Exploring the Relationship between Anemia and Postpartum Depression: Evidence from Malawi
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Participants
2.2. Assessment of Depressive Symptoms
2.3. Hemoglobin Assessment
2.4. Other Covariates
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
3.1. Demographic Characteristics
3.2. Anemia Status
3.3. Depressive Symptoms
3.4. Multivariate Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anokye, R.; Acheampong, E.; Budu-Ainooson, A.; Obeng, E.I.; Akwasi, A.G. Prevalence of postpartum depression and interventions utilized for its management. Ann. Gen. Psychiatry 2018, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dow, A.; Dube, Q.; Pence, B.W.; Van Rie, A. Postpartum Depression and HIV Infection Among Women in Malawi. Am. J. Ther. 2014, 65, 359–365. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Methods and Data Sources for Global Burden of Disease Estimates 2000–2016; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Beghé, C.; Wilson, A.; Ershler, W.B. Prevalence and outcomes of anemia in geriatrics: A systematic review of the literature. Am. J. Med. 2004, 116 (Suppl. 7A), 3S–10S. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Shuai, H.; Cai, Z.; Fu, X.; Liu, Y.; Xiao, X.; Zhang, W.; Krabbendam, E.; Liu, S.; et al. Mapping global prevalence of depression among postpartum women. Transl. Psychiatry 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.-A.; Noori, M.; Nejadghaderi, S.A.; Karamzad, N.; Bragazzi, N.L.; Sullman, M.J.M.; Abdollahi, M.; Collins, G.S.; Kaufman, J.S.; et al. Burden of anemia and its underlying causes in 204 countries and territories, 1990–2019: Results from the Global Burden of Disease Study 2019. J. Hematol. Oncol. 2021, 14, 1–16. [Google Scholar] [CrossRef]
- Azami, M.; Badfar, G.; Khalighi, Z.; Qasemi, P.; Shohani, M.; Soleymani, A.; Abbasalizadeh, S. The association between anemia and postpartum depression: A systematic review and meta-analysis. Casp. J. Intern. Med. 2019, 10, 115–124. [Google Scholar] [CrossRef]
- Maeda, Y.; Ogawa, K.; Morisaki, N.; Tachibana, Y.; Horikawa, R.; Sago, H. Association between perinatal anemia and postpartum depression: A prospective cohort study of Japanese women. Int. J. Gynecol. Obstet. 2019, 148, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Goshtasebi, A.; Alizadeh, M.; Behboudi-Gandevani, S. Association between Maternal Anaemia and Postpartum Depression in an Urban Sample of Pregnant Women in Iran. J. Health Popul. Nutr. 2013, 31, 398–402. [Google Scholar] [CrossRef]
- Abdulghani, H.M.; Alharbi, A. Risk factors associated with postpartum depression in the Saudi population. Neuropsychiatr. Dis. Treat. 2014, 10, 311–316. [Google Scholar] [CrossRef]
- Eckerdal, P.; Kollia, N.; Löfblad, J.; Hellgren, C.; Karlsson, L.; Högberg, U.; Wikström, A.-K.; Skalkidou, A. Delineating the Association between Heavy Postpartum Haemorrhage and Postpartum Depression. PLoS ONE 2016, 11, e0144274. [Google Scholar] [CrossRef]
- Armony-Sivan, R.; Shao, J.; Li, M.; Zhao, G.; Zhao, Z.; Xu, G.; Zhou, M.; Zhan, J.; Bian, Y.; Ji, C.; et al. No Relationship between Maternal Iron Status and Postpartum Depression in Two Samples in China. J. Pregnancy 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.C.; Umar, E.; Tomenson, B.; Creed, F. A cross-sectional study of antenatal depression and associated factors in Malawi. Arch. Women′s Ment. Health 2013, 17, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Harrington, B.J.; Hosseinipour, M.C.; Maliwichi, M.; Phulusa, J.; Jumbe, A.; Wallie, S.; Gaynes, B.N.; Maselko, J.; Miller, W.C.; Pence, B.W. Prevalence and incidence of probable perinatal depression among women enrolled in Option B+ antenatal HIV care in Malawi. J. Affect. Disord. 2018, 239, 115–122. [Google Scholar] [CrossRef]
- Karra, M.; Canning, D. The Effect of Improved Access to Family Planning on Postpartum Women: Protocol for a Randomized Controlled Trial. JMIR Res. Protoc. 2020, 9, e16697. [Google Scholar] [CrossRef]
- Andrews-Fike, C. A Review of Postpartum Depression. Prim. Care Companion J. Clin. Psychiatry 1999, 1, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2011, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Udedi, M.; Muula, A.S.; Stewart, R.C.; Pence, B.W. The validity of the patient health Questionnaire-9 to screen for depression in patients with type-2 diabetes mellitus in non-communicable diseases clinics in Malawi. BMC Psychiatry 2019, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Navarro, R.; Cano-Vindel, A.; Medrano, L.A.; Schmitz, F.; Ruiz-Rodríguez, P.; Abellán-Maeso, C.; Font-Payeras, M.A.; Hermosilla-Pasamar, A.M. Utility of the PHQ-9 to identify major depressive disorder in adult patients in Spanish primary care centres. BMC Psychiatry 2017, 17, 1–9. [Google Scholar] [CrossRef]
- HemoCue. Hemocue Worldwide. POCT for Anemia Screening. 2006. Available online: http://www.hemocue.com/international/POCT_for_Anemia_Screening-1131.html (accessed on 29 October 2019).
- World Health Organization (WHO). Hemoglobin Concentrations for the Diagnosis of Anemia and Assessment of Severity. Department of Nutrition for Health and Development (NHD). World Health Organization: Geneva, Switzerland. 2011. Available online: https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf. (accessed on 29 October 2019).
- Gebremedhin, S.; Asefa, A. Association between type of contraceptive use and haemoglobin status among women of reproductive age in 24 sub-Saharan Africa countries. BMJ Sex. Reprod. Health 2018, 45, 54–60. [Google Scholar] [CrossRef]
- Murray-Kolb, L.E.; Beard, J.L. Iron deficiency and child and maternal health. Am. J. Clin. Nutr. 2009, 89, 946S–950S. [Google Scholar] [CrossRef]
- Dalili, H.; Baghersalimi, A.; Dalili, S.; Pakdaman, F.; Rad, A.H.; Kakroodi, M.A.; Rezvany, S.; Koohmanaei, S. Is there any relation between Duration of breastfeeding and anemia? Iran. J. Pediatr. Hematol. Oncol. 2015, 5, 218–226. [Google Scholar]
- Brazier, Y. Nutritional-Deficiency Anemia: Causes, Symptoms, and Treatment. Available online: https://www.medicalnewstoday.com/articles/188770 (accessed on 29 October 2019).
- Sawyer, A.; Ayers, S.; Smith, H. Pre- and postnatal psychological wellbeing in Africa: A systematic review. J. Affect. Disord. 2010, 123, 17–29. [Google Scholar] [CrossRef]
- Ghaedrahmati, M.; Kazemi, A.; Kheirabadi, G.; Ebrahimi, A.; Bahrami, M. Postpartum depression risk factors: A narrative review. J. Educ. Health Promot. 2017, 6, 60. [Google Scholar]
- Righetti-Veltema, M.; Bousquet, A.; Manzano, J. Impact of postpartum depressive symptoms on mother and her 18-month-old infant. Eur. Child Adolesc. Psychiatry 2003, 12, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.L.; Evindar, A.; Stewart, D.E. The effect of postpartum depression on child cognitive development and behavior: A review and critical analysis of the literature. Arch. Women′s Ment. Health 2003, 6, 263–274. [Google Scholar] [CrossRef]
- Beck, C.T. Predictors of Postpartum Depression: An Update. Nurs. Res. 2001, 50, 275–285. [Google Scholar] [CrossRef]
- American Pregnancy Association. Anemia During Pregnancy. Available online: https://americanpregnancy.org/healthy-pregnancy/pregnancy-concerns/anemia-during-pregnancy/#:~:text=Types%20of%20anemia%20during%20pregnancy (accessed on 31 January 2023).
- Aoki, C.; Imai, K.; Owaki, T.; Kobayashi-Nakano, T.; Ushida, T.; Iitani, Y.; Nakamura, N.; Kajiyama, H.; Kotani, T. The Possible Effects of Zinc Supplementation on Postpartum Depression and Anemia. Medicina 2022, 58, 731. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Antenatal Iron Supplementation. Available online: https://www.who.int/data/nutrition/nlis/info/antenatal-iron-supplementation (accessed on 31 January 2023).
- Kwak, D.-W.; Kim, S.; Lee, S.-Y.; Kim, M.-H.; Park, H.-J.; Han, Y.-J.; Cha, D.-H.; Kim, M.-Y.; Chung, J.-H.; Park, B.; et al. Maternal Anemia during the First Trimester and Its Association with Psychological Health. Nutrients 2022, 14, 3505. [Google Scholar] [CrossRef] [PubMed]


| Variables | All | Analysis Sample | |
|---|---|---|---|
| (N = 829) | (N = 565) | ||
| n (%) | n (%) | ||
| PHQ-9 Total Score | 2.10 (3.56) | 2.21 (3.55) | |
| Mean (standard deviation) | |||
| Depression (PHQ-9 total score, categorical) | |||
| No depression (0) | 454 (54.76) | 295 (52.21) | |
| Minimal (1–4) | 228 (27.50) | 163 (28.85) | |
| Mild (5–9) | 110 (13.27) | 78 (13.81) | |
| Moderate (10–14) | 24 (2.90) | 22 (3.89) | |
| Moderately severe (15–19) | 7 (0.84) | 3 (0.53) | |
| Severe (20–27) | 6 (0.72) | 4 (0.71) | |
| Major depression (shaded areas, binary) | |||
| Yes | 23 (2.77) | 15 (2.65) | |
| No | 806 (97.23) | 550 (97.35) | |
| PHQ-9 score ≥ 10 | |||
| Yes | 37 (4.46) | 29 (5.13) | |
| No | 792 (95.54) | 536 (94.87) | |
| Age (years) | |||
| 15–19 * | 108 (13.03) | 68 (12.04) | |
| 20–24 | 350 (42.22) | 247 (43.72) | |
| 25–29 | 192 (23.16) | 127 (22.48) | |
| 30–39 | 179 (21.59) | 123 (21.77) | |
| Education | |||
| No schooling and primary * | 448 (54.04) | 307 (54.34) | |
| Secondary and higher | 366 (44.15) | 258 (45.66) | |
| Missing | 15 (1.81) | 0 | |
| Employment status | |||
| Not working * | 527 (63.57) | 371 (65.66) | |
| Working | 302 (36.43) | 194 (34.34) | |
| Husband has other wives | |||
| Yes | 47 (5.67) | 31 (5.49) | |
| No * | 751 (90.59) | 534 (94.51) | |
| Missing | 31 (3.74) | 0 | |
| Multiple births | |||
| Yes | 18 (2.17) | 16 (2.83) | |
| No * | 811 (97.83) | 549 (97.17) | |
| Total number of alive children per woman | |||
| Mean (standard deviation) | 2.31(1.25) | 2.34 (1.28) | |
| Anemia | |||
| Yes | 223 (26.90) | 212 (37.52) | |
| No * | 367 (44.27) | 353 (62.48) | |
| Missing | 239 (28.83) | 0 | |
| Anemia severity | |||
| None * | 367 (44.27) | 353 (62.48) | |
| Mild | 127 (15.32) | 120 (21.24) | |
| Moderate | 91 (10.98) | 87 (15.40) | |
| Severe | 5 (0.60) | 5 (0.88) | |
| Missing | 239 (28.83) | 0 | |
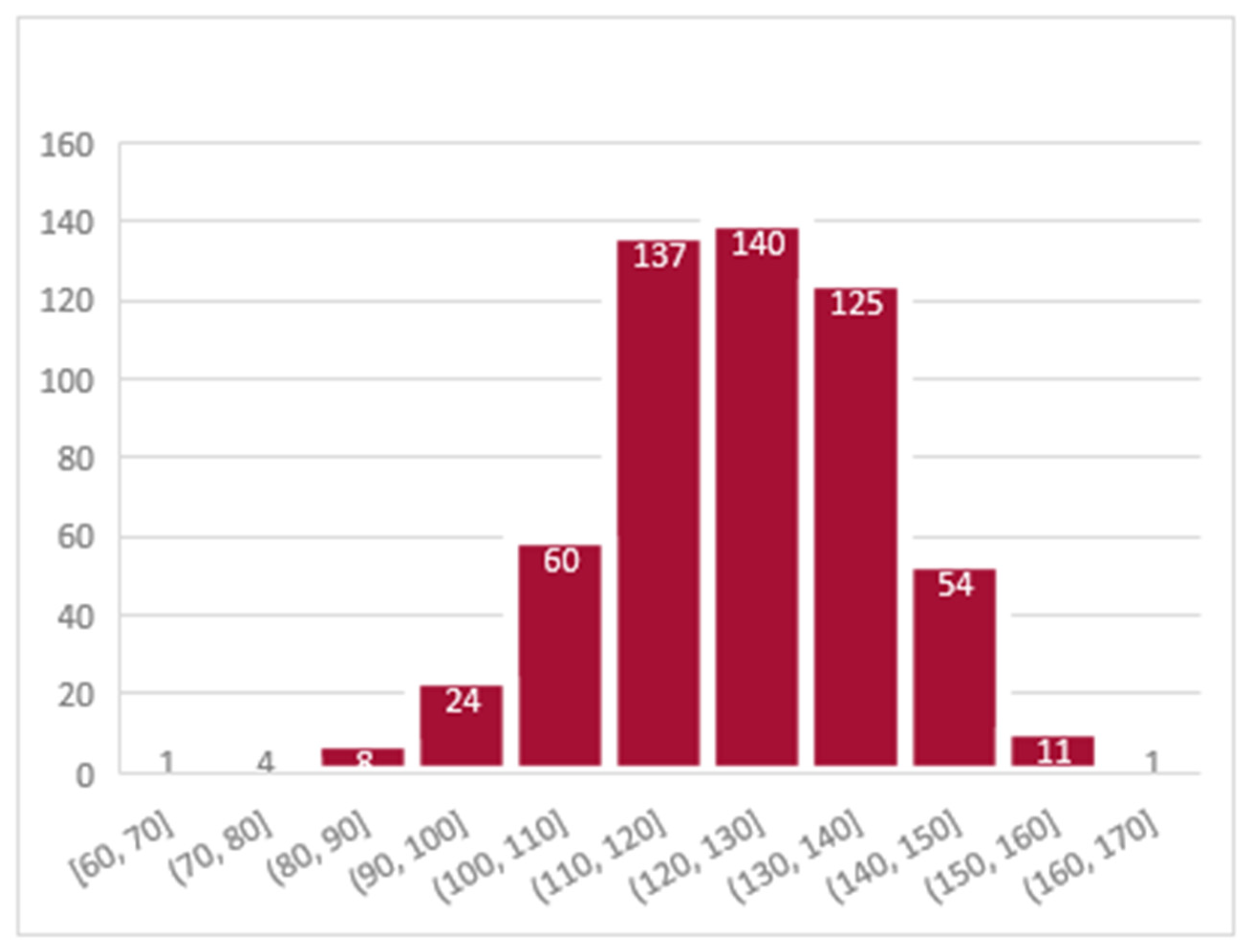
| Hemoglobin (g/L) | |||
| Mean (standard deviation) | 123.23 (14.84) | 123.40 (14.82) | |
| Variable | Summary of PHQ-9 Total Scores | ||||
|---|---|---|---|---|---|
| Anemia | Mean | Std. Dev. | Freq. | ||
| No Anemia | 2.12 | 3.32 | 353 | ||
| Mild | 2.53 | 3.95 | 120 | ||
| Moderate to Severe | 2.12 | 3.89 | 92 | ||
| Total | 2.21 | 3.56 | 565 | ||
| Sum of Squares | DF | Mean Square | F-Statistic | p-Value | |
| Between groups | 15.87 | 2 | 7.93 | 0.63 | 0.5345 |
| Within groups | 7110.07 | 562 | 12.65 | ||
| Total | 7125.94 | 564 | 12.63 | ||
| Major Depressive Disorder | Major Depressive Disorder | Moderate to Severe Depression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (Unadjusted Model) | (Model 1) | (Model 2) | |||||||
| Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | p-Value | |
| Anemia status (g/dL) | |||||||||
| Yes | 3.45 | 1.16 to 10.22 | 0.03 * | 3.48 | 1.15 to 10.57 | 0.03 * | 1.90 | 0.89 to 4.07 | 0.10 |
| Age (years) | |||||||||
| 20–24 | 0.74 | 0.08 to 7.04 | 0.79 | 0.85 | 0.22 to 3.29 | 0.81 | |||
| 25–29 | 0.81 | 0.07 to 9.36 | 0.87 | 0.58 | 0.11 to 2.98 | 0.52 | |||
| 30–39 | 0.31 | 0.02 to 5.73 | 0.43 | 0.54 | 0.08 to 3.44 | 0.51 | |||
| Education | |||||||||
| Secondary and higher | 2.49 | 0.75 to 8.31 | 0.14 | 1.20 | 0.52 to 2.74 | 0.67 | |||
| Employment status | |||||||||
| Working | 1.84 | 0.63 to 5.42 | 0.27 | 1.05 | 0.47 to 2.34 | 0.91 | |||
| Husband has other wives | |||||||||
| Yes | 3.45 | 0.65 to 18.36 | 0.15 | 2.07 | 0.56 to 7.64 | 0.28 | |||
| Multiple births | |||||||||
| Yes | 2.11 | 0.23 to 18.96 | 0.51 | 2.45 | 0.51 to 11.85 | 0.27 | |||
| Total number of alive children per woman | 1.60 | 0.90 to 2.81 | 0.11 | 1.34 | 0.88 to 2.03 | 0.17 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Karra, M.; Guo, M.; Patel, V.; Canning, D. Exploring the Relationship between Anemia and Postpartum Depression: Evidence from Malawi. Int. J. Environ. Res. Public Health 2023, 20, 3178. https://doi.org/10.3390/ijerph20043178
Cheng Z, Karra M, Guo M, Patel V, Canning D. Exploring the Relationship between Anemia and Postpartum Depression: Evidence from Malawi. International Journal of Environmental Research and Public Health. 2023; 20(4):3178. https://doi.org/10.3390/ijerph20043178
Chicago/Turabian StyleCheng, Zijing, Mahesh Karra, Muqi Guo, Vikram Patel, and David Canning. 2023. "Exploring the Relationship between Anemia and Postpartum Depression: Evidence from Malawi" International Journal of Environmental Research and Public Health 20, no. 4: 3178. https://doi.org/10.3390/ijerph20043178
APA StyleCheng, Z., Karra, M., Guo, M., Patel, V., & Canning, D. (2023). Exploring the Relationship between Anemia and Postpartum Depression: Evidence from Malawi. International Journal of Environmental Research and Public Health, 20(4), 3178. https://doi.org/10.3390/ijerph20043178







