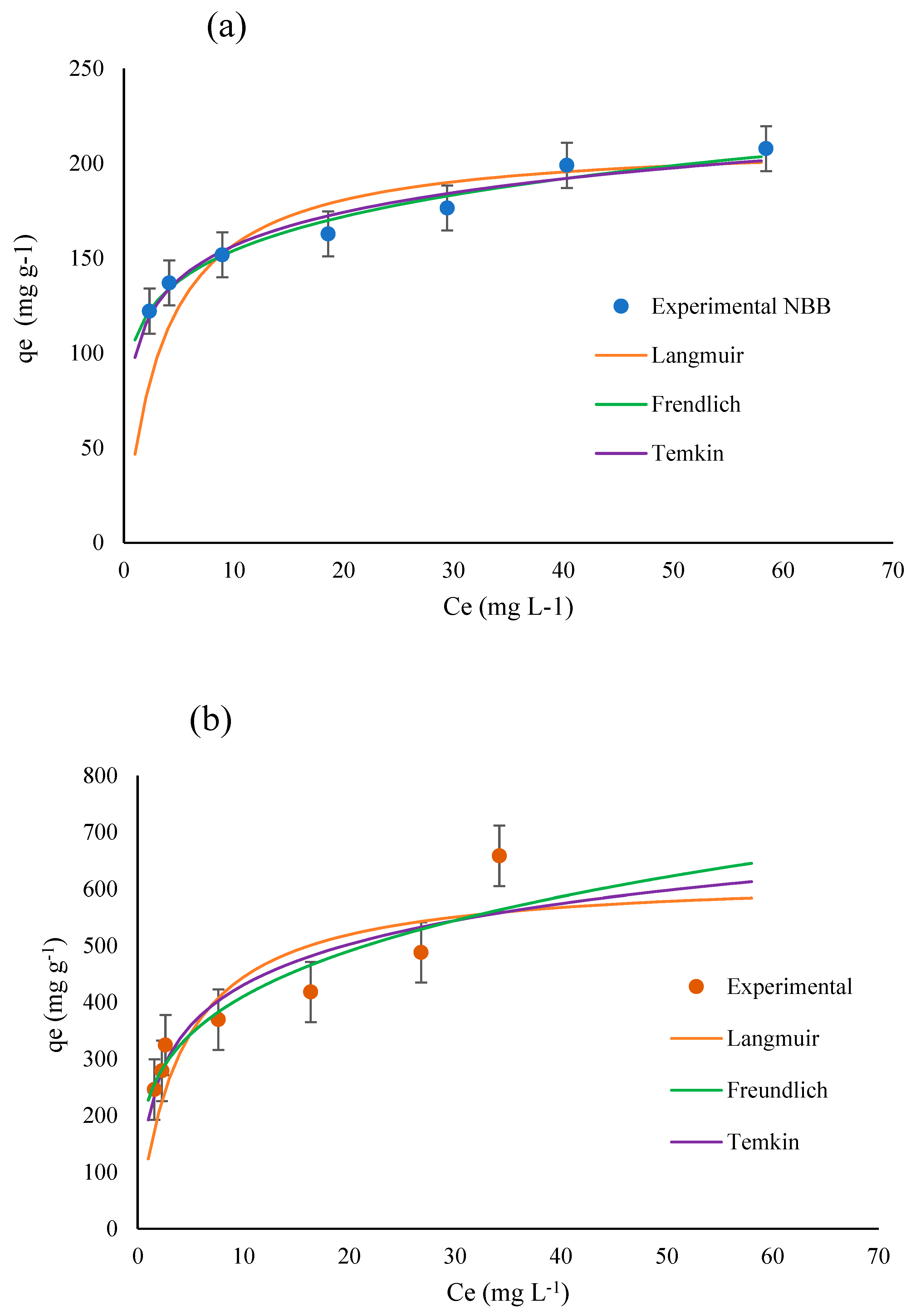
3.3. Isotherm Studies
3.4. Kinetics Study
3.5. Thermodynamics
4. Reusability of ACOC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Visa, M.; Bogatu, C.; Duta, A. Simultaneous Adsorption of Dyes and Heavy Metals from Multicomponent Solutions Using Fly Ash. Appl. Surf. Sci. 2010, 256, 5486–5491. [Google Scholar] [CrossRef]
- Samchetshabam, G.; Hussan, A.; Choudhury, T. Impact of Textile Dyes Waste on Aquatic Environments and Its Treatment. Environ. Ecol. 2016, 35, 2349–2353. [Google Scholar]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile Finishing Dyes and Their Impact on Aquatic Environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Peighambardoust, S.H.; Pateiro, M.; Lorenzo, J.M. Adsorption of Crystal Violet Dye Using Activated Carbon of Lemon Wood and Activated Carbon/Fe3O4 Magnetic Nanocomposite from Aqueous Solutions: A Kinetic, Equilibrium and Thermodynamic Study. Molecules 2021, 26, 2241. [Google Scholar] [CrossRef]
- Đorđević, J.S.; Vladisavljević, G.T.; Trtić-Petrović, T.M. Removal of the Selected Pesticides from a Water Solution by Applying Hollow Fiber Liquid–Liquid Membrane Extraction. Ind. Eng. Chem. Res. 2014, 53, 4861–4870. [Google Scholar] [CrossRef]
- Bakry, A.M.; Alamier, W.M.; Salama, R.S.; Samy El-Shall, M.; Awad, F.S. Remediation of Water Containing Phosphate Using Ceria Nanoparticles Decorated Partially Reduced Graphene Oxide (CeO2-PRGO) Composite. Surf. Interfaces 2022, 31, 102006. [Google Scholar] [CrossRef]
- Mansour, R.A.E.-G.; Simeda, M.G.; Zaatout, A.A. Removal of Brilliant Green Dye from Synthetic Wastewater under Batch Mode Using Chemically Activated Date Pit Carbon. RSC Adv. 2021, 11, 7851–7861. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, S.; Kumar, A.; Lai, C.W.; Naushad, M.; Shehnaz; Iqbal, J.; Stadler, F.J. Activated Carbon as Superadsorbent and Sustainable Material for Diverse Applications. Adsorpt. Sci. Technol. 2022, 2022, e4184809. [Google Scholar] [CrossRef]
- Haji, A.; Mahmoodi, N.M. Soy Meal Hull Activated Carbon: Preparation, Characterization and Dye Adsorption Properties. Desalination Water Treat. 2012, 44, 237–244. [Google Scholar] [CrossRef]
- Boudechiche, N.; Fares, M.; Ouyahia, S.; Yazid, H.; Trari, M.; Sadaoui, Z. Comparative Study on Removal of Two Basic Dyes in Aqueous Medium by Adsorption Using Activated Carbon from Ziziphus Lotus Stones. Microchem. J. 2019, 146, 1010–1018. [Google Scholar] [CrossRef]
- Bhowmik, S.; Chakraborty, V.; Das, P. Batch Adsorption of Indigo Carmine on Activated Carbon Prepared from Sawdust: A Comparative Study and Optimization of Operating Conditions Using Response Surface Methodology. Results Surf. Interfaces 2021, 3, 100011. [Google Scholar] [CrossRef]
- Seidmohammadi, A.; Asgari, G.; Dargahi, A.; Leili, M.; Vaziri, Y.; Hayati, B.; Shekarchi, A.A.; Mobarakian, A.; Bagheri, A.; Nazari; et al. A Comparative Study for the Removal of Methylene Blue Dye from Aqueous Solution by Novel Activated Carbon Based Adsorbents. Prog. Color Color. Coat. 2019, 12, 133–144. [Google Scholar] [CrossRef]
- Djilani, C.; Zaghdoudi, R.; Djazi, F.; Bouchekima, B.; Lallam, A.; Modarressi, A.; Rogalski, M. Adsorption of Dyes on Activated Carbon Prepared from Apricot Stones and Commercial Activated Carbon. J. Taiwan Inst. Chem. Eng. 2015, 53, 112–121. [Google Scholar] [CrossRef]
- Astuti, F.; Sari, N.; Maghfirohtuzzoimah, V.L.; Asih, R.; Baqiya, M.A.; Darminto, D. Study of the Formation of Amorphous Carbon and RGO-like Phases from Palmyra Sugar by Variation of Calcination Temperature. J. Fis. Dan Apl. 2020, 16, 91. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Kinetics and Equilibrium Study for the Adsorption of Textile Dyes on Coconut Shell Activated Carbon. Arab. J. Chem. 2017, 10, S3381–S3393. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an Anionic Dye (Congo Red) from Aqueous Solutions by Pine Bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Chowdhury, S. Insight into Adsorption Thermodynamics. Thermodynamics 2011, 16, 349–364. [Google Scholar] [CrossRef]

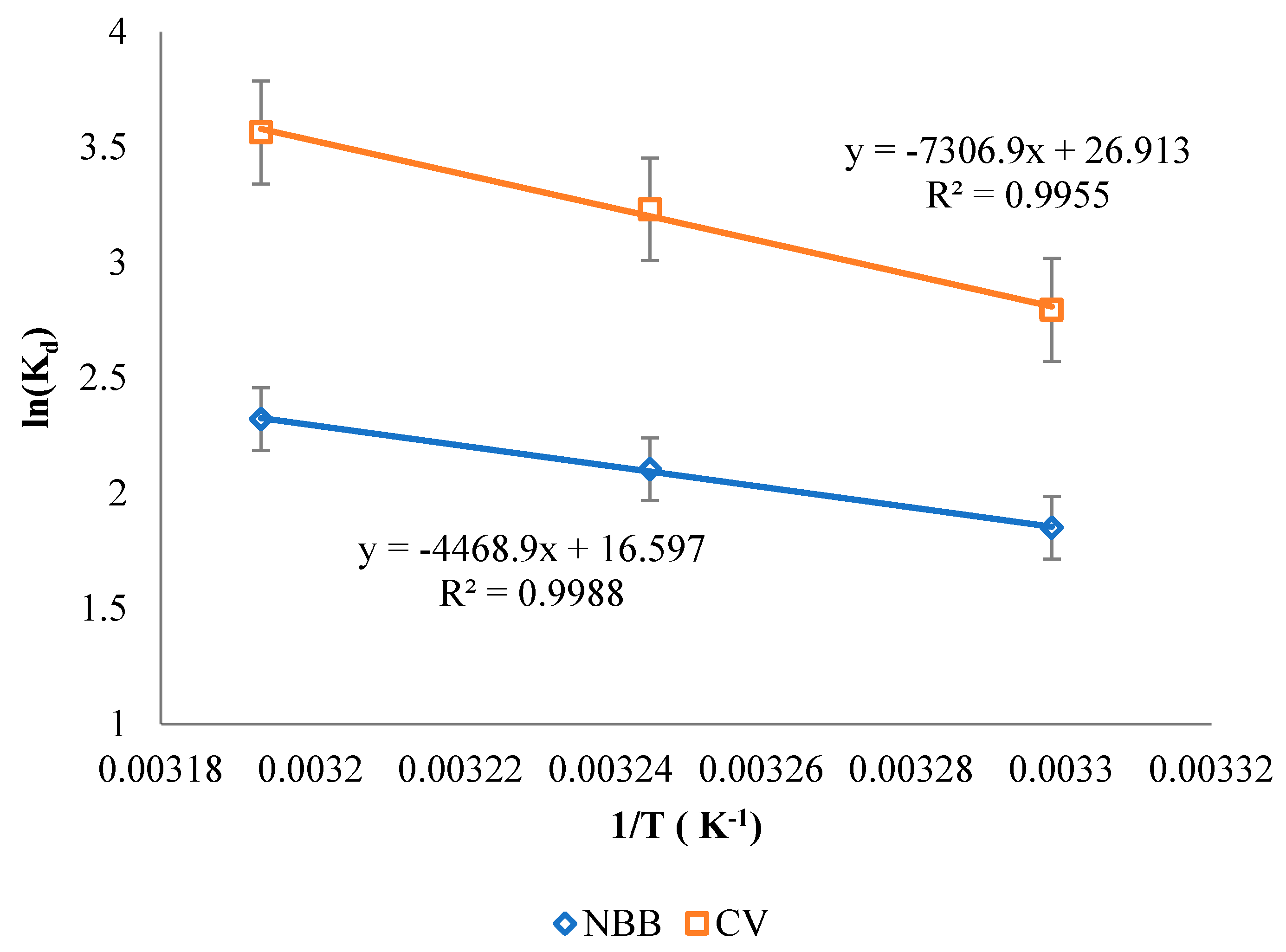

| Model | Name | Nonlinear Equation | Linear Equation | Parameters |
|---|---|---|---|---|
| Isotherm | Langmuir | Ce: Concentration of dye at equilibrium (mg L−1) qe: Amount of adsorbate adsorbed per unit mass of adsorbent at equilibrium (mg g−1) qm: Theoretical maximum adsorption capacity (mg g−1) kl: Langmuir constant related to the rate of adsorption 1/n and KF: Freundlich constants kt: Temkin constant | ||
| Freundlich | ||||
| Temkin | ||||
| Kinetics | Pseudo-first order | qt: Amount of adsorbate adsorbed per unit mass of adsorbent at time t constant (mg g−1) k1p: Pseudo-first order rate constant k2: Second order rate constant k2p: Second order rate constant kint: Intraparticle diffusion rate constant | ||
| Second order | ||||
| Pseudo-second order | ||||
| Intraparticle diffusion | ||||
| Model | Parameter | NBB | CV |
|---|---|---|---|
| Langmuir | qm (mg g−1) | 213 | 625 |
| kl | 0.2814 | 0.2462 | |
| R2 | 0.992 | 0.935 | |
| Freundlich | 1/n | 0.1585 | 0.2572 |
| Kf | 106.9 | 227 | |
| R2 | 0.9768 | 0.9164 | |
| Temkin | qm (mg g−1) | 25.5 | 103.5 |
| kT | 46.0 | 6.41 | |
| R2 | 0.9570 | 0.8475 |
| Model | Parameter | NBB | CV |
|---|---|---|---|
| Pseudo-first order | K1p | 0.0173 | 0.0202 |
| qe | 125 | 66.3 | |
| R2 | 0.9917 | R2 = 0.9439 | |
| Second order | k2 | −(4 × 10−5) | −(2 × 10−6) |
| qe | 91.7 | 294 | |
| R2 | 0.571 | 0.4997 | |
| Pseudo-second order | k2p | 3.08 × 10−5 | 8.35 × 10−4 |
| qe | 278 | 333 | |
| R2 | R2 = 0.5981 | R2 = 1 | |
| Intraparticle diffusion | kint | 0.0861 | 6.6788 |
| R2 | 0.9469 | 0.7128 |
| Dye | ΔH (KJ/mol) | ΔS (J/mol) | ΔG (KJ/mol) | ||
|---|---|---|---|---|---|
| 303 K | 308 K | 313 K | |||
| NBB | 37.15 | 137.99 | −4.68 | −5.37 | −6.06 |
| CV | 60.75 | 223.75 | −11.42 | −12.61 | −13.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhabbas, M.; Al-Ma’abreh, A.M.; Edris, G.; Saleh, T.; Alhmood, H. Adsorption of Anionic and Cationic Dyes on Activated Carbon Prepared from Oak Cupules: Kinetics and Thermodynamics Studies. Int. J. Environ. Res. Public Health 2023, 20, 3280. https://doi.org/10.3390/ijerph20043280
Alkhabbas M, Al-Ma’abreh AM, Edris G, Saleh T, Alhmood H. Adsorption of Anionic and Cationic Dyes on Activated Carbon Prepared from Oak Cupules: Kinetics and Thermodynamics Studies. International Journal of Environmental Research and Public Health. 2023; 20(4):3280. https://doi.org/10.3390/ijerph20043280
Chicago/Turabian StyleAlkhabbas, Manal, Alaa M. Al-Ma’abreh, Gada Edris, Tasneem Saleh, and Heba Alhmood. 2023. "Adsorption of Anionic and Cationic Dyes on Activated Carbon Prepared from Oak Cupules: Kinetics and Thermodynamics Studies" International Journal of Environmental Research and Public Health 20, no. 4: 3280. https://doi.org/10.3390/ijerph20043280
APA StyleAlkhabbas, M., Al-Ma’abreh, A. M., Edris, G., Saleh, T., & Alhmood, H. (2023). Adsorption of Anionic and Cationic Dyes on Activated Carbon Prepared from Oak Cupules: Kinetics and Thermodynamics Studies. International Journal of Environmental Research and Public Health, 20(4), 3280. https://doi.org/10.3390/ijerph20043280







