The Involvement of Prolactin in Stress-Related Disorders
Abstract
1. Introduction
2. Prolactin and Its Receptors
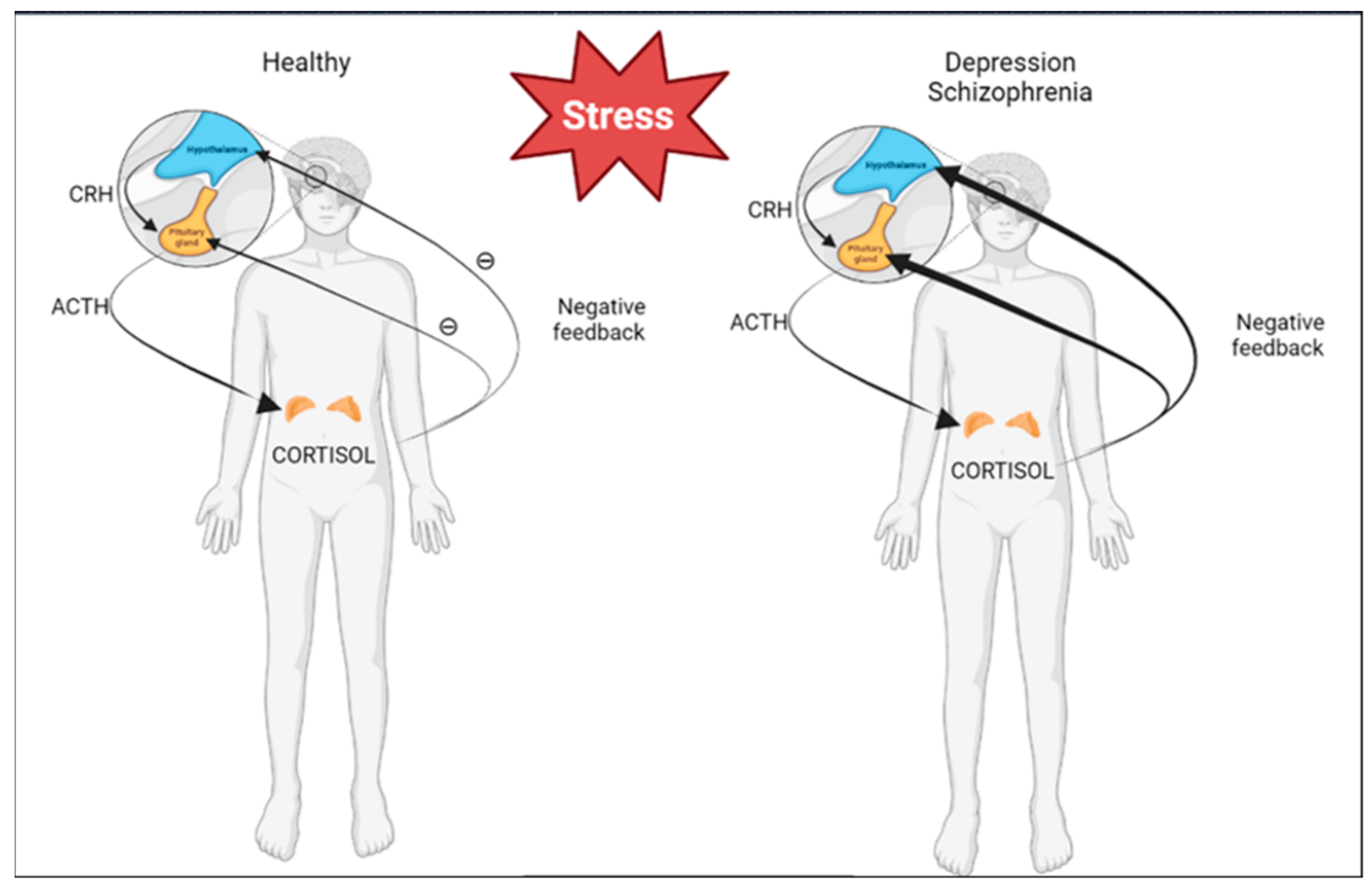
3. PRL and Stress
4. PRL and Neuropsychiatric Disorders
4.1. Depression
4.2. Schizophrenia
5. Novel Aspects of PRL Action in CNS
5.1. Neurogenesis
5.2. Blood Brain Barrier and PRL
6. PRL and Other Disorders
6.1. Hyperprolactinemia, Sexual Health, and Infertility
6.2. PRL and Carcinogenesis
6.3. PRL and Artherosclerotic Vascular Disease
6.4. PRL and Autoimmune Diseases
6.5. PRL and Virus Infection
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Truong, A.T.; Duez, C.; Belayew, A.; Renard, A.; Pictet, R.; Bell, G.I.; Martial, J.A. Isolation and characterization of the human prolactin gene. EMBO J. 1984, 3, 429–437. [Google Scholar] [CrossRef]
- Cooke, N.E.; Coit, D.; Shine, J.; Baxter, J.D.; Martial, J.A. Human prolactin. cDNA structural analysis and evolutionary comparisons. J. Biol. Chem. 1981, 256, 4007–4016. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [CrossRef] [PubMed]
- Garnier, P.E.; Aubert, M.L.; Kaplan, S.L.; Grumbach, M.M. Heterogeneity of pituitary and plasma prolactin in man: Decreased affinity of “Big” prolactin in a radioreceptor assay and evidence for its secretion. J. Clin. Endocrinol. Metab. 1978, 47, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Aisaka, K.; Shimatsu, A. A possible cause of the variable detectability of macroprolactin by different immunoassay systems. Clin. Chem. Lab. Med. 2016, 54, 603–608. [Google Scholar] [CrossRef]
- Ben-Jonathan, N.; LaPensee, C.R.; LaPensee, E.W. What can we learn from rodents about prolactin in humans? Endocr. Rev. 2008, 29, 1–41. [Google Scholar] [CrossRef]
- Ignacak, A.; Kasztelnik, M.; Sliwa, T.; Korbut, R.A.; Rajda, K.; Guzik, T.J. Prolactin--not only lactotrophin. A “new” view of the “old” hormone. J. Physiol. Pharmacol. 2012, 63, 435–443. [Google Scholar]
- Faron-Górecka, A.; Kuśmider, M.; Kolasa, M.; Zurawek, D.; Gruca, P.; Papp, M.; Szafran, K.; Solich, J.; Pabian, P.; Romańska, I.; et al. Prolactin and its receptors in the chronic mild stress rat model of depression. Brain Res. 2014, 1555, 48–59. [Google Scholar] [CrossRef]
- Walsh, R.J.; Slaby, F.J.; Posner, B.I. A receptor-mediated mechanism for the transport of prolactin from blood to cerebrospinal fluid. Endocrinology 1987, 120, 1846–1850. [Google Scholar] [CrossRef]
- Brown, R.S.; Wyatt, A.K.; Herbison, R.E.; Knowles, P.J.; Ladyman, S.R.; Binart, N.; Banks, W.A.; Grattan, D.R. Prolactin transport into mouse brain is independent of prolactin receptor. FASEB J. 2016, 30, 1002–1010. [Google Scholar] [CrossRef]
- Costa-Brito, A.R.; Quintela, T.; Gonçalves, I.; Duarte, A.C.; Costa, A.R.; Arosa, F.A.; Cavaco, J.E.; Lemos, M.C.; Santos, C.R.A. The Choroid Plexus Is an Alternative Source of Prolactin to the Rat Brain. Mol. Neurobiol. 2021, 58, 1846–1858. [Google Scholar] [CrossRef]
- Donner, N.; Bredewold, R.; Maloumby, R.; Neumann, I.D. Chronic intracerebral prolactin attenuates neuronal stress circuitries in virgin rats. Eur. J. Neurosci. 2007, 25, 1804–1814. [Google Scholar] [CrossRef]
- Torner, L.; Toschi, N.; Pohlinger, A.; Landgraf, R.; Neumann, I.D. Anxiolytic and anti-stress effects of brain prolactin: Improved efficacy of antisense targeting of the prolactin receptor by molecular modeling. J. Neurosci. 2001, 21, 3207–3214. [Google Scholar] [CrossRef] [PubMed]
- Torner, L.; Toschi, N.; Nava, G.; Clapp, C.; Neumann, I.D. Increased hypothalamic expression of prolactin in lactation: Involvement in behavioral and neuroendocrine stress responses. Eur. J. Neurosci. 2002, 15, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; Higuchi, T.; Matsuura, M. Immobilization stress and prolactin secretion in male rats. Possible roles of dopamine and TRH. Neuroendocrinology 1979, 29, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, W.; Li, C.; Su, Z.; Guo, Z.; Li, Z.; Wang, C.; Xu, F. Sex differences in peripheral monoamine transmitter and related hormone levels in chronic stress mice with a depression-like phenotype. PeerJ 2022, 10, e14014. [Google Scholar] [CrossRef]
- Herzog, C.J.; Czéh, B.; Corbach, S.; Wuttke, W.; Schulte-Herbrüggen, O.; Hellweg, R.; Flügge, G.; Fuchs, E. Chronic social instability stress in female rats: A potential animal model for female depression. Neuroscience 2009, 159, 982–992. [Google Scholar] [CrossRef]
- Seggie, J.A.; Brown, G.M. Stress response patterns of plasma corticosterone, prolactin, and growth hormone in the rat, following handling or exposure to novel environment. Can. J. Physiol. Pharmacol. 1975, 53, 629–637. [Google Scholar] [CrossRef]
- Nicholson, G.; Greeley, G.H., Jr.; Humm, J.; Youngblood, W.W.; Kizer, J.S. Prolactin in cerebrospinal fluid a probable site of prolactin autoregulation. Brain Res. 1980, 190, 447–457. [Google Scholar] [CrossRef]
- Fujikawa, T.; Soya, H.; Tamashiro, K.L.; Sakai, R.R.; McEwen, B.S.; Nakai, N.; Ogata, M.; Suzuki, I.; Nakashima, K. Prolactin prevents acute stress-induced hypocalcemia and ulcerogenesis by acting in the brain of rat. Endocrinology 2004, 145, 2006–2013. [Google Scholar] [CrossRef]
- Muccioli, G.; Di Carlo, R. Modulation of prolactin receptors in the rat hypothalamus in response to changes in serum concentration of endogenous prolactin or to ovine prolactin administration. Brain Res. 1994, 663, 244–250. [Google Scholar] [CrossRef]
- Nunes, M.C.; Sobrinho, L.G.; Calhaz-Jorge, C.; Santos, M.A.; Mauricio, J.C.; Sousa, M.F. Psychosomatic factors in patients with hyperprolactinemia and/or galactorrhea. Obstet. Gynecol. 1980, 55, 591–595. [Google Scholar] [PubMed]
- Jergović, M.; Bendelja, K.; Savić Mlakar, A.; Vojvoda, V.; Aberle, N.; Jovanovic, T.; Rabatić, S.; Sabioncello, A.; Vidović, A. Circulating levels of hormones, lipids, and immune mediators in post-traumatic stress disorder—A 3-month follow-up study. Front. Psychiatry 2015, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Olff, M.; Güzelcan, Y.; de Vries, G.J.; Assies, J.; Gersons, B.P. HPA- and HPT-axis alterations in chronic postraumatic stress disorder. Psychoneuroendocrinology 2006, 31, 1220–1230. [Google Scholar] [CrossRef]
- Dinan, T.G.; Barry, S.; Yatham, L.N.; Mobayed, M.; Brown, I. A pilot study of a neuroendocrine test battery in posttraumatic stress disorder. Biol. Psychiatry 1990, 28, 665–672. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, D.; Wang, X. Increased serum cortisol and growth hormone levels in earthquake survivors with PTSD or subclinical PTSD. Psychoneuroendocrinology 2008, 33, 1155–1159. [Google Scholar] [CrossRef]
- Schweitzer, I.; Morris, P.; Hopwood, M.; Maguire, K.; Norman, T. Prolactin response to d-fenfluramine in combat-related post-traumatic stress disorder. Int. J. Neuropsychopharmacol 2004, 7, 291–298. [Google Scholar] [CrossRef]
- Vidović, A.; Gotovac, K.; Vilibić, M.; Sabioncello, A.; Jovanović, T.; Rabatić, S.; Folnegović-Šmalć, V.; Dekaris, D. Repeated assessments of endocrine- and immune-related changes in posttraumatic stress disorder. Neuroimmunomodulation 2011, 18, 199–211. [Google Scholar] [CrossRef]
- Grossman, R.; Yehuda, R.; Boisoneau, D.; Schmeidler, J.; Giller, E.L., Jr. Prolactin response to low-dose dexamethasone challenge in combat-exposed veterans with and without posttraumatic stress disorder and normal controls. Biol. Psychiatry 1996, 40, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- de Vries, G.J.; Mocking, R.; Assies, J.; Schene, A.; Olff, M. Plasma lipoproteins in posttraumatic stress disorder patients compared to healthy controls and their associations with the HPA- and HPT-axis. Psychoneuroendocrinology 2017, 86, 209–217. [Google Scholar] [CrossRef]
- Corcoran, C.; Walker, E.; Huot, R.; Mittal, V.; Tessner, K.; Kestler, L.; Malaspina, D. The stress cascade and schizophrenia: Etiology and onset. Schizophr. Bull. 2003, 29, 671–692. [Google Scholar] [CrossRef] [PubMed]
- Cherian, K.; Schatzberg, A.F.; Keller, J. HPA axis in psychotic major depression and schizophrenia spectrum disorders: Cortisol, clinical symptomatology, and cognition. Schizophr. Res. 2019, 213, 72–79. [Google Scholar] [CrossRef]
- Grace, A.A. Dysregulation of the dopaminę system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef]
- Elgellaie, A.; Larkin, T.; Kaelle, J.; Mills, J.; Thomas, S. Plasma prolactin is higher in major depressive disorder andes, and associated with anxiety, hostility, somatization, psychotic symptoms, and heart rate. Compr. Psychoneuroendocrinol. 2021, 6, 100049. [Google Scholar] [CrossRef] [PubMed]
- Faron-Górecka, A.; Kuśmider, M.; Szafran-Pilch, K.; Kolasa, M.; Żurawek, D.; Gruca, P.; Papp, M.; Solich, J.; Pabian, P.; Dziedzicka-Wasylewska, M. Basal prolactin levels in rat plasma correlates with response to antidepressant treatment in animal model of depression. Neurosci. Lett. 2017, 647, 147–152. [Google Scholar] [CrossRef]
- Malone, K.M.; Thase, M.E.; Mieczkowski, T.; Myers, J.E.; Stull, S.D.; Cooper, T.B.; Mann, J.J. Fenfluramine challenge test as a predictor of outcome in major depression. Psychopharmacol. Bull. 1993, 29, 155–161. [Google Scholar] [PubMed]
- Porter, R.J.; Gallagher, P.; Watson, S.; Smith, M.S.; Young, A.H. Elevated prolactin responses to L-tryptophan infusion in medication-free depressed patients. Psychopharmacology 2003, 169, 77–83. [Google Scholar] [CrossRef]
- Coker, F.; Taylor, D. Antidepressant-induced hyperprolactinaemia: Incidence, mechanisms and management. CNS Drugs 2010, 24, 563–574. [Google Scholar] [CrossRef]
- Molitch, M.E. Drugs and prolactin. Pituitary 2008, 11, 209–218. [Google Scholar] [CrossRef]
- Reeves, K.W.; Okereke, O.I.; Qian, J.; Tworoger, S.S.; Rice, M.S.; Hankinson, S.E. Antidepressant use and circulating prolactin levels. Cancer Causes Control. 2016, 27, 853–861. [Google Scholar] [CrossRef]
- Nordin, C.; Siwers, B.; Bertilsson, L. Bromocriptine treatment of depressive disorders. Clinical and biochemical effects. Acta Psyciatr. Scand 1981, 64, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Raap, D.K.; Garcia, F.; Serres, F.; Ma, Q.; Battaglia, G.; Van de Kar, L.D. Long-term fluoxetine produces behavioral anxiolytic effects without inhibiting neuroendocrine responses to conditioned stress in rats. Brain Res. 2000, 855, 58–66. [Google Scholar] [CrossRef]
- Tebeka, S.; Strat, Y.L.; Dubertret, C. Developmental trajectories of pregnant and postpartum depression in an epidemiologic survey. J. Affect. Disord. 2016, 203, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.C.; Greenwood, R.J.; Woolridge, M.W. Hormonal predictors of postnatal depression at 6 months in breastfeeding women. J. Reprod. Infant Psychol. 2003, 21, 61–68. [Google Scholar] [CrossRef]
- Cheng, B.; Hu, X.; Roberts, N.; Zhao, Y.; Xu, X.; Zhou, Y.; Tan, X.; Chen, S.; Meng, Y.; Wang, S.; et al. Prolactin mediates the relationship between regional gray matter volume and postpartum depression symptoms. J Affect. Disord. 2022, 301, 253–259. [Google Scholar] [CrossRef]
- Dunn, R.T.; Kimbrell, T.A.; Ketter, T.A.; Frye, M.A.; Willis, M.W.; Luckenbaugh, D.A.; Post, R.M. Principal components of the Beck Depression Inventory and regional cerebral metabolism in unipolar and bipolar depression. Biol. Psychiatry 2002, 51, 387–399. [Google Scholar] [CrossRef]
- Wonch, K.E.; de Medeiros, C.B.; Barrett, J.A.; Dudin, A.; Cunningham, W.A.; Hall, G.B.; Steiner, M.; Fleming, A.S. Postpartum depression and brain response to infants: Differential amygdala response and connectivity. Soc. Neurosci. 2016, 11, 600–617. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rizo, C.; Fernandez-Egea, E.; Oliveira, C.; Justicia, A.; Parellada, E.; Bernardo, M.; Kirkpatrick, B. Prolactin concentrations in newly diagnosed, antipsychotic-naïve patients with nonaffective psychosis. Schizophr. Res. 2012, 134, 16–19. [Google Scholar] [CrossRef]
- Riecher-Rössler, A.; Rybakowski, J.K.; Pflueger, M.O.; Beyrau, R.; Kahn, R.S.; Malik, P.; Fleischhacker, W.W.; EUFEST Study Group. Hyperprolactinemia in antipsychotic-naive patients with first-episode psychosis. Psychol. Med. 2013, 43, 2571–2582. [Google Scholar] [CrossRef]
- Petrikis, P.; Tigas, S.; Tzallas, A.T.; Archimandriti, D.T.; Skapinakis, P.; Mavreas, V. Prolactin levels in drug-naïve patients with schizophrenia and other psychotic disorders. Int. J. Psychiatry Clin. Pract. 2016, 20, 165–169. [Google Scholar] [CrossRef]
- Delgado, M.; Tordesillas-Gutierrez, D.; Ayesa-Arriola, R.; Canal, M.; de la Foz, V.O.; Labad, J.; Crespo-Facorro, B. Plasma prolactin levels are associated with the severity of illness in drug-naive first-episode psychosis female patients. Arch. Womens Ment. Health 2019, 22, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Vuk Pisk, S.; Matić, K.; Gereš, N.; Ivezić, E.; Ruljančić, N.; Filipčić, I. Hyperprolactinemia—Side effect or part of the illness. Psychiatr. Danub. 2019, 31 (Suppl. 2), 148–152. [Google Scholar] [PubMed]
- Studerus, E.; Ittig, S.; Beck, K.; Del Cacho, N.; Vila-Badia, R.; Butjosa, A.; Usall, J.; Riecher-Rössler, A. Relation between self-perceived stress, psychopathological symptoms and the stress hormone prolactin in emerging psychosis. J. Psychiatr. Res. 2021, 136, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Kapur, S. The dopamine hypothesis of schizophrenia: Version III—The final common pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef]
- Riecher-Rössler, A. Oestrogens, prolactin, hypothalamic-pituitary-gonadal axis, and schizophrenic psychoses. Lancet Psychiatry 2017, 4, 63–72. [Google Scholar] [CrossRef]
- Labad, J. The role of cortisol and prolactin in the pathogenesis and clinical expression of psychotic disorders. Psychoneuroendocrinology 2019, 102, 24–36. [Google Scholar] [CrossRef]
- Pompili, M.; Gibiino, S.; Innamorati, M.; Serafini, G.; Del Casale, A.; De Risio, L.; Palermo, M.; Montebovi, F.; Campi, S.; De Luca, V.; et al. Prolactin and thyroid hormone levels are associated with suicide attempts in psychiatric patients. Psychiatry Res. 2012, 200, 389–394. [Google Scholar] [CrossRef]
- Rajkumar, R.P. Prolactin and psychopathology in schizophrenia: A literature review and reappraisal. Schizophr. Res. Treat. 2014, 2014, 175360. [Google Scholar] [CrossRef]
- Huhn, M.; Nikolakopoulou, A.; Schneider-Thoma, J.; Krause, M.; Samara, M.; Peter, N.; Arndt, T.; Bäckers, L.; Rothe, P.; Cipriani, A.; et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: A systematic review and network meta-analysis. Lancet 2019, 394, 939–951. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, C.; Siafis, S.; Zhuo, K.; Zhu, D.; Wu, H.; Liu, D.; Jiang, K.; Wang, J.; Leucht, S.; et al. Prolactin levels influenced by antipsychotic drugs in schizophrenia: A systematic review and network meta-analysis. Schizophr. Res. 2021, 237, 20–25. [Google Scholar] [CrossRef]
- Miura, I.; Zhang, J.P.; Hagi, K.; Lencz, T.; Kane, J.M.; Yabe, H.; Malhotra, A.K.; Correll, C.U. Variants in the DRD2 locus and antipsychotic-related prolactin levels: A meta-analysis. Psychoneuroendocrinology 2016, 72, 1–10. [Google Scholar] [CrossRef]
- Cao, Y.L.; Zhu, L.; Zhang, H.; Meng, J.H.; Wu, H.J.; Wang, X.; Wu, J.H.; Zou, J.L.; Fang, M.S.; An, J.; et al. Total Barley Maiya Alkaloids Prevent Increased Prolactin Levels Caused by Antipsychotic Drugs and Reduce Dopamine Receptor D2 via Epigenetic Mechanisms. Front. Pharmacol. 2022, 13, 888522. [Google Scholar] [CrossRef]
- Torner, L. Actions of Prolactin in the Brain: From Physiological Adaptations to Stress and Neurogenesis to Psychopathology. Front. Endocrinol. 2016, 7, 25. [Google Scholar] [CrossRef]
- Martínez-Alarcón, O.; García-López, G.; Guerra-Mora, J.R.; Molina-Hernández, A.; Diaz-Martínez, N.E.; Portillo, W.; Díaz, N.F. Prolactin from Pluripotency to Central Nervous System Development. Neuroendocrinology 2022, 112, 201–214. [Google Scholar] [CrossRef]
- Larsen, C.M.; Grattan, D.R. Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology 2010, 151, 3805–3814. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.M.; Voogt, J.L. Comparison of plasma corticosterone and prolactin levels in cycling and lactating rats. Neuroendocrinology 1973, 13, 173–181. [Google Scholar] [CrossRef]
- Bonnin, F. Cortisol levels in saliva and mood changes in early puerperium. J. Affect. Disord. 1992, 26, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Leuner, B.; Mirescu, C.; Noiman, L.; Gould, E. Maternal experience inhibits the production of immature neurons in the hippocampus during the postpartum period through elevations in adrenal steroids. Hippocampus 2007, 17, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.L.; Vukovic, J.; Koudijs, M.M.; Blackmore, D.G.; Mackay, E.W.; Sykes, A.M.; Overall, R.W.; Hamlin, A.S.; Bartlett, P.F. Prolactin stimulates precursor cells in the adult mouse hippocampus. PLoS ONE 2012, 7, e44371. [Google Scholar] [CrossRef] [PubMed]
- Torner, L.; Karg, S.; Blume, A.; Kandasamy, M.; Kuhn, H.G.; Winkler, J.; Aigner, L.; Neumann, I.D. Prolactin prevents chronic stress-induced decrease of adult hippocampal neurogenesis and promotes neuronal fate. J. Neurosci. 2009, 29, 1826–1833. [Google Scholar] [CrossRef]
- Lajud, N.; Gonzalez-Zapien, R.; Roque, A.; Tinajero, E.; Valdez, J.J.; Clapp, C.; Torner, L. Prolactin administration during early postnatal life decreases hippocampal and olfactory bulb neurogenesis and results in depressive-like behavior in adulthood. Horm. Behav. 2013, 64, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, N.; Higashi, T.; Furuse, M. Localization of angulin-1/LSR and tricellulin at tricellular contacts of brain and retinal endothelial cells in vivo. Cell Struct. Funct. 2014, 39, 1–8. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017, 20, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Hanley, N.; Campbell, M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl. Psychiatry 2020, 10, 373. [Google Scholar] [CrossRef]
- Greene, C.; Kealy, J.; Humphries, M.M.; Gong, Y.; Hou, J.; Hudson, N.; Cassidy, L.M.; Martiniano, R.; Shashi, V.; Hooper, S.R.; et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol. Psychiatry 2017, 23, 2156–2166. [Google Scholar] [CrossRef]
- Brand, B.A.; de Boer, J.N.; Sommer, I.E.C. Estrogens in schizophrenia: Progress, current challenges and opportunities. Curr. Opin. Psychiatry 2021, 34, 228–237. [Google Scholar] [CrossRef]
- Rosas-Hernandez, H.; Ramirez, M.; Ramirez-Lee, M.A.; Ali, S.F.; Gonzalez, C. Inhibition of prolactin with bromocriptine for 28 days increases blood-brain barrier permeability in the rat. Neuroscience 2015, 301, 61–70. [Google Scholar] [CrossRef]
- O’Brien, F.E.; O’Connor, R.M.; Clarke, G.; Dinan, T.G.; Griffin, B.T.; Cryan, J.F. P-glycoprotein inhibition increases the brain distribution and antidepressant-like activity of escitalopram in rodents. Neuropsychopharmacology 2013, 38, 2209–2219. [Google Scholar] [CrossRef]
- Redzic, Z.B.; Preston, J.E.; Duncan, J.A.; Chodobski, A.; Szmydynger-Chodobska, J. The choroid plexus-cerebrospinal fluid system: From development to aging. Curr. Top. Dev. Biol. 2005, 71, 1–52. [Google Scholar] [CrossRef]
- Redman, B.; Kitchen, C.; Johnson, K.W.; Bezwada, P.; Kelly, D.L. Levels of prolactin and testosterone and associated sexual dysfunction and breast abnormalities in men with schizophrenia treated with antipsychotic medications. J. Psychiatr. Res. 2021, 143, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, M.; Cugati, G.; Singh, A.K. Hyperprolactinemia: An often missed cause of male infertility. J. Hum. Reprod. Sci. 2011, 4, 102–103. [Google Scholar] [CrossRef]
- Patel, S.S.; Bamigboye, V. Hyperprolactinaemia. J. Obstet. Gynaecol. 2007, 27, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Kokay, I.C.; Petersen, S.L.; Grattan, D.R. Identification of prolactin-sensitive GABA and kisspeptin neurons in regions of the rat hypothalamus involved in the control of fertility. Endocrinology 2011, 152, 526–535. [Google Scholar] [CrossRef]
- Kaiser, U.B. Hyperprolactinemia and infertility: New insights. J. Clin. Investig. 2012, 122, 3467–3468. [Google Scholar] [CrossRef] [PubMed]
- Sonigo, C.; Bouilly, J.; Carré, N.; Tolle, V.; Caraty, A.; Tello, J.; Simony-Conesa, F.J.; Millar, R.; Young, J.; Binart, N. Hyperprolactinemia-induced ovarian acyclicity is reversed by kisspeptin administration. J. Clin. Investig. 2012, 122, 3791–3795. [Google Scholar] [CrossRef]
- Liby, K.; Neltner, B.; Mohamet, L.; Menchen, L.; Ben-Jonathan, N. Prolactin overexpression by MDA-MB-435 human breast cancer cells accelerates tumor growth. Breast Cancer Res. Treat. 2003, 79, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.; Montone, K.T.; Powell, C.M.; Tomaszewski, J.E.; Clevenger, C.V. Expression of prolactin and its receptor in human breast carcinoma. Endocrinology 1997, 138, 5555–5560. [Google Scholar] [CrossRef] [PubMed]
- Clevenger, C.V. Role of prolactin/prolactin receptor signaling in human breast cancer. Breast Dis. 2003, 18, 75–86. [Google Scholar] [CrossRef]
- Tworoger, S.S.; Eliassen, A.H.; Rosner, B.; Sluss, P.; Hankinson, S.E. Plasma prolactin concentrations and risk of postmenopausal breast cancer. Cancer Res. 2004, 64, 6814–6819. [Google Scholar] [CrossRef]
- Taipale, H.; Solmi, M.; Lähteenvuo, M.; Tanskanen, A.; Correll, C.U.; Tiihonen, J. Antipsychotic use and risk of breast cancer in women with schizophrenia: A nationwide nested case-control study in Finland. Lancet Psychiatry 2021, 8, 883–891. [Google Scholar] [CrossRef]
- Nevalainen, M.T.; Valve, E.M.; Ingleton, P.M.; Nurmi, M.; Martikainen, P.M.; Harkonen, P.L. Prolactin and prolactin receptors are expressed and functioning in human prostate. J. Clin. Investig. 1997, 99, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Leav, I.; Merk, F.B.; Lee, K.F.; Loda, M.; Mandoki, M.; McNeal, J.E.; Ho, S.M. Prolactin receptor expression in the developing human prostate and in hyperplastic, dysplastic, and neoplastic lesions. Am. J. Pathol. 1999, 154, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Goffin, V.; Hoang, D.T.; Bogorad, R.L.; Nevalainen, M.T. Prolactin regulation of the prostate gland: A female player in a male game. Nat. Rev. Urol. 2011, 8, 597–607. [Google Scholar] [CrossRef]
- Molitch, M.E.; Drummond, J.; Korbonits, M. Prolactinoma Management. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2022. [Google Scholar]
- Stumpe, K.O.; Kolloch, R.; Higuchi, M.; Krück, F.; Vetter, H. Hyperprolactinaemia and antihypertensive effect of bromocriptine in essential hypertension. Identification of abnormal central dopamine control. Lancet 1977, 2, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Georgiopoulos, G.A.; Stamatelopoulos, K.S.; Lambrinoudaki, I.; Lykka, M.; Kyrkou, K.; Rizos, D.; Creatsa, M.; Christodoulakos, G.; Alevizaki, M.; Sfikakis, P.P.; et al. Prolactin and preclinical atherosclerosis in menopausal women with cardiovascular risk factors. Hypertension 2009, 54, 98–105. [Google Scholar] [CrossRef]
- Zhang, L.; Curhan, G.C.; Forman, J.P. Plasma prolactin level and risk of incident hypertension in postmenopausal women. J. Hypertens. 2010, 28, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Szkróbka, W.; Okopień, B. Different Effects of Atorvastatin on Cardiometabolic Risk Factors in Young Women With and Without Hyperprolactinemia. J. Clin. Pharmacol. 2019, 59, 83–89. [Google Scholar] [CrossRef]
- Shelly, S.; Boaz, M.; Orbach, H. Prolactin and autoimmunity. Autoimmun. Rev. 2012, 11, A465–A470. [Google Scholar] [CrossRef] [PubMed]
- Borba, V.V.; Zandman-Goddard, G.; Shoenfeld, Y. Prolactin and autoimmunity: The hormone as an inflammatory cytokine. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101324. [Google Scholar] [CrossRef]
- Jara, L.J.; Medina, G.; Saavedra, M.A.; Vera-Lastra, O.; Navarro, C. Prolactin and autoimmunity. Clin. Rev. Allergy Immunol. 2011, 40, 50–59. [Google Scholar] [CrossRef]
- Suarez, A.L.; López-Rincón, G.; Neri, P.A.; Estrada-Chávez, C. Prolactin in inflammatory response. Recent Adv. Prolact. Res. 2015, 2015, 243–264. [Google Scholar]
- Matera, L. Action of pituitary and lymphocyte prolactin. Neuroimmunomodulation 1997, 4, 171–180. [Google Scholar] [CrossRef]
- Matera, L.; Mori, M. Cooperation of pituitary hormone prolactin with interleukin-2 and interleukin-12 on production of interferon-gamma by natural killer and T cells. Ann. N. Y. Acad. Sci. 2000, 917, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Collazos, J.; Ibarra, S.; Martínez, E.; Mayo, J. Serum prolactin concentrations in patients infected with human immunodeficiency virus. HIV Clin. Trials 2002, 3, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Collazos, J.; Esteban, M. Has prolactin a role in the hypogonadal status of HIV-infected patients? J. Int. Assoc. Phys. AIDS Care 2009, 8, 43–46. [Google Scholar] [CrossRef]
- Montero, A.; Bottasso, O.A.; Luraghi, M.R.; Giovannoni, A.G.; Sen, L. Association between high serum prolactin levels and concomitant infections in HIV-infected patients. Hum. Immunol. 2001, 62, 191–196. [Google Scholar] [CrossRef]
- Graef, A.S.; Gonzalez, S.S.; Baca, V.R.; Ramirez, M.L.; Daza, L.B.; Blanco, F.F.; Ortiz, O.A.; Lavalle, C.M. High serum prolactin levels in asymptomatic HIV-infected patients and in patients with acquired immunodeficiency syndrome. Clin. Immunol. Immunopathol. 1994, 72, 390–393. [Google Scholar] [CrossRef]
- Hofny, E.R.; Ali, M.E.; Taha, E.A.; Nafeh, H.M.; Sayed, D.S.; Abdel-Azeem, H.G.; Abdou, E.F.; Kamal, G.M.; Mostafa, T. Semen and hormonal parameters in men with chronic hepatitis C infection. Fertil. Steril. 2011, 95, 2557–2559. [Google Scholar] [CrossRef] [PubMed]
- Kiciak, S.; Fota-Markowska, H.; Borowicz, I.; Modrzewska, R.; Przybyła, A. Prolactin concentration in the serum of male patients with chronic hepatitis C. Ann. Univ. Mariae Curie Sklodowska Med. 2002, 57, 210–216. [Google Scholar]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Butnariu, M.; Batiha, G.E. The crucial role of prolactin-lactogenic hormone in COVID-19. Mol. Cell Biochem. 2022, 477, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xie, W.; Li, D.; Shi, L.; Mao, Y.; Xiong, Y.; Zhang, Y.; Zhang, M. Effect of SARS-CoV-2 infection upon male gonadal function: A single center-based study. medRxiv 2020. [Google Scholar] [CrossRef]
| Gender | Symptoms |
|---|---|
| Women |
|
| Men |
|
| Symptoms occurring in both sexes | |
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faron-Górecka, A.; Latocha, K.; Pabian, P.; Kolasa, M.; Sobczyk-Krupiarz, I.; Dziedzicka-Wasylewska, M. The Involvement of Prolactin in Stress-Related Disorders. Int. J. Environ. Res. Public Health 2023, 20, 3257. https://doi.org/10.3390/ijerph20043257
Faron-Górecka A, Latocha K, Pabian P, Kolasa M, Sobczyk-Krupiarz I, Dziedzicka-Wasylewska M. The Involvement of Prolactin in Stress-Related Disorders. International Journal of Environmental Research and Public Health. 2023; 20(4):3257. https://doi.org/10.3390/ijerph20043257
Chicago/Turabian StyleFaron-Górecka, Agata, Katarzyna Latocha, Paulina Pabian, Magdalena Kolasa, Iwona Sobczyk-Krupiarz, and Marta Dziedzicka-Wasylewska. 2023. "The Involvement of Prolactin in Stress-Related Disorders" International Journal of Environmental Research and Public Health 20, no. 4: 3257. https://doi.org/10.3390/ijerph20043257
APA StyleFaron-Górecka, A., Latocha, K., Pabian, P., Kolasa, M., Sobczyk-Krupiarz, I., & Dziedzicka-Wasylewska, M. (2023). The Involvement of Prolactin in Stress-Related Disorders. International Journal of Environmental Research and Public Health, 20(4), 3257. https://doi.org/10.3390/ijerph20043257







