Predictive Value of Cardiovascular Health Score for Health Outcomes in Patients with PCI: Comparison between Life’s Simple 7 and Life’s Essential 8
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Procedure
2.2. Life’s Simple 7
2.3. Life’s Essential 8
2.4. Outcome Variables
2.5. Statistical Analysis
3. Results
3.1. Differences in Baseline Characteristics among the MACE and Non-MACE Groups of the Study Population
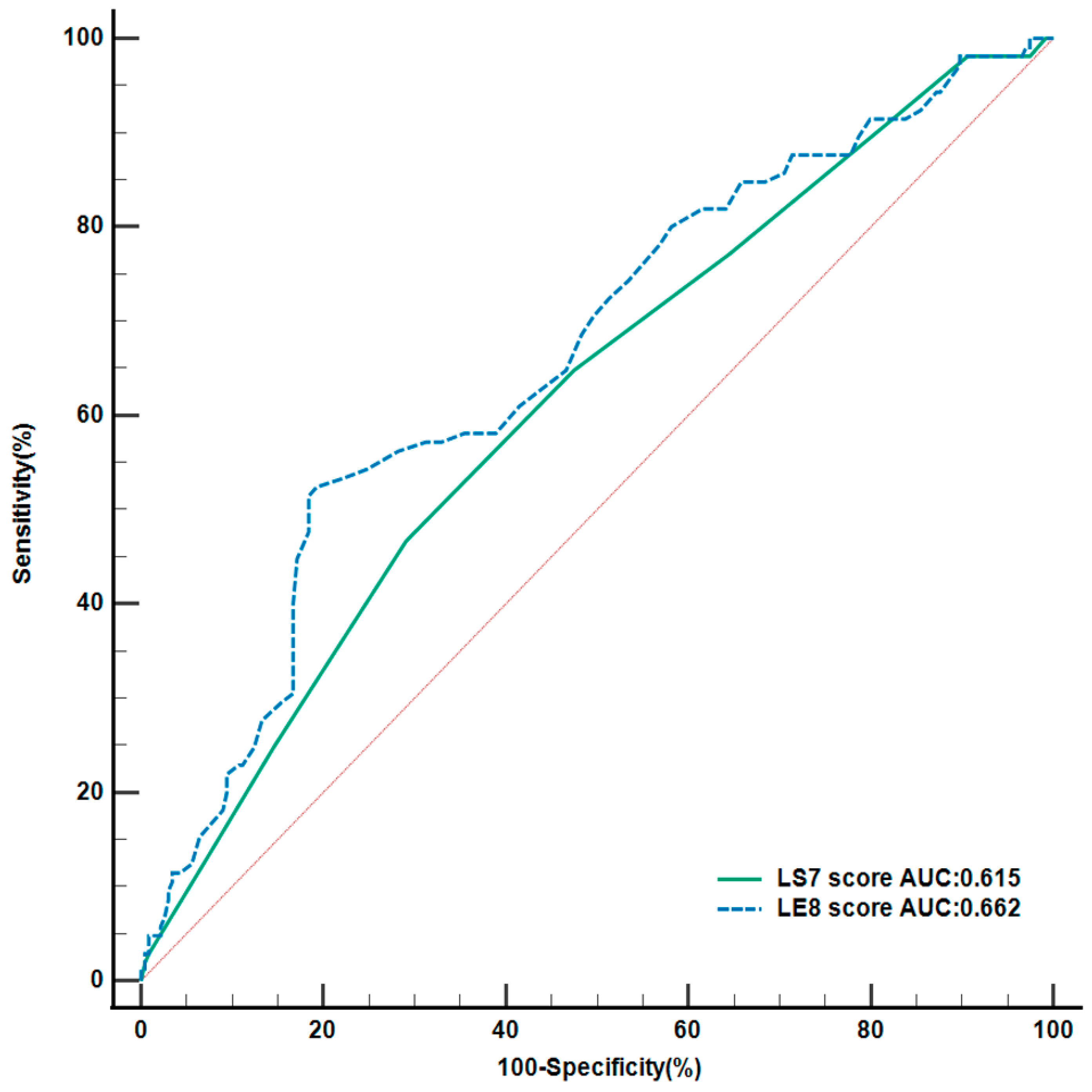
3.2. Predictive Value of Life’s Essential 8 and Life’s Simple 7
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steen, D.L.; Khan, I.; Andrade, K.; Koumas, A.; Giugliano, R.P. Event Rates and Risk Factors for Recurrent Cardiovascular Events and Mortality in a Contemporary Post Acute Coronary Syndrome Population Representing 239 234 Patients during 2005 to 2018 in the United States. J. Am. Heart Assoc. 2022, 11, e022198. [Google Scholar] [CrossRef] [PubMed]
- Ding, E.Y.; Mehawej, J.; Abu, H.; Lessard, D.; Saczynski, J.S.; McManus, D.D.; Kiefe, C.I.; Goldberg, R.J. Cardiovascular Health Metrics in Patients Hospitalized with an Acute Coronary Syndrome. Am. J. Med. 2021, 134, 1396–1402.e1. [Google Scholar] [CrossRef]
- Hoole, S.P.; Bambrough, P. Recent advances in percutaneous coronary intervention. Heart 2020, 106, 1380–1386. [Google Scholar] [CrossRef]
- Park, J.Y.; Rha, S.-W.; Noh, Y.-K.; Choi, B.G.; Hong, J.Y.; Choi, J.-W.; Ryu, S.K.; Park, S.-H.; Kim, Y.H.; Jeong, M.H. Real-World Three-Year Clinical Outcomes of Biolimus-Eluting Stents versus Other Contemporary Drug-Eluting Stents in Patients with Acute Myocardial Infarction Patients: Data from the Korea Acute Myocardial Infarction Registry (KAMIR). J. Interv. Cardiol. 2021, 2021, 6698582. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Sun, D.; Cheng, Y.; Wang, D.; Wang, F.; Wang, L.; Li, W.; Shen, D.; Guo, D.; Zhang, Z.; et al. Post-operative blood pressure and 3-year major adverse cardiac events in Chinese patients undergoing PCI. BMC Cardiovasc. Disord. 2021, 21, 623. [Google Scholar] [CrossRef]
- Staszczak, B.; Siudak, Z.; Malinowski, K.P.; Jędrychowska, M.; Zabojszcz, M.; Dolecka-Ślusarczyk, M.; Janion-Sadowska, A.; Susuł, M.; Tokarek, T.; Bartuś, J.; et al. Clinical outcomes in patients with acute myocardial infarction treated with primary percutaneous coronary intervention stratified according to duration of pain-to-balloon time and type of myocardial infarction. Cardiol. J. 2021, VM/OJS/J/69815. [Google Scholar] [CrossRef]
- Hasbani, N.R.; Ligthart, S.; Brown, M.R.; Heath, A.S.; Bebo, A.; Ashley, K.E.; Boerwinkle, E.; Morrison, A.C.; Folsom, A.R.; Aguilar, D.; et al. American Heart Association’s Life’s Simple 7: Lifestyle Recommendations, Polygenic Risk, and Lifetime Risk of Coronary Heart Disease. Circulation 2022, 145, 808–818. [Google Scholar] [CrossRef]
- Nguyen, A.T.H.; Saeed, A.; Bambs, C.E.; Swanson, J.; Emechebe, N.; Mansuri, F.; Talreja, K.; Reis, S.E.; Kip, K.E. Usefulness of the American Heart Association’s Ideal Cardiovascular Health Measure to Predict Long-term Major Adverse Cardiovascular Events (from the Heart SCORE Study). Am. J. Cardiol. 2021, 138, 20–25. [Google Scholar] [CrossRef]
- Wang, Y.; Xian, Y.; Chen, T.; Zhao, Y.; Yang, J.; Xu, B.; Li, W. Effect of Lifestyle Changes after Percutaneous Coronary Intervention on Revascularization. BioMed Res. Int. 2020, 2020, 2479652. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Deo, S.; Celis-Morales, C.; Ho, F.K.; Bahuguna, P.; McAllister, D.; Sattar, N.; Pell, J.P. An Opportunity for Prevention: Associations Between the Life’s Essential 8 Score and Cardiovascular Incidence Using Prospective Data from UK Biobank. Curr. Probl. Cardiol. 2023, 48, 101540. [Google Scholar] [CrossRef]
- Mok, Y.; Sang, Y.; Ballew, S.H.; Rebholz, C.M.; Rosamond, W.D.; Heiss, G.; Folsom, A.R.; Coresh, J.; Matsushita, K. American Heart Association’s Life’s Simple 7 at Middle Age and Prognosis After Myocardial Infarction in Later Life. J. Am. Heart Assoc. 2018, 7, e007658. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction: The American Heart Association’s Strategic Impact Goal through 2020 and Beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Allen, N.B.; Anderson, C.A.M.; Black, T.; Brewer, L.C.; Foraker, R.E.; Grandner, M.A.; Lavretsky, H.; Perak, A.M.; Sharma, G.; et al. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory from the American Heart Association. Circulation 2022, 146, e18–e43. [Google Scholar] [CrossRef] [PubMed]
- Calling, S.; Johansson, S.-E.; Wolff, M.; Sundquist, J.; Sundquist, K. Total cholesterol/HDL-C ratio versus non-HDL-C as predictors for ischemic heart disease: A 17-year follow-up study of women in southern Sweden. BMC Cardiovasc. Disord. 2021, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Harvey, A.G.; Lockley, S.W.; Dijk, D.-J. Circadian rhythms and disorders of the timing of sleep. Lancet 2022, 400, 1061–1078. [Google Scholar] [CrossRef]
- Makarem, N.; Castro-Diehl, C.; St-Onge, M.; Redline, S.; Shea, S.; Lloyd-Jones, D.; Ning, H.; Aggarwal, B. Redefining Cardiovascular Health to Include Sleep: Prospective Associations with Cardiovascular Disease in the MESA Sleep Study. J. Am. Heart Assoc. 2022, 11, e025252. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S73–S84. [Google Scholar] [CrossRef]
- Yuan, S. Fat Intake and Hypertension Among Adults in China: The Modifying Effects of Fruit and Vegetable Intake. Am. J. Prev. Med. 2020, 58, 29–301. [Google Scholar] [CrossRef]
- Xu, L.; Tian, Z.; Chen, H.; Zhao, Y.; Yang, Y. Anthocyanins, Anthocyanin-Rich Berries, and Cardiovascular Risks: Systematic Review and Meta-Analysis of 44 Randomized Controlled Trials and 15 Prospective Cohort Studies. Front. Nutr. 2021, 8, 747884. [Google Scholar] [CrossRef]
- Lian, Z.; Perrard, X.D.; Peng, X.; Raya, J.L.; Hernandez, A.A.; Johnson, C.G.; Lagor, W.R.; Pownall, H.J.; Hoogeveen, R.C.; Simon, S.I.; et al. Replacing Saturated Fat with Unsaturated Fat in Western Diet Reduces Foamy Monocytes and Atherosclerosis in Male Ldlr−/− Mice. ATVB 2020, 40, 72–85. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, X.; Stevanovic, S.; Wu, X.; Xiang, Z.; Yu, M.; Liu, J. Characterization particulate matter from several Chinese cooking dishes and implications in health effects. J. Environ. Sci. 2018, 72, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.M. Sleep function: An evolutionary perspective. Lancet Neurol. 2022, 21, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-Y.; Hu, H.-L.; Tang, G.-M.; Sun, J.-C.; Zheng, H.-X.; Zhai, C.-L.; He, C.-J. Sleep Quality, Sleep Duration, and the Risk of Adverse Clinical Outcomes in Patients with Myocardial Infarction with Non-obstructive Coronary Arteries. Front. Cardiovasc. Med. 2022, 9, 834169. [Google Scholar] [CrossRef]
- Sadeghi, M.; Daneshpour, M.S.; Khodakarim, S.; Momenan, A.A.; Akbarzadeh, M.; Soori, H. Impact of secondhand smoke exposure in former smokers on their subsequent risk of coronary heart disease: Evidence from the population-based cohort of the Tehran Lipid and Glucose Study. Epidemiol. Health 2020, 42, e2020009. [Google Scholar] [CrossRef]
- Hoshi, R.A.; Liu, Y.; Luttmann-Gibson, H.; Tiwari, S.; Giulianini, F.; Andres, A.M.; Watrous, J.D.; Cook, N.R.; Costenbader, K.H.; Okereke, O.I.; et al. Association of Physical Activity with Bioactive Lipids and Cardiovascular Events. Circ. Res. 2022, 131, e84–e99. [Google Scholar] [CrossRef]
- Park, S.; Han, K.; Lee, S.; Kim, Y.; Lee, Y.; Kang, M.W.; Park, S.; Kim, Y.C.; Han, S.S.; Lee, H.; et al. Cardiovascular or mortality risk of controlled hypertension and importance of physical activity. Heart 2021, 107, 1472–1479. [Google Scholar] [CrossRef]
- Park, S.; Han, K.; Lee, S.; Kim, Y.; Lee, Y.; Kang, M.W.; Park, S.; Kim, Y.C.; Han, S.S.; Lee, H.; et al. Association Between Moderate-to-Vigorous Physical Activity and the Risk of Major Adverse Cardiovascular Events or Mortality in People with Various Metabolic Syndrome Status: A Nationwide Population-Based Cohort Study Including 6 Million People. J. Am. Heart Assoc. 2020, 9, e016806. [Google Scholar] [CrossRef]
- Guy, J.-M.; Wilson, M.; Schnell, F.; Chevalier, L.; Verdier, J.-C.; Corone, S.; Doutreleau, S.; Kervio, G.; Carré, F. Incidence of major adverse cardiac events in men wishing to continue competitive sport following percutaneous coronary intervention. Arch. Cardiovasc. Dis. 2019, 112, 226–233. [Google Scholar] [CrossRef]
- National Health and Nutrition Examination Survey. Physical activity and physical fitness: PAQ-K. 2019. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2019-2020/questionnaires/PAQ_K.pdf. (accessed on 15 February 2022).
- Barrett, E.M.; Amoutzopoulos, B.; Batterham, M.J.; Ray, S.; Beck, E.J. Whole grain intake compared with cereal fibre intake in association to CVD risk factors: A cross-sectional analysis of the National Diet and Nutrition Survey (UK). Public Health Nutr. 2020, 23, 1392–1403. [Google Scholar] [CrossRef]
- Barrett, E.M.; Batterham, M.J.; Beck, E.J. Whole grain and cereal fibre intake in the Australian Health Survey: Associations to CVD risk factors. Public Health Nutr. 2020, 23, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total Population | MACEs (+) | MACEs (-) | p Value |
|---|---|---|---|---|
| n = 339 | n = 105 | n = 234 | ||
| Age (y) † | 59.57 ± 11.18 | 59.42 ± 11.21 | 59.64 ± 11.19 | 0.869 |
| Sex | 0.959 | |||
| Male | 246 (72.6) | 76 (72.4) | 170 (72.6) | |
| Habitation | 0.815 | |||
| City | 197 (58.1) | 62 (59.0) | 135 (57.7) | |
| Rural | 142 (41.9) | 43 (41.0) | 99 (42.3) | |
| Living pattern | 0.035 | |||
| Alone | 29 (8.6) | 14 (13.3) | 15 (6.4) | |
| Not-Alone | 310 (91.4) | 91 (86.7) | 219 (93.6) | |
| Marital status | 0.566 | |||
| Have spouse | 34 (10.0) | 12 (11.4) | 22 (9.4) | |
| No spouse | 305 (90.0) | 93 (88.6) | 212 (90.6) | |
| Educational attainment | 0.200 | |||
| ≤Junior high | 195 (57.5) | 55 (52.4) | 140 (59.8) | |
| ≥Senior high | 144 (42.5) | 50 (47.6) | 94 (40.2) | |
| Personal income (RMB/month) | 0.865 | |||
| ≤2500 | 172 (50.7) | 54 (51.4) | 118 (50.4) | |
| >2500 | 167 (49.3) | 51 (48.6) | 116 (49.6) | |
| Medications | ||||
| Dual Antithrombotic therapy | 0.730 | |||
| No | 10 (2.9) | 4 (3.8) | 6 (2.6) | |
| Yes | 329 (97.1) | 101 (96.2) | 228 (97.4) | |
| Statins | 1.000 | |||
| No | 2 (0.6) | 1 (1.0) | 1 (0.4) | |
| Yes | 337 (99.4) | 104 (99.0) | 233 (99.6) | |
| β-blockers | 0.079 | |||
| No | 96 (28.3) | 23 (21.9) | 73 (31.2) | |
| Yes | 243 (71.7) | 82 (78.1) | 161 (68.8) | |
| ACEI/ARB | 0.795 | |||
| No | 165 (48.7) | 50 (47.6) | 115 (49.1) | |
| Yes | 174 (51.3) | 55 (52.4) | 119 (50.9) | |
| Number of lesions † | 2.49 ± 0.82 | 2.65 ± 0.78 | 2.41 ± 0.83 | 0.015 |
| Number of stents † | 1.38 ± 0.97 | 1.59 ± 1.17 | 1.28 ± 0.85 | 0.006 |
| LS7 † | 6.42 ± 2.08 | 5.83 ± 2.01 | 6.68 ± 2.06 | <0.001 |
| LE8 † | 55.88 ± 12.12 | 51.21 ± 11.81 | 57.97 ± 11.69 | <0.001 |
| Variables | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p Value | HR | 95%CI | p Value | |
| Number of lesions | 1.317 | 1.021–1.699 | 0.034 | 1.312 | 1.024–1.681 | 0.032 |
| Number of stents | 1.197 | 0.999–1.434 | 0.051 | 1.153 | 0.961–1.384 | 0.126 |
| Living pattern (Not-Alone) | 0.611 | 0.348–1.075 | 0.088 | 0.623 | 0.354–1.095 | 0.100 |
| LS7 Score | 0.857 | 0.780–0.943 | 0.001 | - | - | - |
| LE8 Score | - | - | - | 0.964 | 0.948–0.980 | <0.001 |
| Variables | HR | 95%CI | p Value |
|---|---|---|---|
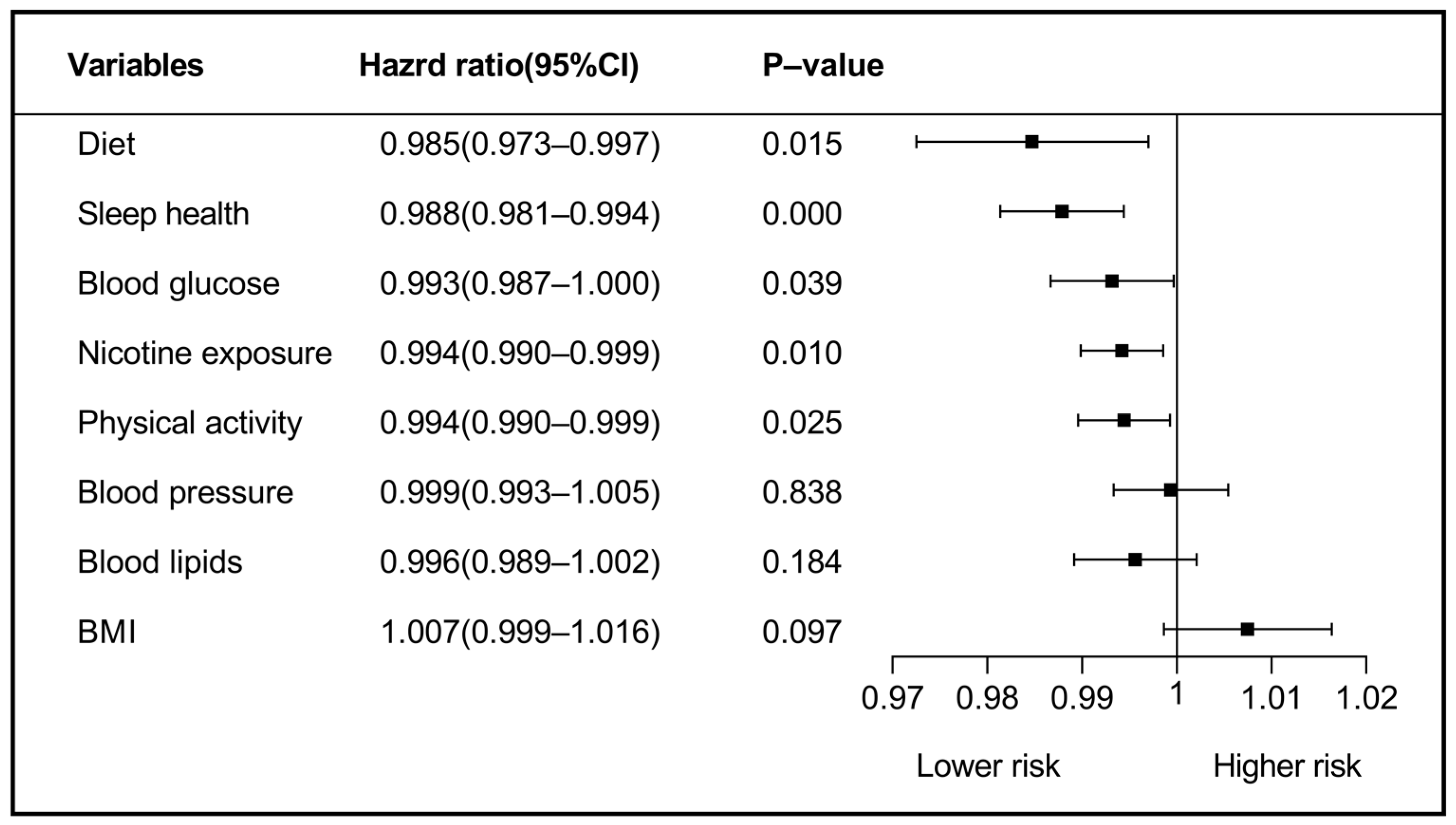
| Blood lipids | |||
| Model 1: Diet a1 | 0.9997 | 0.9996–0.9999 | 0.005 |
| Model 2: Sleep health a2 | 0.9998 | 0.9997–0.9999 | <0.001 |
| Model 3: Nicotine exposure a3 | 0.9999 | 0.9998–0.9999 | 0.028 |
| BMI | |||
| Model 1: Diet b1 | 0.9997 | 0.9995–0.9999 | 0.007 |
| Model 2: Sleep health b2 | 0.9998 | 0.9997–0.9999 | <0.001 |
| Model 3: Nicotine exposure b3 | 0.9999 | 0.9998–0.9999 | 0.019 |
| Model 4: Physical activity b4 | 0.9999 | 0.9998–0.9999 | 0.047 |
| BP | |||
| Model 1: Blood glucose c1 | 0.9998 | 0.9996–0.9999 | 0.009 |
| Model 2: Sleep health c2 | 0.9998 | 0.9996–0.9999 | 0.012 |
| Model 3: Nicotine exposure c3 | 0.9998 | 0.9997–0.9999 | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Ma, X.; Lin, P.; Wang, Y.; Zhao, Z.; Zhang, R.; Yu, B.; Hao, Y. Predictive Value of Cardiovascular Health Score for Health Outcomes in Patients with PCI: Comparison between Life’s Simple 7 and Life’s Essential 8. Int. J. Environ. Res. Public Health 2023, 20, 3084. https://doi.org/10.3390/ijerph20043084
Gao X, Ma X, Lin P, Wang Y, Zhao Z, Zhang R, Yu B, Hao Y. Predictive Value of Cardiovascular Health Score for Health Outcomes in Patients with PCI: Comparison between Life’s Simple 7 and Life’s Essential 8. International Journal of Environmental Research and Public Health. 2023; 20(4):3084. https://doi.org/10.3390/ijerph20043084
Chicago/Turabian StyleGao, Xueqin, Xinrui Ma, Ping Lin, Yini Wang, Zhenjuan Zhao, Rui Zhang, Bo Yu, and Yanhua Hao. 2023. "Predictive Value of Cardiovascular Health Score for Health Outcomes in Patients with PCI: Comparison between Life’s Simple 7 and Life’s Essential 8" International Journal of Environmental Research and Public Health 20, no. 4: 3084. https://doi.org/10.3390/ijerph20043084
APA StyleGao, X., Ma, X., Lin, P., Wang, Y., Zhao, Z., Zhang, R., Yu, B., & Hao, Y. (2023). Predictive Value of Cardiovascular Health Score for Health Outcomes in Patients with PCI: Comparison between Life’s Simple 7 and Life’s Essential 8. International Journal of Environmental Research and Public Health, 20(4), 3084. https://doi.org/10.3390/ijerph20043084






