Effect of Non-Pharmacological Methods in the Reduction of Neonatal Pain: Systematic Review and Meta-Analysis
Abstract
1. Introduction
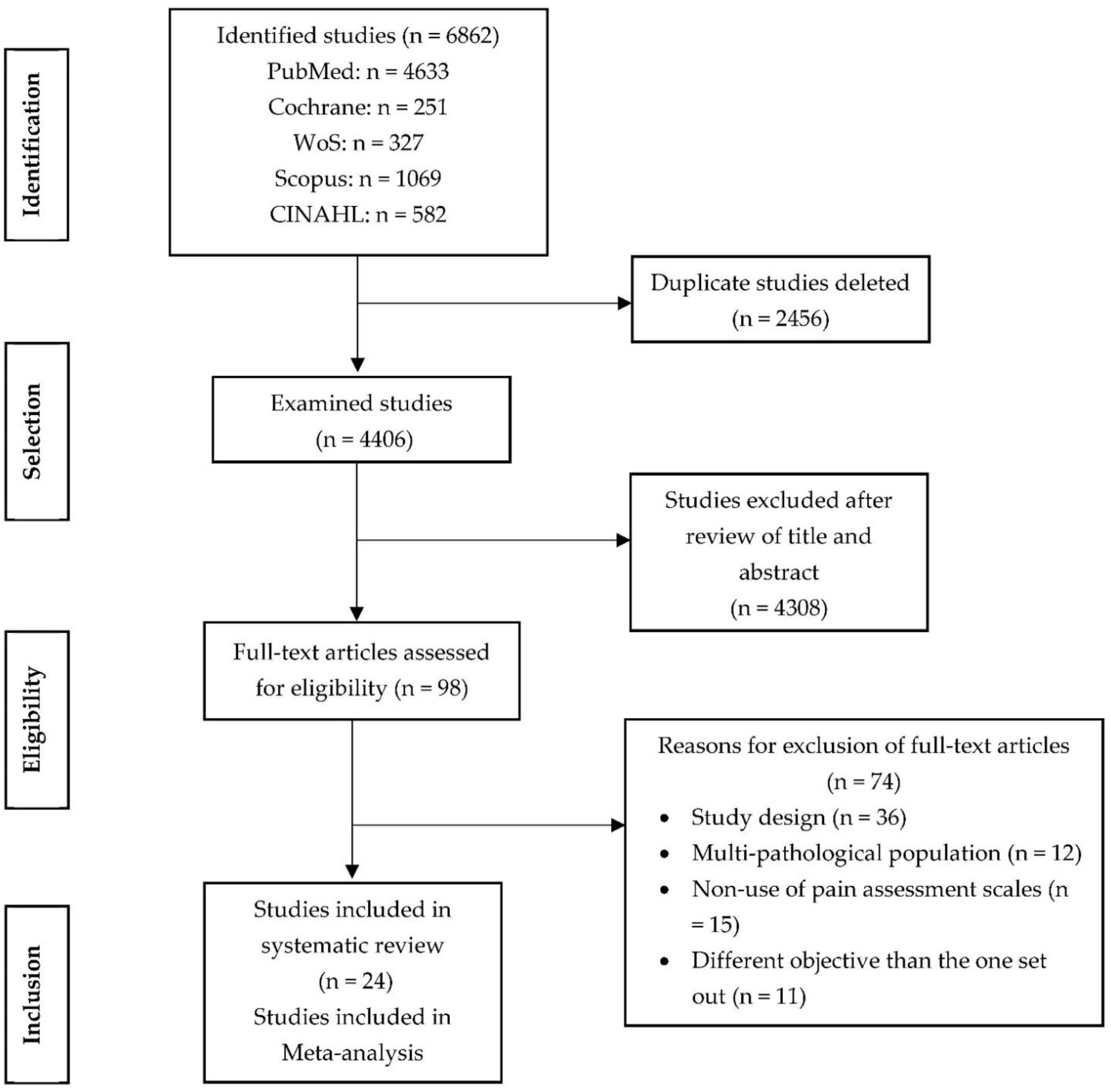
2. Materials and Methods
3. Results
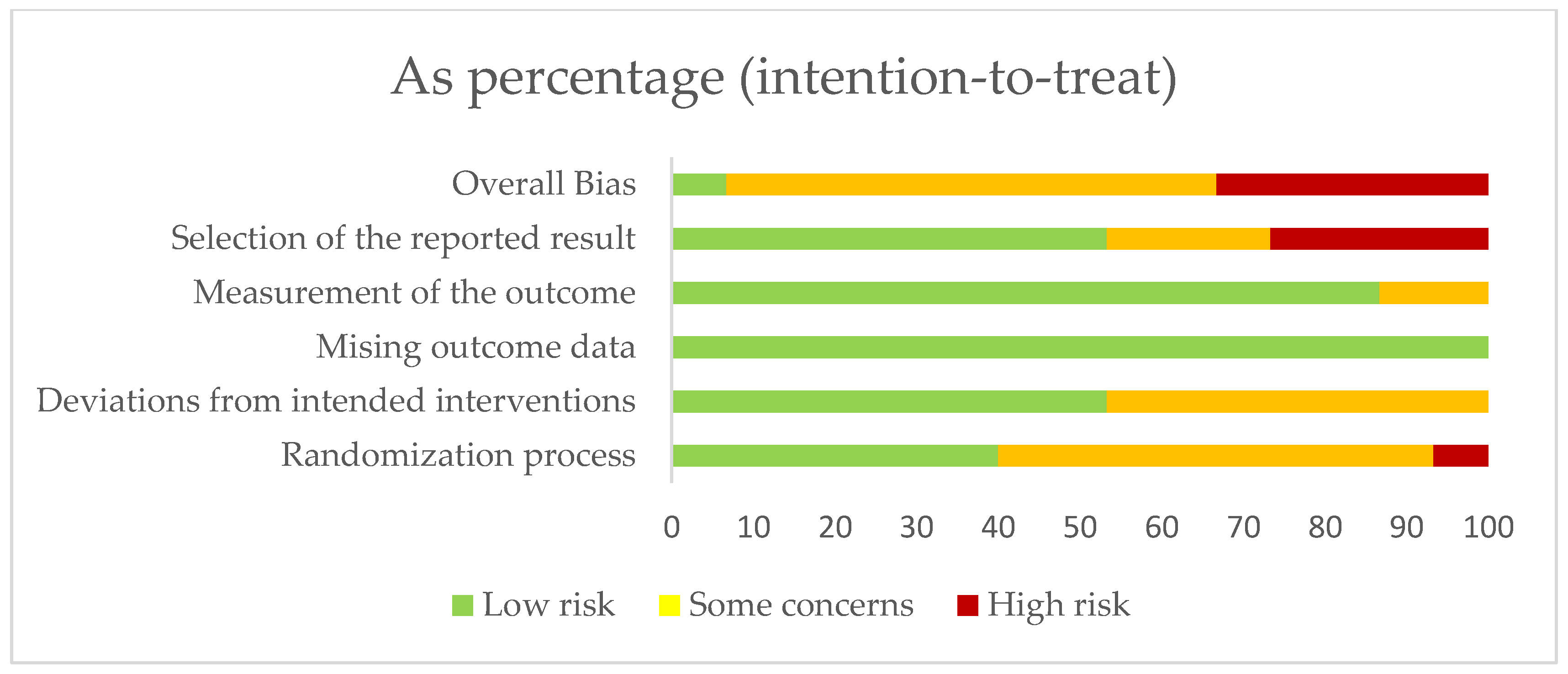
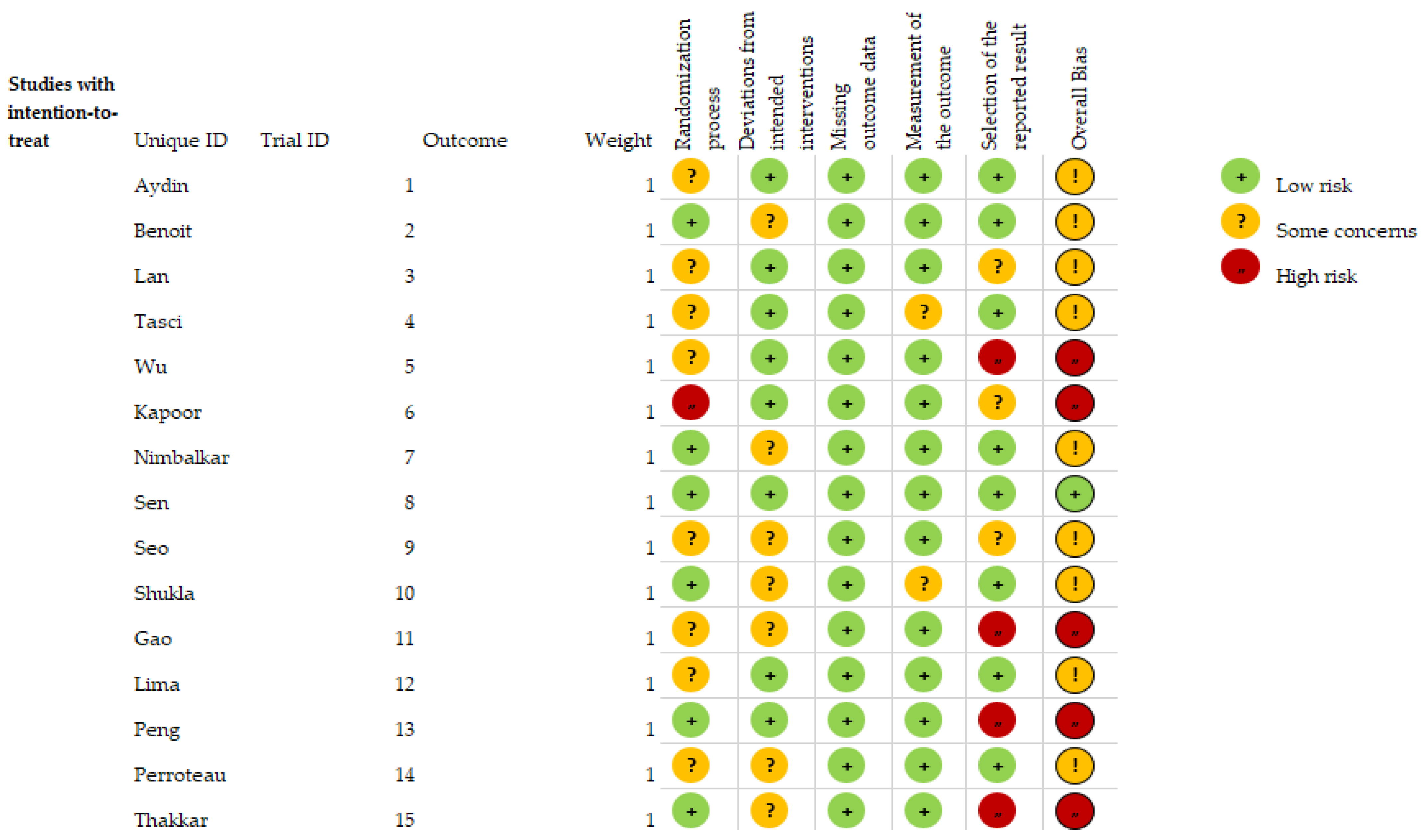
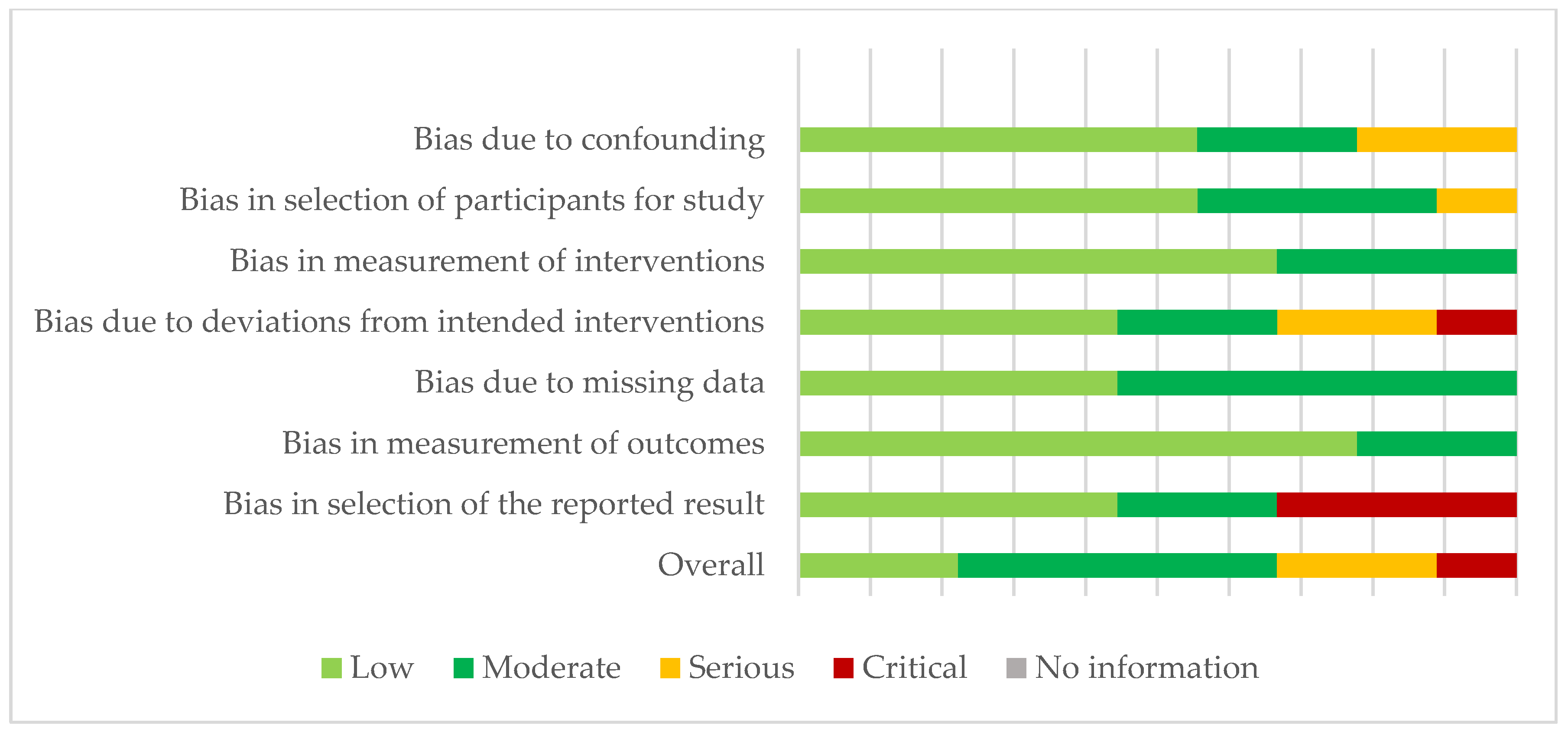
3.1. Risk of Bias in the Studies
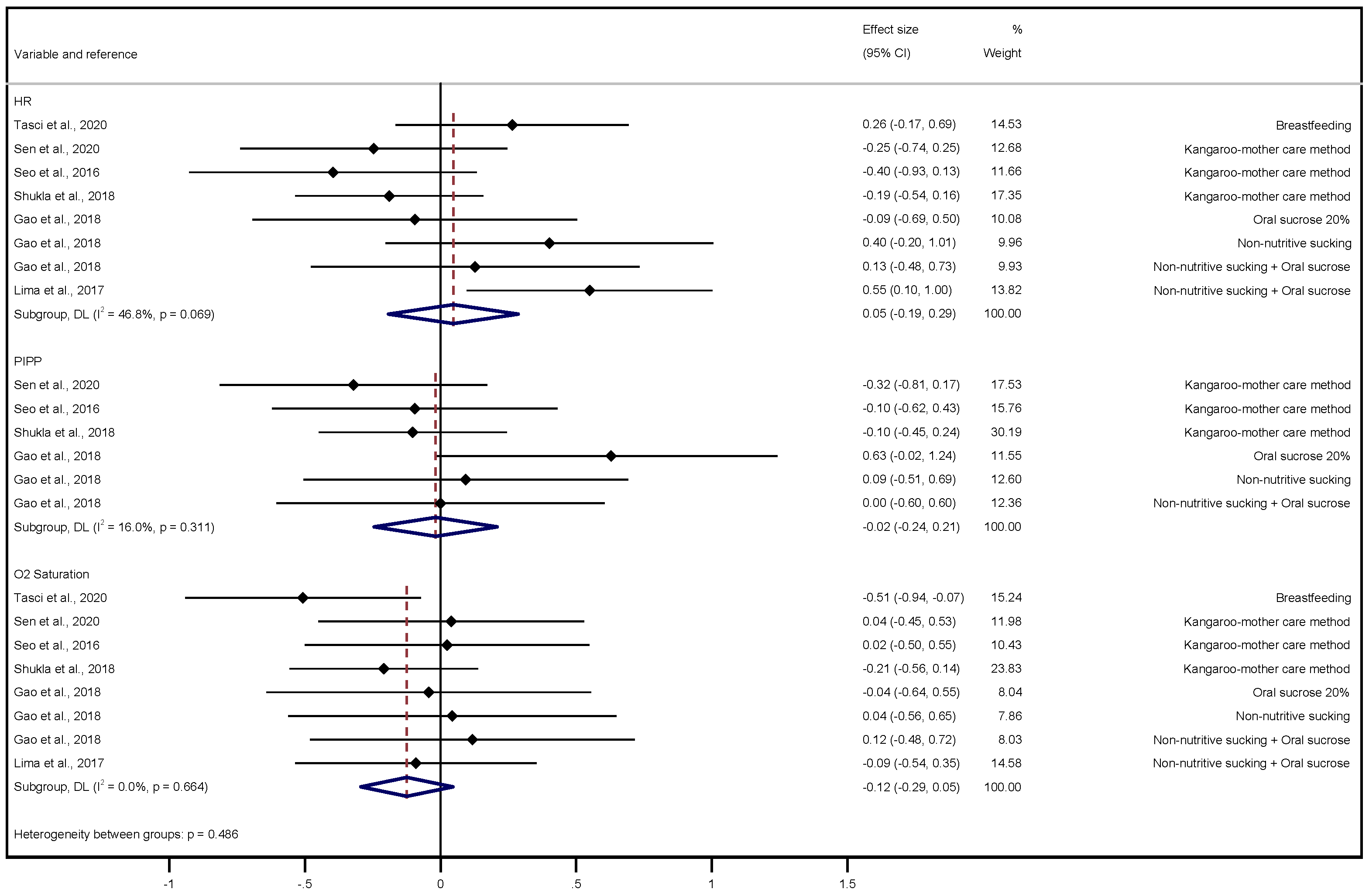
3.2. Meta-Analysis
3.3. Sensitivity Analysis
3.4. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obeidat, H.M.; Dwairej, D.A.; Aloweidi, A.S. Pain in preterm infants: Different perspectives. J. Perinat. Educ. 2021, 30, 185–195. [Google Scholar] [PubMed]
- Johnston, C. Neonatal pain: A journey spanning three decades. Paediatr. Neonatal Pain 2020, 2, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Brewer, C.L.; Baccei, M.L. The development of pain circuits and unique effects of neonatal injury. J. Neural Transm. 2020, 127, 467–479. [Google Scholar] [CrossRef]
- Perry, M.; Tan, Z.; Chen, J.; Weidig, T.; Xu, W.; Cong, X.S. Neonatal pain perceptions and current practice. Crit. Care Nurs. Clin. N. Am. 2018, 30, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Vinall, J.; Grunau, R.E. Impact of repeated procedural pain related stress in infants born very preterm. Pediatr. Res. 2014, 75, 584–587. [Google Scholar] [CrossRef]
- Fernández, M.G.E.; González-Pacheco, N.; Sánchez-Redondo, M.D.; Cernada, M.; Martín, A.; Pérez-Muñuzuri, A.; Boix, H.; Couce, M.L. Sedoanalgesia en las unidades neonatales. In Anales de Pediatría; Elsevier Doyma: Barcelona, Spain, 2020; Volume 95, pp. 126.e1–126.e11. [Google Scholar] [CrossRef]
- Vogel, J.P.; Chawanpaiboon, S.; Moller, A.-B.; Watananirun, K.; Bonet, M.; Lumbiganon, P. The global epidemiology of preterm birth. Best Pr. Res. Clin. Obstet. Gynaecol. 2018, 52, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Estadística. Nacimientos Por Tipo de Parto, Tiempo de Gestación y Grupo de Edad de la Madre; Instituto Nacional de Estadística: Madrid, Spain, 2021. [Google Scholar]
- Shiff, I.; Bucsea, O.; Pillai Riddell, R. Psychosocial and neurobiological vulnerabilities of the hospitalized preterm infant and relevant non-pharmacological pain mitigation strategies. Front. Pediatr. 2021, 9, 988. [Google Scholar] [CrossRef] [PubMed]
- Haraldsdottir, K.; Watson, A.M.; Goss, K.N.; Beshish, A.; Pegelow, D.F.; Palta, M.; Tetri, L.H.; Barton, G.P.; Brix, M.D.; Centanni, R.M.; et al. Impaired autonomic function in adolescents born preterm. Physiol. Rep. 2018, 6, e13620. [Google Scholar] [CrossRef]
- Collados-Gómez, L.; Camacho-Vicente, V.; Gónzalez-Villalba, M.; Sanz-Prades, G.; Bellón-Vaquerizo, B. Neonatal nurses’ perceptions of pain management. Enferm. Intensiv. 2018, 29, 41–47. [Google Scholar] [CrossRef] [PubMed]
- McPherson, C.; Miller, S.P.; El-Dib, M.; Massaro, A.N.; Inder, T.E. The influence of pain, agitation, and their management on the immature brain. Pediatr. Res. 2020, 88, 168–175. [Google Scholar] [CrossRef]
- Harrillo-Acevedo, F.D.; Rico-Becerra, J.I.; López-Martínez, A. The philosophy of the Developmental centered care of the premature infant (NIDCAP): A literature review. Enferm. Glob. 2017, 16, 577–589. [Google Scholar]
- Sheldon, R.E. Developmental care for preemies and their families: One Neonatologist’s journey toward NIDCAP practice. Neo Rev. 2017, 18, 568–575. [Google Scholar] [CrossRef]
- Ohlsson, A.; Jacbos, S.E. NIDCAP: A systematic review and meta-analyses of randomized controlled trials. Pediatrics 2013, 131, 881–893. [Google Scholar] [CrossRef]
- Mangat, A.K.; Oei, J.L.; Chen, K.; Quah-Smith, I.; Schmözler, G.M. A review of non-pharmacological treatments for pain management in newborn infants. Children 2018, 5, 130. [Google Scholar] [CrossRef] [PubMed]
- AAP; Committee on Fetus and Newborn; Section on Anesthesiology and Pain Medicine. Prevention and management of procedural pain in the neonate: An update. Pediatrics 2016, 137, 1–13. [Google Scholar]
- Aguilar-Cordero, M.J.; Baena-García, L.; Sánchez-López, A.M.; Mur-Villar, N.; Fernández-Castillo, R.; García, I. Non pharmacological methods to reduce pain in newborns: A systematic review. Nutr. Hosp. 2015, 32, 2496–2507. [Google Scholar]
- Olsson, E.; Ahl, H.; Bengtsson, K.; Vejayaram, D.N.; Norman, E.; Bruschettini, M.; Eriksson, M. The use and reporting of neonatal pain scales: A systematic review of randomized trials. Pain 2020, 162, 353–360. [Google Scholar] [CrossRef]
- Gibbins, S.; Stevens, B.J.; Yamada, J.; Dionne, K.; Campbell-Yeo, M.; Lee, G.; Caddell, K.; Johnston, C.; Taddio, A. Validation of the Premature Infant Pain Profile-Revised (PIPP-R). Early Hum. Dev. 2014, 90, 189–193. [Google Scholar] [CrossRef]
- Sarkaria, E.; Gruszfeld, D. Assessing neonatal pain with NIPS and COMFORT-B: Evaluation of NICU’s staff competences. Pain Res. Manag. 2022, 16, 8545372. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffman, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomized trials. BMJ 2019, 366, 48–98. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016, 355, 19–49. [Google Scholar] [CrossRef]
- Lachenbruch, P.A.; Cohen, J. Statistical power analysis for the behavioural sciences (2nd ed.). J. Am. Stat. Assoc. 1989, 84, 1096. [Google Scholar] [CrossRef]
- Sterne, J.A.; Egger, M.; Smith, G.D. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001, 323, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Avcin, E.; Kucukoglu, S. The effect of breastfeeding, kangaroo care, and facilitated tucking positioning in reducing the pain during heel stick in neonates. J. Pediatr. Nurs. 2021, 61, 410–416. [Google Scholar] [CrossRef]
- Aydin, D.; İnal, S. Effects of breastfeeding and heel warming on pain levels during heel stick in neonates. Int. J. Nurs. Pract. 2019, 25, 127–134. [Google Scholar] [CrossRef]
- Bembich, S.; Cont, G.; Causin, E.; Paviotti, G.; Marzari, P.; Demarini, S. Infant analgesia with a combination of breast milk, glucose, or maternal holding. Pediatrics 2018, 142, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Benoit, B.; Newman, A.; Martin-Misener, R.; Latimer, M.; Campbell-Yeo, M. The influence of breastfeeding on cortical and bio-behavioural indicators of procedural pain in newborns: Findings of a randomized controlled trial. Early Hum. Dev. 2021, 154, 105–308. [Google Scholar] [CrossRef]
- Lan, H.Y.; Yang, L.; Lin, C.H.; Hsieh, K.H.; Chang, Y.C.; Yin, T. Breastmilk as a multisensory intervention for relieving pain during newborn screening procedures: A randomized control trial. Int. J. Environ. Res. Public Health 2021, 18, 123–130. [Google Scholar] [CrossRef]
- Tasci, B.; Kuzlu Ayyildiz, T. The calming effect of maternal breast milk odor on term infant: A randomized controlled trial. Breastfeed Med. 2020, 15, 724–730. [Google Scholar] [CrossRef]
- Wu, H.P.; Yin, T.; Hsieh, K.H.; Lan, H.Y.; Feng, R.C.; Chang, Y.C.; Liaw, J.J. Integration of different sensory interventions from mother’s breast milk for preterm infant pain during peripheral venipuncture procedures: A prospective randomized controlled trial. J. Nurs. Scholarsh 2020, 52, 75–84. [Google Scholar] [CrossRef]
- Cong, X.; Ludington-Hoe, S.M.; Walsh, S. Randomized crossover trial of kangaroo care to reduce biobehavioral pain responses in preterm infants: A pilot study. Biol. Res. Nurs. 2011, 13, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Khan, M.A.; Beohar, V. Pain relief in late preterm neonates: A comparative study of kangaroo mother care, oral dextrose 50%, and supine nesting position. Int. J. Appl. Basic Med. Res. 2021, 11, 188–191. [Google Scholar] [CrossRef]
- Murmu, J.; Venkatnarayan, K.; Thapar, R.K.; Shaw, S.C.; Dalal, S.S. When alternative female kangaroo care is provided by other immediate postpartum mothers, it reduces postprocedural pain in preterm babies more than swaddling. Acta Paediatr. 2017, 106, 411–415. [Google Scholar] [CrossRef]
- Nimbalkar, S.M.; Chaudhary, N.S.; Gadhavi, K.V.; Phatak, A. Kangaroo mother care in reducing pain in preterm neonates on heel prick. Indian J. Pediatr. 2013, 80, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Nimbalkar, S.; Shukla, V.V.; Chauhan, V.; Phatak, A.G.; Patel, D.; Chapla, A.; Nimbalkar, A. Blinded randomized crossover trial: Skin-to-skin care vs. sucrose for preterm neonatal pain. J. Perinatol. 2020, 40, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Sen, E.; Manav, G. Effect of Kangaroo Care and Oral Sucrose on Pain in Premature Infants: A Randomized Controlled Trial. Pain Manag. Nurs. 2020, 21, 556–564. [Google Scholar] [CrossRef]
- Seo, Y.S.; Lee, J.; Ahn, H.Y. Effects of Kangaroo Care on Neonatal Pain in South Korea. J. Trop. Pediatr. 2016, 62, 246–249. [Google Scholar] [CrossRef]
- Shukla, V.; Chapla, A.; Uperiya, J.; Nimbalkar, A.; Phatak, A.; Nimbalkar, S. Sucrose vs. skin to skin care for preterm neonatal pain control: A randomized control trial. J. Perinatol. 2018, 38, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.V.; Chaudhari, A.J.; Nimbalkar, S.M.; Phatak, A.G.; Patel, D.V.; Nimbalkar, A.S. Skin-to-skin care by mother vs. father for preterm neonatal pain: A randomized control trial. Int. J. Pediatr. 2021, 88, 68–87. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, M.; Gao, H.; Xu, G.; Li, F.; Zhou, J.; Zou, Y.; Jiang, H. Effect of non-nutritive sucking and sucrose alone and in combination for repeated procedural pain in preterm infants: A randomized controlled trial. Int. J. Nurs. Stud. 2018, 83, 25–33. [Google Scholar] [CrossRef]
- Kumari, S.; Datta, V.; Rehan, H. Comparison of the efficacy of oral 25% glucose with oral 24% sucrose for pain relief during heel lance in preterm neonates: A double blind randomized controlled trial. J. Trop. Pediatr. 2017, 63, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.G.; Santos, V.S.; Nunes, M.S.; Barreto, J.A.; Ribeiro, C.J.; Carvalho, J.; Ribeiro, M.C.O. Glucose solution is more effective in relieving pain in neonates than non-nutritive sucking: A randomized clinical trial. Eur. J. Pain 2017, 21, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-F.; Yin, T.; Yang, L.; Wang, C.; Chang, Y.-C.; Jeng, M.-J.; Liaw, J.-J. Non-nutritive sucking, oral breast milk, and facilitated tucking relieve preterm infant pain during heel-stick procedures: A prospective, randomized controlled trial. Int. J. Nurs. Stud. 2018, 77, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Perroteau, A.; Nanquette, M.-C.; Rousseau, A.; Renolleau, S.; Bérard, L.; Mitanchez, D.; Leblanc, J. Efficacy of facilitated tucking combined with non-nutritive sucking on very preterm infants’ pain during the heel-stick procedure: A randomized controlled trial. Int. J. Nurs. Stud. 2018, 86, 29–35. [Google Scholar] [CrossRef]
- Silveira, A.; Christoffel, M.M.; Velarde, L.G.C.; Rodrigues, E.D.C.; Magesti, B.N.; Souza, R.O. Effect of glucose and non-nutritive sucking on puncture pain in premature infants: A crossover clinical trial. Rev. Esc. Enferm. USP 2021, 55, 32–37. [Google Scholar]
- Stevens, B.; Yamada, J.; Campbell-Yeo, M.; Gibbins, S.; Harrison, D.; Dionne, K.; Taddio, A.; McNair, C.; Willan, A.; Ballantyne, M.; et al. The minimally effective dose of sucrose for procedural pain relief in neonates: A randomized controlled trial. BMC Pediatr. 2018, 18, 85–93. [Google Scholar] [CrossRef]
- Thakkar, P.; Arora, K.; Goyal, K.; Das, R.R.; Javadekar, B.; Aiyer, S.; Panigrahi, S. To evaluate and compare the efficacy of combined sucrose and non-nutritive sucking for analgesia in newborns undergoing minor painful procedure: A randomized controlled trial. J. Perinatol. 2015, 36, 67–70. [Google Scholar] [CrossRef]
- Harrison, D.; Reszel, J.; Bueno, M.; Sampson, M.; Shah, V.S.; Taddio, A.; Larocque, C.; Turner, L. Breastfeeding for procedural pain in infants beyond the neonatal period. Cochrane Database Syst. Rev. 2016, 10, 112–148. [Google Scholar]
- Johnston, C.; Campbell-Yeo, M.; Fernandes, A.; Inglis, D.; Streiner, D.; Zee, R. Skin-to-skin care for procedural pain in neonates. Cochrane Database Syst. Rev. 2017, 2, 1–83. [Google Scholar] [CrossRef]
- Boundy, E.O.; Dastjerdi, R.; Spiegelman, D.; Fawzi, W.W.; Missmer, S.A.; Lieberman, E.; Kajeepeta, S.; Wall, S.; Chan, G.J. Kangaroo Mother Care and Neonatal Outcomes: A Meta-analysis. Pediatrics 2016, 137, e20152238. [Google Scholar] [CrossRef]
- Olsson, E.; Ahlsén, G.; Eriksson, M. Skin-to-skin contact reduces near-infrared spectroscopy pain responses in premature infants during blood sampling. Acta Paediatr. 2016, 105, 376–380. [Google Scholar] [CrossRef]
- Gao, H.; Gao, H.; Xu, G.; Li, M.; Du, S.; Li, F.; Zhang, H.; Wang, D. Efficacy and safety of repeated oral sucrose for repeated procedural pain in neonates: A systematic review. Int. J. Nurs. Stud. 2016, 62, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.J.D.; Medeiros, K.S.; Sarmento, A.C.A.; Oliveira, F.J.D.; Costa, A.P.F.; Souza, N.L.; Gonçalves, A.K.; Silva, M.D.L.C. Use of glucose for pain management in premature neonates: A systematic review and meta-analysis protocol. BMJ Open 2021, 11, e052901. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; Yamada, J.; Ohlsson, A.; Haliburton, S.; Shorkey, A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst. Rev. 2016, 7, 1–256. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, X.; Zhou, X.; Wu, T.; Sun, J. Efficacy and safety of combined nonpharmacological interventions for repeated procedural pain in preterm neonates: A systematic review of randomized controlled trials. Int. J. Nurs. Stud. 2019, 102, 103471. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, L.A.; Murphy, N.; Karp, K.; Polomano, R.C. A systematic review of behavioural and environmental interventions for procedural pain management in preterm infants. J. Pediatr. Nurs. 2019, 44, 22–30. [Google Scholar] [CrossRef]
- Badiee, Z.; Asghari, M.; Mohammadizadeh, M. The calming effect of maternal breast milk odor on premature infants. Pediatr. Neonatol. 2013, 54, 322–325. [Google Scholar] [CrossRef]
- Gómez-Cantarino, S.; García-Valdivieso, I.; Moncunill-Martínez, E.; Yáñez-Araque, B.; Ugarte Gurrutxaga, M.I. Developing a family-centered care model in the neonatal intensive care unit (NICU): A new vision to manage healthcare. Int. J. Environ. Res. Public Health 2020, 17, 7197. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cantarino, S.; García-Valdivieso, I.; Dios-Aguado, M.; Yáñez-Araque, B.; Molina Gallego, B.; Moncunill-Martínez, E. Nursing perspective of the humanized care of the neonate and family: A systematic review. Children 2021, 8, 35. [Google Scholar] [CrossRef] [PubMed]
| Author and Year | Country | Sample Characteristics | Intervention Characteristics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (Girls) | Gestational Age (Weeks) | Birth Weight (kg) | Apgar Test 5′ | Pain Measurement | Intervention | Characteristics | Dose | Certainty of the Evidence GRADE | ||
| Avcin et al., 2021 [28] | Turkey | IG1: 35 (15) IG2: 35 (18) IG3: 35 (15) CG: 35 (18) | IG1: 38.48 IG2: 38.62 IG3: 38.42 CG: 38.28 | IG1: 3.184 IG2: 3.293 IG3: 3.261 CG: 3.361 | NA | NIPS scale IG1: 2.64/6.02/2.88 IG2: 2.85/6.20/3.03 IG3: 2.60/5.97/2.40 CG: 2.66/6.31/3.45 | IG1: BF IG2: KMC IG3: Contention CG: Routine care | BF 5 min before, KMC 15 min before, lateral decubitus restraint 1 min before, during and after. NIPS scale 1 min before, during and after heel prick. | BF on demand | High |
| Aydin et al., 2019 [29] | Turkey | IG1: 50 (25) IG2: 50 (25) CG: 50 (25) | IG1: 39.14 IG2: 39.36 CG: 38.98 | IG1: 3.344 IG2: 3.394 CG: 3.241 | NA | NIPS scale IG1: 6.10 IG2: 4.44 CG: 6.42 | IG1: Heel warm-up IG2: BF CG: Routine care | 3–5 min before thermal bag water at 40° on the heel. 1 min before BF up to 2 min after. NIPS scale during heel prick. | BF on demand | Moderate |
| Bembich et al., 2018 [30] | Italy | IG1: 20 (11) IG2: 20 (12) IG2: 20 (11) IG4: 20 (8) | IG1: 39.6 IG2: 39.8 IG3: 39.8 IG4: 39.9 | IG1: 3.354 IG2: 3.155 IG3: 3.429 IG4: 3.298 | NA | NIPS scale IG1: 5.00 IG2: 5.50 IG3: 2.00 IG4: 2.50 | IG1: Oral glucose 20% IG2: Expressed breast milk IG3: KMC + oral glucose 20% IG4: BF | 2 min before glucose. Expressed breast milk 2 min before. KMC during. BF 2 min before and during the puncture. NIPS scale during heel prick. | 2 mL glucose 20% 2 mL BF | Moderate |
| Benoit et al., 2021 [31] | Canada | IG: 18 (6) CG: 19 (8) | IG: 39.3 CG: 39.5 | IG: 3.508 CG: 3.318 | IG: 9 CG: 9 | PIPP scale IG: 4.7/4.7/5.0/4.4 CG: 4.1/4.5/3.9/4.3 | IG: BF + KMC CG: Oral sucrose 24% + NNS | BF and sucrose 2 min before. PIPP scale at 30, 60, 90 and 120 s after heel prick. | BF on demand 0.24 mL sucrose 24% | Moderate |
| Lan et al., 2021 [32] | Taiwan | IG2: 40 (18) IG3: 40 (20) CG: 40 (23) | IG1: 39.07 IG2: 38.91 CG: 39.42 | IG1: 3.109 IG2: 3.070 CG: 3.121 | IG1: 8.98 IG2: 8.98 CG: 9 | NIPS scale IG1: 0.46 IG2: 0.42 CG: 0.61 | IG1: BF odour + Routine care IG2: Odour + BF taste + Routine care CG: Routine care | 3 min before, cotton wool impregnated with BF near the nose, removed 5 min later. 2 min before and during heel prick, BF by drip syringe. NIPS scale 5 min before, during and after. | 3 mL BF | Moderate |
| Tasci et al., 2020 [33] | Turkey | IG: 42 (ND) CG: 42 (ND) | IG: 38–42 CG: 38–42 | IG: 2.5–4 CG: 2.5–4 | IG: 8 CG: 8 | NIPS scale IG: 0.02/2.00/0.36 CG: 0.12/4.62/1.83 | IG: BF odour CG: Formula milk odour | Filter paper impregnated with milk near the nose 3 min before, removed 9 min after. NIPS scale before, during and 2 min after heel prick. | 2 mL BF 2 mL formula milk | Moderate |
| Wu et al., 2020 [34] | Taiwan | IG1: 34 (ND) IG2: 34 (ND) IG3: 36 (ND) CG: 36 (ND) | IG1: 32.25 IG2: 32.51 IG3: 32.33 CG: 32.64 | IG1: 1.774 IG2: 1.795IG3: 1.767 CG: 1.790 | IG1: 8.09 IG2: 7.91 IG3: 7.97 CG: 8.03 | PIPP scale IG1: 0/13.0/7.0 IG2: 0/13.5/7.5 IG3: 0/10.0/5.0 CG: 0/14.5/8.0 | IG1: BF odour or taste IG2: BF odour or taste + Heartbeat sound IG3: BF odour or taste + Heartbeat sound + NNS CG: Routine care | 3 min before cotton wool impregnated with BF near the nose, removed 10 min later. 2 min before breast milk. Reproduction of mother’s heartbeat of each newborn 3 min before, up to 10 min after. Oral–tactile stimulation. PIPP scale 5 min before, during and after the heel prick. | 2 mL BF | Low |
| Cong et al., 2011 [35] | USA | IG1: 18 (6) IG2: 10 (5) | IG1: 31.5 IG2: 32 | IG1: 1.779 IG2: 1.577 | NA | Escala PIPP IG1: 13.63/17.05 vs. 13.25/16.09 IG2: 8.60/10.60 vs. 9.75 14.33 | IG1: KMC 80 vs. Incubator IG2: KMC 30 vs. Incubator | KMC 60 min before and 20 min after vs. 60 min incubator rest. KMC 10 min before and 20 min after vs. 30 min incubator rest. PIPP scale at 30, 60 s after heel prick. | KMC 80 and 30 min | High |
| Kapoor et al., 2021 [36] | India | IG1: 45 (22) IG2: 54 (23) CG: 50 (22) | IG1: 36 IG2: 36 CG: 35 | NA | IG1: >5 IG2: >5 CG: >5 | Escala PIPP IG1: 8.42 IG2: 8.76 CG: 13.08 | IG1: KMC IG2: Oral sucrose 50% CG: Contention | KMC before and sucrose 2 min before. Supine restraint. PIPP scale 30 s after heel prick. | KMC 30 min 0.5 mL sucrose 50% | Low |
| Murmu et al., 2017 [37] | India | IG1: 17 (6) IG2: 17 (6) IG3: 17 (6) | IG1: 32 IG2: 32 IG3: 32 | IG1: 1.824 IG2: 1.824 IG3: 1.824 | IG1: 8 IG2: 8 IG3: 8 | Escala PIPP IG1: 10.59 IG2: 11.24 IG3: 12.96 | IG1: KMC + alternative Kangaroo + Contention IG2: Alternative Kangaroo + Contention + KMC IG3: Contention + KMC + alternative Kangaroo | KMC and alternative kangaroo before and during heel prick. Prone restraint. PIPP scale 30 s after heel prick. | KMC 30 min | Moderate |
| Nimbalkar et al., 2013 [38] | India | IG: 19 (ND) CG: 28 (ND) | IG: 34.02 CG: 34.02 | IG: 1.730 CG: 1.730 | NA | Escala PIPP IG: 5.38 CG: 10.23 | IG: KMC CG: Contention | KMC before, during and after. Prone restraint 15 min. PIPP scale 30 s after heel prick. | KMC 15 +15 min | Moderate |
| Nimbalkar et al., 2020 [39] | India | IG1: 50 (22) IG2: 50 (23) | IG1: 33.60 IG2: 33.60 | IG1: 1.604 IG2: 1.604 | NA | Escala PIPP IG1: 3.40/6.98/3.57 IG2: 2.75/6.84/3.11 | IG1: KMC + oral sucrose 24% IG2: Oral sucrose 24% + KMC | KMC before, during and after 1st heel prick, sucrose 2 min before 2nd heel prick, and vice versa. PIPP scale before, 1 min and 5 min after heel prick. | KMC 15 min 0.5–1 mL sucrose 24% | Moderate |
| Sen et al., 2020 [40] | Turkey | IG: 32 (18) CG: 32 (14) | IG: 34.38 CG: 34.95 | IG: 2.102 CG: 2.244 | NA | Escala PIPP IG: 4/7/3 CG: 5/7/4 | IG: KMC CG: Oral sucrose 24% | KMC before heel prick and sucrose 2 min before. PIPP scale before, during and 2 min after heel prick. | KMC 15 min 0.5 mL sucrose 24% | High |
| Seo et al., 2016 [41] | South Korea | IG: 26 (10) CG: 30 (17 | IG: 38.66 CG: 38.36 | IG: 3.203 CG: 3.043 | IG: 8.7 CG: 8.9 | Escala PIPP IG: 2.0/4.1/4.5/2.9 CG: 2.4/6.3/9.8/8.2 | IG: KMC CG: Routine care | KMC before, during and after. PIPP scale before, during, 1 min and 2 min after heel prick. | KMC 10 + 3 min | Moderate |
| Shukla et al., 2018 [42] | India | IG: 50 (18) CG: 50 (30) | IG: 31.68 CG: 33.90 | IG: 1.460 CG: 1.780 | NA | Escala PIPP IG: 7.74 CG: 8.1 | IG: KMC CG: Oral sucrose 24% | KMC before, during and after. Sucrose 2 min before. PIPP scale 30 s after heel prick. | KMC 10 min 0.2 mL sucrose 24% | Moderate |
| Shukla et al., 2021 [43] | India | IG1: 32 (ND) IG2: 32 (ND) | IG1: 34.28 IG2: 34.28 | IG1: 1.665 IG2: 1.665 | NA | Escala PIPP IG1: 3.20/8.59/3.80 IG2: 3.02/8.27/3.94 | IG1: KMC + Father kangaroo IG2: Father kangaroo + MMC | KMC before and during 1st heel prick, parent-kangaroo before and during 2nd heel prick, and vice versa. PIPP scale before, 1 and 5 min after. | KMC 15 min | Moderate |
| Gao et al., 2018 [44] | China | IG1: 22 (7) IG2: 21 (11) IG3: 22 (8) CG: 21 (8) | IG1: 31.9 IG2: 31.7 IG3: 32.0 CG: 31.3 | IG1: 1.767 IG2: 1.780 IG3: 1.697 CG: 1.682 | IG1: 8.8 IG2: 8.9 IG3: 8.8 CG: 8.7 | PIPP scale IG1: 9.3/6.8 IG2: 10.1/7.4 IG3: 4.4/3.0 CG: 13.3/10.6 | IG1: NNS IG2: Oral sucrose 20% IG3: NNS + Oral sucrose 20% CG: Routine care | NNS and sucrose 2 min before. PIPP scale 15 s before and 30 s after heel prick. | NNS 2 min 1 mL sucrose 20% | Low |
| Kumari et al., 2017 [45] | India | IG1: 47 (29) IG2: 47 (26) | IG1: 35.56 IG2: 35.53 | IG1: 2.417 IG2: 2.685 | IG1: >7 IG2: >7 | PIPP scale IG1: 1.13/3.0/1.7/2.0 IG2: 1.49/2.74/1.72/1.74 | IG1: Oral sucrose 24% IG2: Oral glucose 25% | 2 min before sucrose or glucose. PIPP scale before, 30 s, 1 min and 2 min after heel prick. | 1 mL sucrose 24% 1 mL glucose 25% | High |
| Lima et al., 2017 [46] | Brazil | IG: 40 (24) CG: 38 (18) | IG: 39.0 CG: 39.05 | IG: 3.300 CG: 3.230 | IG: 9.8 CG: 9.7 | NIPS scale IG: 3.3 CG: 5.6 | IG: Oral glucose 25% CG: NNS | Glucose 2 min before. NNS before and during. NIPS scale 2 min before and during heel prick. | NNS 2 min 2 mL glucose 25% | Moderate |
| Peng et al., 2018 [47] | Taiwan | IG1: 37 (ND) IG2: 36 (ND) CG: 36 (ND) | IG1: 31.28 IG2: 31.31 CG: 31.16 | IG1: 1.572 IG2: 1.559 CG: 1.556 | IG1: 7.38 IG2: 7.32 CG: 7.28 | PIPP scale IG1: 2.6/4.3/1.4 IG2: 2.6/3.6/1.1 CG: 5.6/6.9/2.6 | IG1: NNS + BF IG2: NNS + BF + Contention CG: Routine care | NNS y BF 2 min before. PIPP scale 10 min before, during and 10 min after heel prick. | NNS 2 min 0.5–2.0 mL BF | Low |
| Perroteau et al., 2018 [48] | France | IG: 30 (15) CG: 29 (16) | IG: 29.0 CG: 30.0 | IG: 1.330 CG: 1.280 | NA | PIPP scale IG: 6.0/12.0 CG: 7.0/13.0 | IG: NNS + Contention CG: NNS | NNS after. Contention before, during and after. PIPP scale 15 s before and 30 s after heel prick. | NNS 3 min | Moderate |
| Silveira et al., 2021 [49] | Brazil | IG1: 34 (17) IG2: 34 (17) IG3: 34 (17) | IG1: 33.48 IG2: 33.48 IG3: 33.48 | IG1: 1.851 IG2: 1.851 IG3: 1.851 | IG1: ≥7 IG2: ≥7 IG3: ≥7 | PIPP scale IG1: 3.3/5.5 IG2: 3.3/4.6 IG3: 4.0/4.2 | IG1: NNS IG2: Oral glucose 25% IG3: NNS + Oral glucose 25% | NNS before and during, frequency > 32 suctions/min. Glucose 2 min before. PIPP scale 30 s before and 5 min after heel prick. | NNS 2 min 1 mL glucose 25% | High |
| Stevens et al., 2018 [50] | Canada | IG1: 81 (44) IG2: 81 (32) IG3: 81 (41) | IG1: 32.6 IG2: 32.5 IG3: 32.7 | IG1: 2.002 IG2: 1.933 IG3: 2.055 | NA | PIPP scale IG1: 6.8/7.0 IG2: 6.8/6.9 IG3: 6.7/6.7 | IG1: 0,1 mL oral sucrose 24% IG2: 0.5 mL oral sucrose 24% IG3: 1 mL oral sucrose 24% | Sucrose and NNS 2 min before. PIPP scale at 30 s and 60 s after heel prick. | NNS 2 min 0.1–0.5–1 mL sucrose 24% | High |
| Thakkar et al., 2016 [51] | India | IG1: 45 (25) IG2: 45 (20) IG3: 45 (18) CG: 45 (14) | IG1: 38.68 IG2: 38.66 IG3: 38.8 CG: 38.8 | IG1: ≥2.200 IG2: ≥2.200 IG3: ≥2.200 CG: ≥2.200 | NA | PIPP scale IG1: 7 IG2: 9 IG3: 3 CG: 13 | IG1: Oral sucrose 30% IG2: NNS IG3: Oral sucrose 30% + NNS CG: Routine care | Sucrose and NNS 2 min before. PIPP scale after heel prick. | NNS 2 min 2 mL sucrose 30% | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Valdivieso, I.; Yáñez-Araque, B.; Moncunill-Martínez, E.; Bocos-Reglero, M.J.; Gómez-Cantarino, S. Effect of Non-Pharmacological Methods in the Reduction of Neonatal Pain: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 3226. https://doi.org/10.3390/ijerph20043226
García-Valdivieso I, Yáñez-Araque B, Moncunill-Martínez E, Bocos-Reglero MJ, Gómez-Cantarino S. Effect of Non-Pharmacological Methods in the Reduction of Neonatal Pain: Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2023; 20(4):3226. https://doi.org/10.3390/ijerph20043226
Chicago/Turabian StyleGarcía-Valdivieso, Inmaculada, Benito Yáñez-Araque, Eva Moncunill-Martínez, M. Jesús Bocos-Reglero, and Sagrario Gómez-Cantarino. 2023. "Effect of Non-Pharmacological Methods in the Reduction of Neonatal Pain: Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 20, no. 4: 3226. https://doi.org/10.3390/ijerph20043226
APA StyleGarcía-Valdivieso, I., Yáñez-Araque, B., Moncunill-Martínez, E., Bocos-Reglero, M. J., & Gómez-Cantarino, S. (2023). Effect of Non-Pharmacological Methods in the Reduction of Neonatal Pain: Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 20(4), 3226. https://doi.org/10.3390/ijerph20043226








