Anaerobic Storage Completely Removes Suspected Fungal Pathogens but Increases Antibiotic Resistance Gene Levels in Swine Wastewater High in Sulfonamides
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater
2.2. SWW Storage Experiment
2.3. Measurement of Wastewater Properties
2.4. High-Throughput Sequencing
2.5. Quantitative PCR and Quantification of ARGs
2.6. Data Analyses
3. Results and Discussion
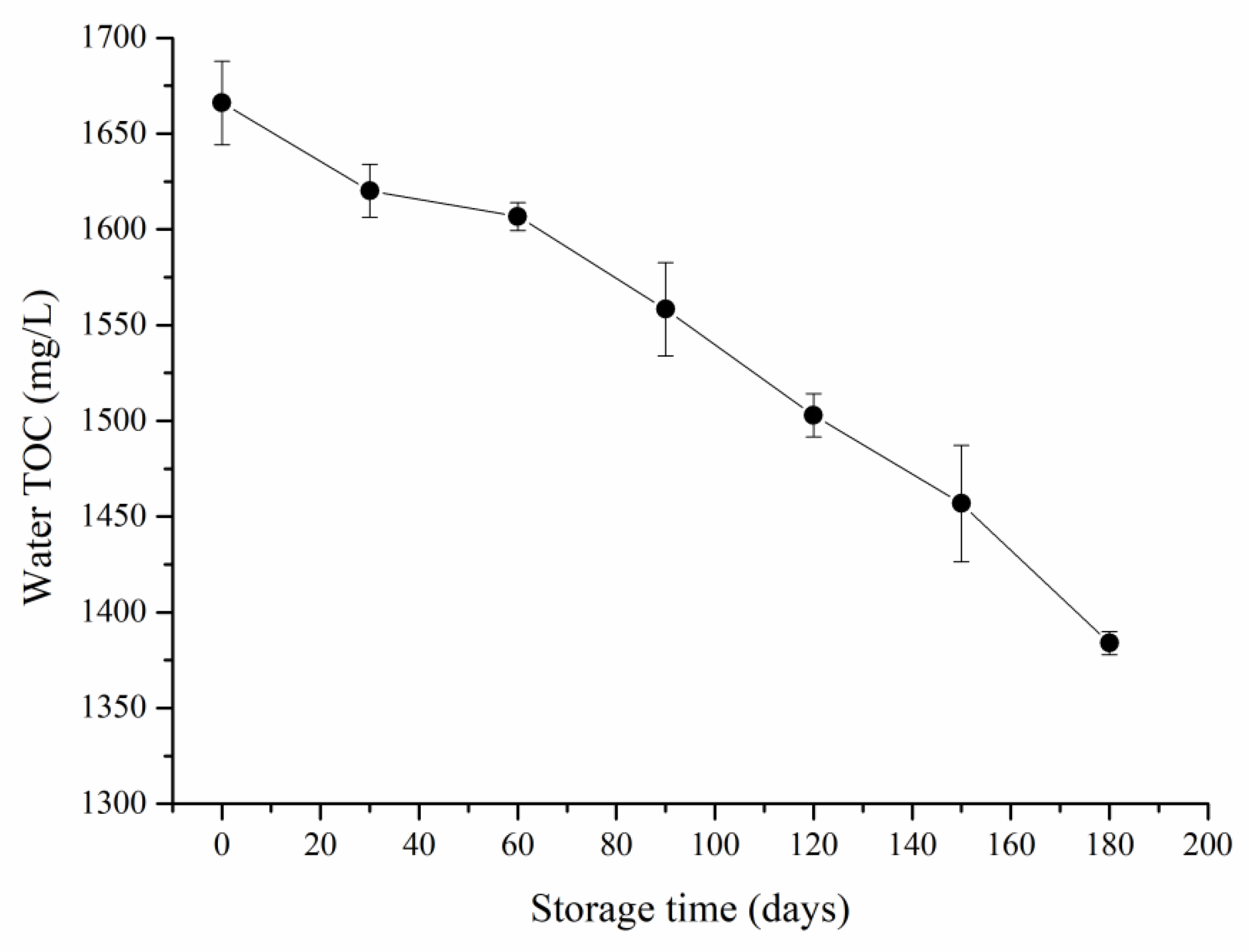
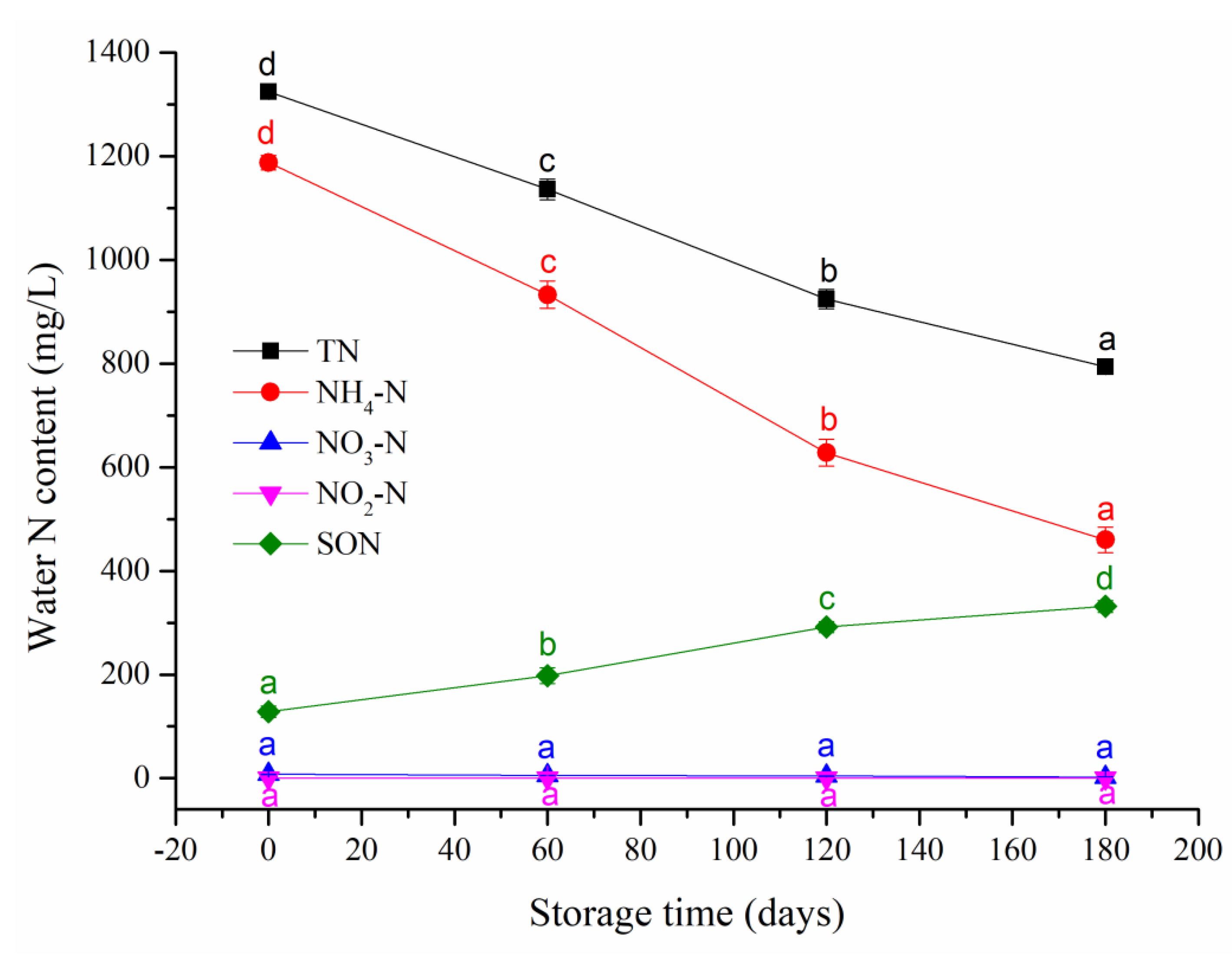
3.1. SWW Properties
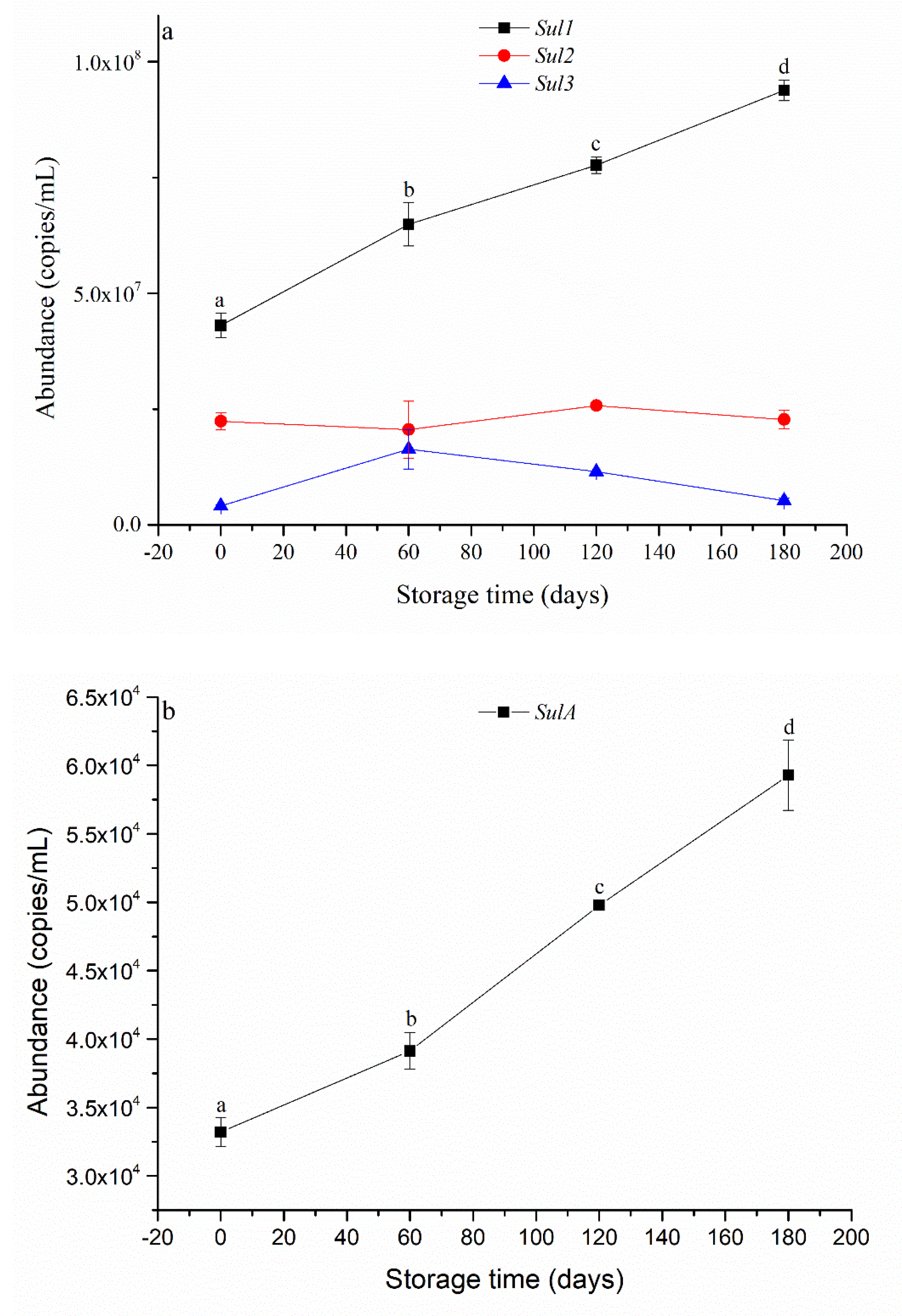
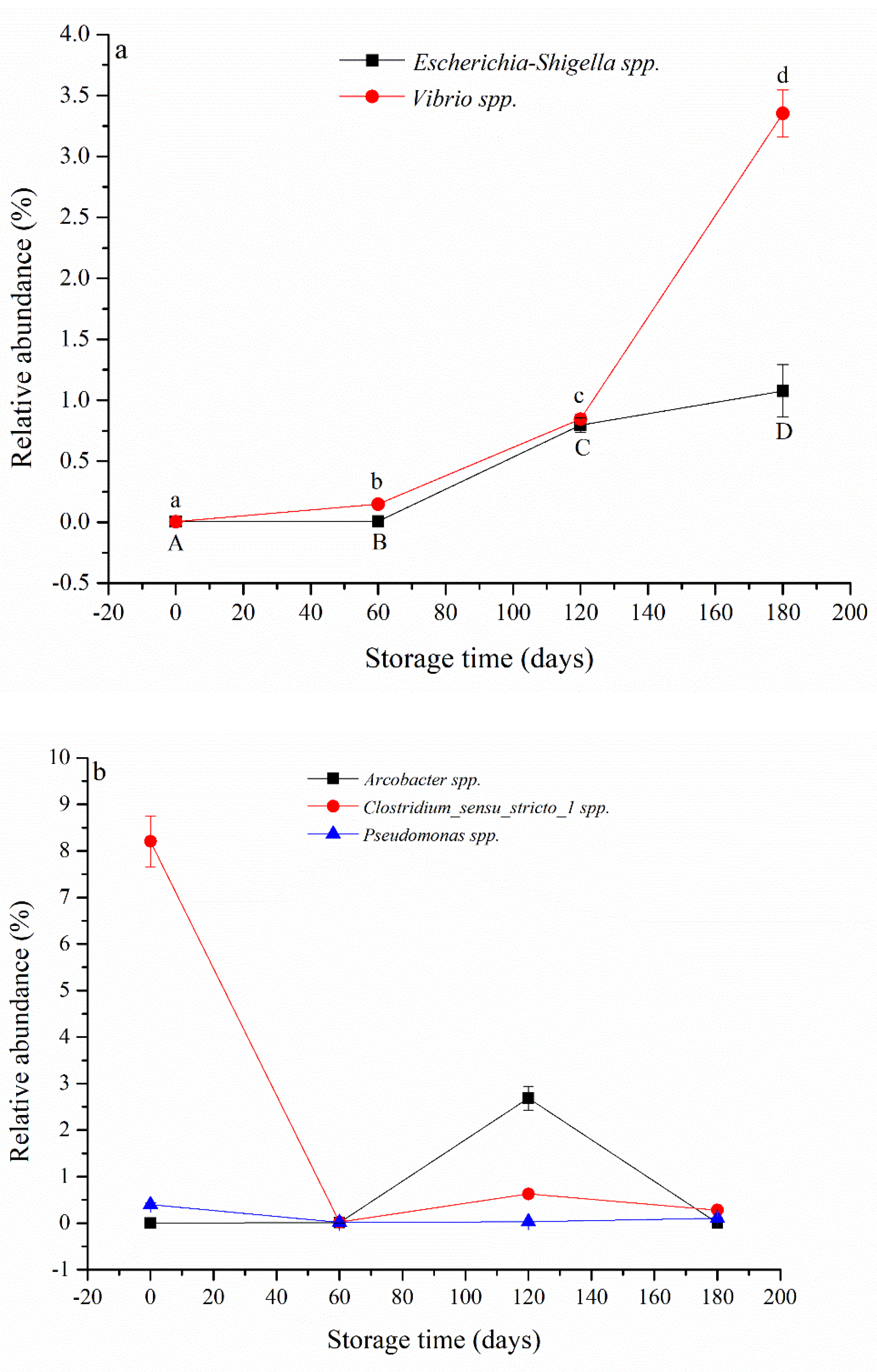
3.2. Microbial Abundance and Level of ARGs during SWW Storage
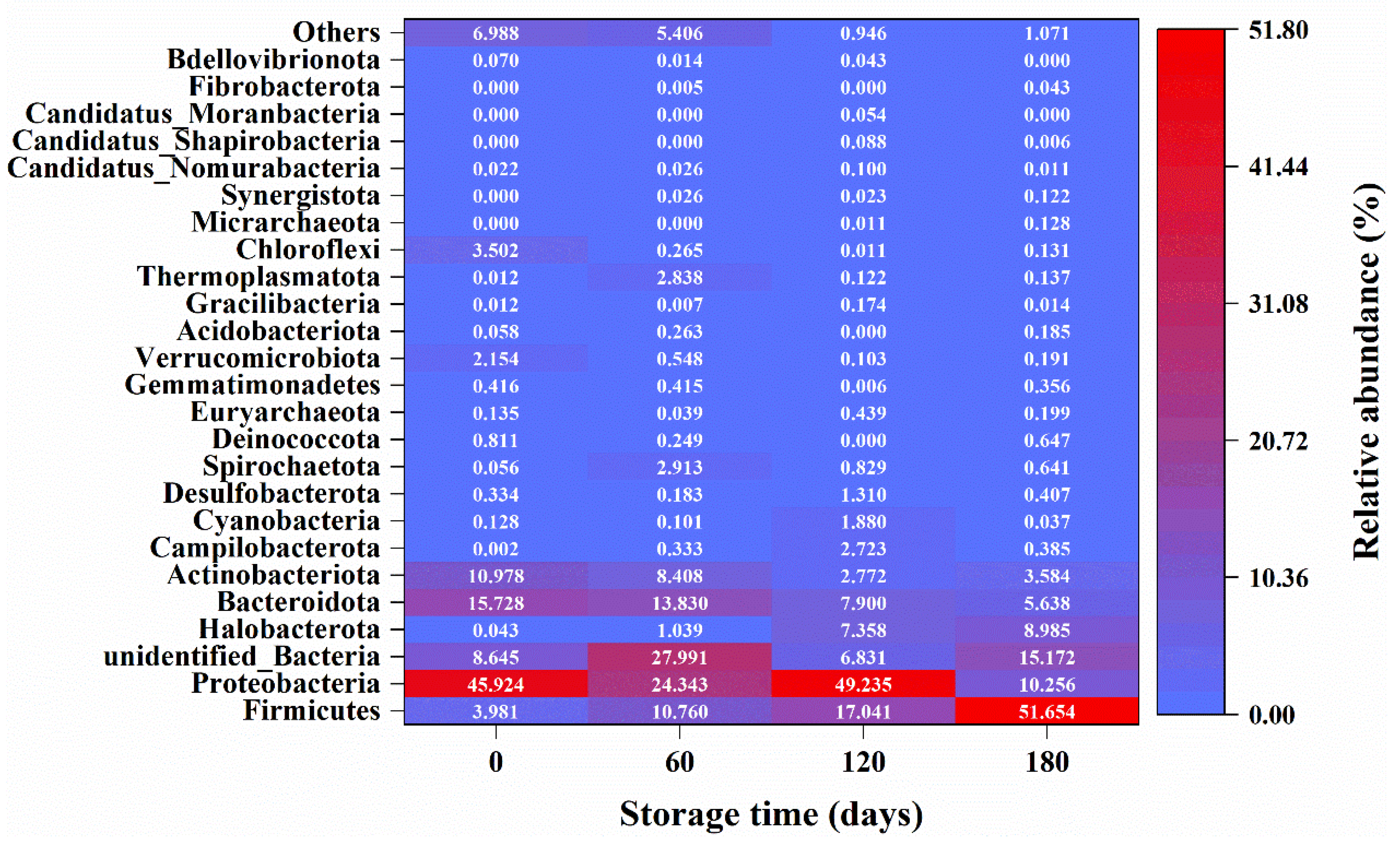
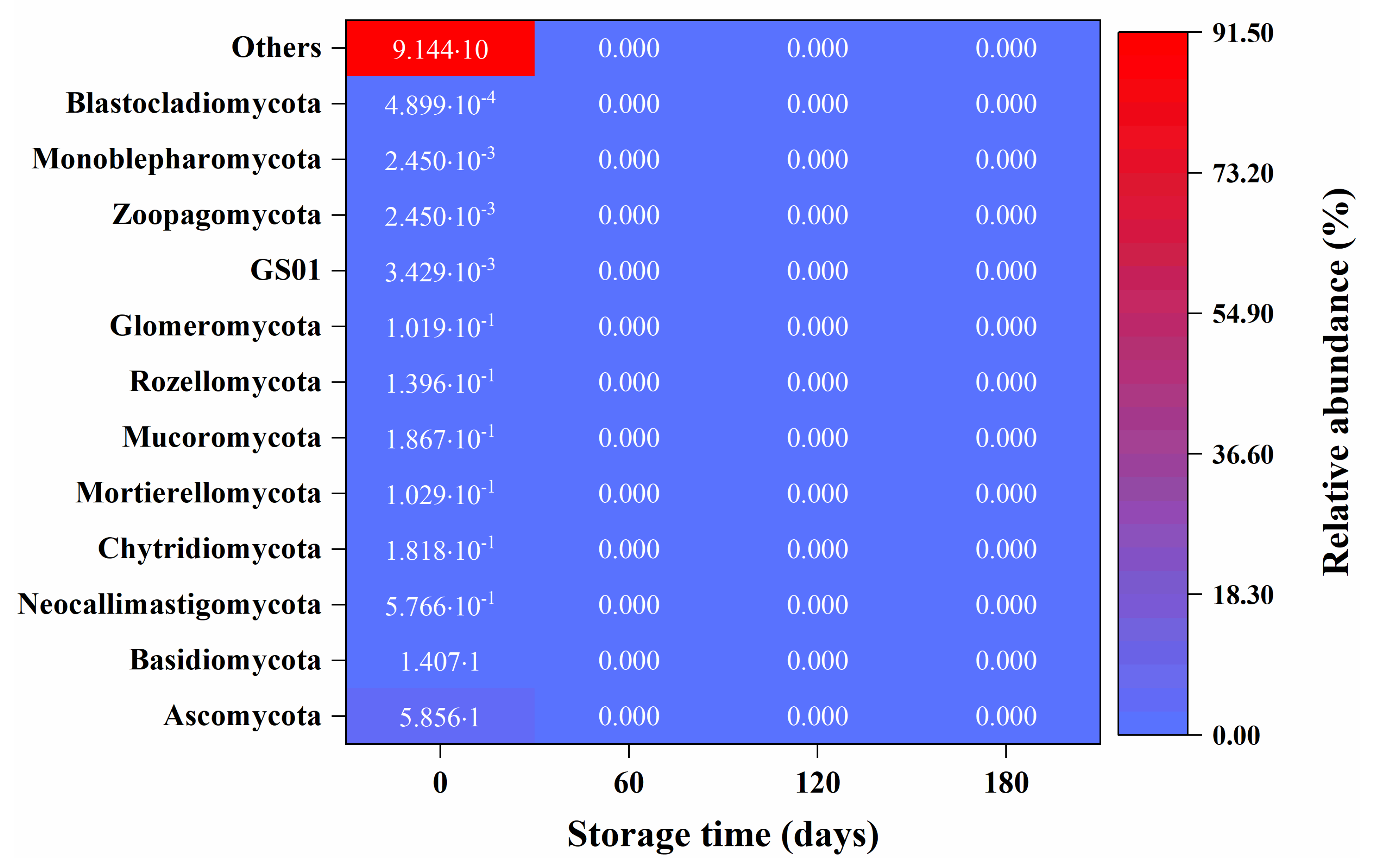
3.3. Microbial Community
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, Z.; Zheng, P.; Ding, A.; Zhang, M.; Abbas, G.; Li, W. Source analysis of organic matter in swine wastewater after anaerobic digestion with EEM-PARAFAC. Environ. Sci. Pollut. Res. 2017, 24, 6770–6778. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.L.; Xu, L.G.; Cheng, F.M.; Pan, G.; Zhou, Q.F. Bicarbonate-rich wastewater as a carbon fertilizer for culture of Dictyosphaerium sp. of a giant pyrenoid. J. Clean. Prod. 2018, 202, 439–443. [Google Scholar]
- Wang, X.J.; Ni, X.; Cheng, Q.L.; Xu, L.G.; Zhou, Q.F. Vetiver and Dictyosphaerium sp. co-culture for rapid removal of nutrients and ecological inactivation of pathogens in swine wastewater. J. Adv. Res. 2019, 20, 71–78. [Google Scholar]
- Hu, H.; Li, X.; Wu, S.; Yang, C. Sustainable livestock wastewater treatment via phytoremediation: Current status and future perspectives. Bioresour. Technol. 2020, 315, 123809. [Google Scholar] [CrossRef]
- Zhai, Y.; Yuan, Q.; Hu, N. Study on structure variation and influencing factors of microbial community in swine wastewater treatment system. Water Resour. Prot. 2018, 34, 88–94. [Google Scholar]
- Tao, C.-W.; Hsu, B.-M.; Ji, W.-T.; Hsu, T.-K.; Kao, P.-M.; Hsu, C.-P.; Shen, S.-M.; Shen, T.-Y.; Wan, T.-J.; Huang, Y.-L. Evaluation of five antibiotic resistance genes in wastewater treatment systems of swine farms by real-time PCR. Sci. Total Environ. 2014, 496, 116–121. [Google Scholar] [CrossRef]
- Pu, C.; Liu, H.; Ding, G.; Sun, Y.; Yu, X.; Chen, J.; Ren, J.; Gong, X. Impact of direct application of biogas slurry and residue in fields: In situ analysis of antibiotic resistance genes from pig manure to fields. J. Hazard. Mater. 2018, 344, 441–449. [Google Scholar] [CrossRef]
- He, L.-Y.; He, L.-K.; Liu, Y.-S.; Zhang, M.; Zhao, J.-L.; Zhang, Q.-Q.; Ying, G.-G. Microbial diversity and antibiotic resistome in swine farm environments. Sci. Total Environ. 2019, 685, 197–207. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.-S.; Zhao, J.-L.; Liu, W.-R.; Chen, J.; Zhang, Q.-Q.; He, L.-Y.; Ying, G.-G. Variations of antibiotic resistome in swine wastewater during full-scale anaerobic digestion treatment. Environ. Int. 2021, 155, 106694. [Google Scholar] [CrossRef]
- Krishnasamy, V.; Otte, J.; Silbergeld, E. Antimicrobial use in Chinese swine and broiler poultry production. Antimicrob. Resist. Infect. Control. 2015, 4, 17. [Google Scholar] [CrossRef]
- Ai, S.; Liu, H.; Wu, M.; Zeng, G.; Yang, C. Roles of acid-producing bacteria in anaerobic digestion of waste activated sludge. Front. Environ. Sci. Eng. 2018, 12, 3. [Google Scholar] [CrossRef]
- Zhu, N.; Jin, H.; Ye, X.; Liu, W.; Li, D.; Shah, G.M.; Zhu, Y. Fate and driving factors of antibiotic resistance genes in an integrated swine wastewater treatment system: From wastewater to soil. Sci. Total Environ. 2020, 721, 137654. [Google Scholar] [CrossRef]
- Topp, E.; Scott, A.; Lapen, D.; Lyautey, E.; Duriez, P. Livestock waste treatment systems for reducing environmental exposure to hazardous enteric pathogens: Some considerations. Bioresour. Technol. 2009, 100, 5395–5398. [Google Scholar] [CrossRef]
- Fongaro, G.; Kunz, A.; Magri, M.E.; Schissi, C.D.; Viancelli, A.; Philippi, L.S.; Barardi, C.R.M. Settling and survival profile of enteric pathogens in the swine effluent for water reuse purpose. Int. J. Hyg. Environ. Health 2016, 219, 883–889. [Google Scholar] [CrossRef]
- Hung Nguyen-viet, H.N.V.; Phuc Pham-duc, P.P.D.; Vi Nguyen, V.N.; Tanner, M.; Odermatt, P.; Tu Vu-van, T.V.V. A one health perspective for integrated human and animal sanitation and nutrient recycling. In One Health: The Theory and Practice of Integrated Health Approaches; Zinsstag, J., Schelling, E., Waltner-Toews, D., Whittaker, M., Tanner, M., Eds.; Cabi: Boston, MA, USA, 2015; pp. 96–107. [Google Scholar]
- Li, D.; Ye, J.; Yang, P.; Zhang, H.; Xue, S. Wastewater treatment technology for livestock and poultry breeding industry. Environ. Impact Assess. 2018, 40, 80–84. [Google Scholar]
- Daigger, G.T. Oxygen and carbon requirements for biological nitrogen removal processes accomplishing nitrification, nitritation, and anammox. Water Environ. Res. 2014, 86, 204–209. [Google Scholar] [CrossRef]
- Lackner, S.; Gilbert, E.M.; Vlaeminck, S.E.; Joss, A.; Horn, H.; van Loosdrecht, M.C. Full-scale partial nitritation/anammox experiences—An application survey. Water Res. 2014, 55, 292–303. [Google Scholar] [CrossRef]
- Svehla, P.; Caceres, L.M.V.; Michal, P.; Tlustos, P. Nitrification of the liquid phase of digestate can help with the reduction of nitrogen losses. Environ. Technol. Innov. 2020, 17, 100514. [Google Scholar] [CrossRef]
- Shao, Y.H.; Wu, J.H. Comammox nitrospira species dominate in an efficient partial nitrification–anammox bioreactor for treating ammonium at low loadings. Environ. Sci. Technol. 2021, 55, 2087–2098. [Google Scholar] [CrossRef]
- SEPA (The Chinese State Environmental Protection Agency). Water and Wastewater Analyzing Methods, 4th ed.; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Li, S.J.; Yao, J. Pollution characteristics and ecological risk assessment of antibiotics in wastewater from a large-scale piggery. J. Agro-Environ. Sci. 2021, 40, 884–893. [Google Scholar]
- Shi, F.; Zhao, X.; Cheng, Q.; Lin, H.; Zheng, H.; Zhou, Q. High-energy-density organic amendments enhance soil health. Int. J. Environ. Res. Public Health 2022, 19, 12212. [Google Scholar] [CrossRef]
- Prokhorova, A.; Kainuma, M.; Hiyane, R.; Boerner, S.; Goryanin, I. Concurrent treatment of raw and aerated swine wastewater using an electrotrophic denitrification system. Bioresour. Technol. 2021, 322, 124508. [Google Scholar] [CrossRef]
- Guo, X.; Sun, C.; Lin, R.; Xia, A.; Huang, Y.; Zhu, X.; Show, P.-L.; Murphy, J.D. Effects of foam nickel supplementation on anaerobic digestion: Direct interspecies electron transfer. J. Hazard. Mater. 2020, 399, 122830. [Google Scholar] [CrossRef]
- Du, R.; Cao, S.; Zhang, H.; Li, X.; Peng, Y. Flexible nitrite supply alternative for mainstream anammox: Advances in enhancing process stability. Environ. Sci. Technol. 2020, 54, 6353–6364. [Google Scholar] [CrossRef]
- Chao, A.; Bunge, J. Estimating the number of species in a stochastic abundance model. Biometrics 2002, 58, 531–539. [Google Scholar] [CrossRef]
- Chi, Z.; Hou, L.; Li, H.; Wu, H.; Yan, B. Indigenous bacterial community and function in phenanthrene-polluted coastal wetlands: Potential for phenanthrene degradation and relation with soil properties. Environ. Res. 2021, 199, 111357. [Google Scholar] [CrossRef]
- Holmes, D.E.; Bond, D.R.; O’Neil, R.A.; Reimers, C.E.; Tender, L.R.; Lovely, D.R. Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments. Microb. Ecol. 2004, 48, 178–190. [Google Scholar] [CrossRef]
- Chu, L.; Ding, P.; Ding, M. Pilot-scale microaerobic hydrolysis-acidification and anoxic-oxic processes for the treatment of petrochemical wastewater. Environ. Sci. Pollut. Res. Int. 2021, 28, 58677–58687. [Google Scholar] [CrossRef]
- Gryaznova, M.V.; Dvoretskaya, Y.D.; Syromyatnikov, M.Y.; Shabunin, S.V.; Parshin, P.A.; Mikhaylov, E.V.; Strelnikov, N.A.; Popov, V.N. Changes in the Microbiome Profile in Different Parts of the Intestine in Piglets with Diarrhea. Animals 2022, 12, 320. [Google Scholar] [CrossRef]
- Wesley, I.V. Encyclopedia of Food Microbiology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Kiu, R.; Hall, L.J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect. 2018, 7, 141. [Google Scholar] [CrossRef]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium Pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef]
- Djamei, A.; Kahmann, R. Ustilago maydis: Dissecting the Molecular Interface between Pathogen and Plant. PLoS Pathog. 2012, 8, e1002955. [Google Scholar] [CrossRef]
- Lu, P.; Guo, L.; Wang, Z.; Li, B.; Li, J.; Li, Y.; Qiu, D.; Shi, W.; Yang, L.; Wang, N.; et al. A rare gain of function mutation in a wheat tandem kinase confers resistance to powdery mildew. Nat. Commun. 2020, 11, 680. [Google Scholar] [CrossRef]

| pH | TOC (mg/L) | NH4-N (mg/L) | SAs (μg/L) |
|---|---|---|---|
| 8.23 ± 0.02 | 1666.1 ± 21.8 | 1187.5 ± 14.2 | 4653.2 ± 33.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zhang, M.; Sun, Z.; Zheng, H.; Zhou, Q. Anaerobic Storage Completely Removes Suspected Fungal Pathogens but Increases Antibiotic Resistance Gene Levels in Swine Wastewater High in Sulfonamides. Int. J. Environ. Res. Public Health 2023, 20, 3135. https://doi.org/10.3390/ijerph20043135
Zhao X, Zhang M, Sun Z, Zheng H, Zhou Q. Anaerobic Storage Completely Removes Suspected Fungal Pathogens but Increases Antibiotic Resistance Gene Levels in Swine Wastewater High in Sulfonamides. International Journal of Environmental Research and Public Health. 2023; 20(4):3135. https://doi.org/10.3390/ijerph20043135
Chicago/Turabian StyleZhao, Xinyue, Mengjie Zhang, Zhilin Sun, Huabao Zheng, and Qifa Zhou. 2023. "Anaerobic Storage Completely Removes Suspected Fungal Pathogens but Increases Antibiotic Resistance Gene Levels in Swine Wastewater High in Sulfonamides" International Journal of Environmental Research and Public Health 20, no. 4: 3135. https://doi.org/10.3390/ijerph20043135
APA StyleZhao, X., Zhang, M., Sun, Z., Zheng, H., & Zhou, Q. (2023). Anaerobic Storage Completely Removes Suspected Fungal Pathogens but Increases Antibiotic Resistance Gene Levels in Swine Wastewater High in Sulfonamides. International Journal of Environmental Research and Public Health, 20(4), 3135. https://doi.org/10.3390/ijerph20043135







