Effects of Aging on Adsorption of Tetracycline Hydrochloride by Humin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples and Chemical Reagents
2.2. Humin Extraction
2.3. Humin Aging Processing
2.4. Adsorption Experiment
- (1)
- Adsorption kinetics: 0.3 g fresh and aged HM samples were weighed and placed into two 50 mL centrifuge tubes. The tubes were added with 30 mL TC solution (20 mg·L−1) and then sealed with Parafilm and vibrated under 120 rpm at 25 °C. Then, 10 mL samples were collected after a certain time to undergo 5 min vibration under 8000 rpm at 25 °C. Supernatant was collected and filtered with 0.45 μm film before being analyzed with a visible spectrophotometer (SP-195, Spectrum, Shanghai, China) at a 355 nm wavelength in order to determine TC concentration [4]. There were 3 parallel samples, and the results were averaged. Each absorbent was mixed with 0.01 mol·L−1 calcium chloride solution to form a control group to be processed under the same conditions as described above.
- (2)
- Adsorption isotherm: 0.3 g fresh and aged HM samples were taken and then placed into two 50 mL centrifuge tubes to be added with 20 mL TC solutions of different concentrations (in a range of 5–50 mg·L−1). Tubes were sealed with Parafilm and vibrated under 120 rpm at 25 °C for 24 h adsorption. Then, 10 mL samples were collected to undergo 5 min centrifugation under 8000 rpm. In the meanwhile, the supernatant was collected and filtered with 0.45 μm film to determine TC’s light adsorption at a 355 nm wavelength [23]. There were 3 parallel samples, and the results were averaged. Each absorbent was mixed with 0.01 mol·L−1 calcium chloride solution to form a control group to be processed under same conditions as described above.
- (3)
- Adsorption models: pseudo-first-order (1) and pseudo-second-order (2) kinetical models as well as a Weber and Morris intraparticle diffusion model (3) were employed to calculate kinetic parameters. Their mathematical formulas are shown below [7]:
3. Result and Discussion
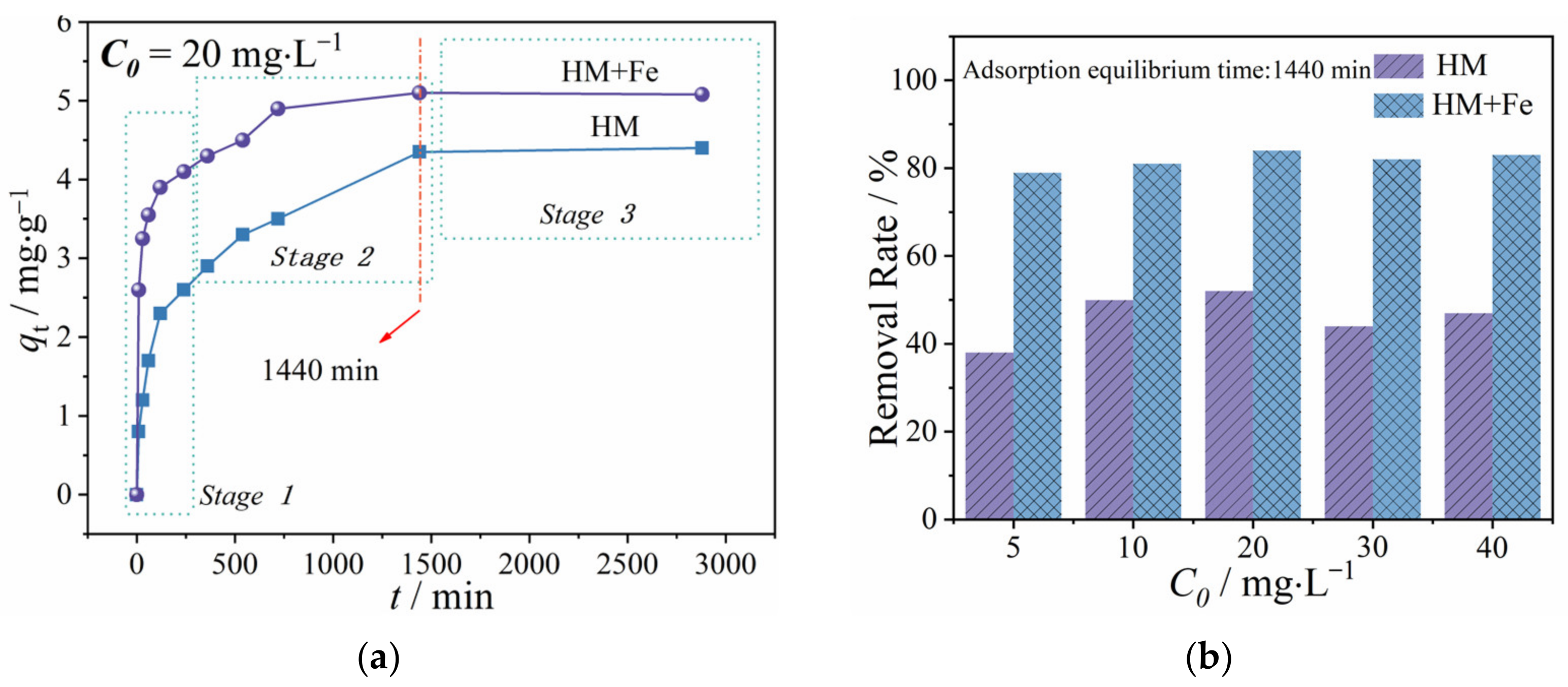
3.1. Time for Achieving Adsorption Equilibrium
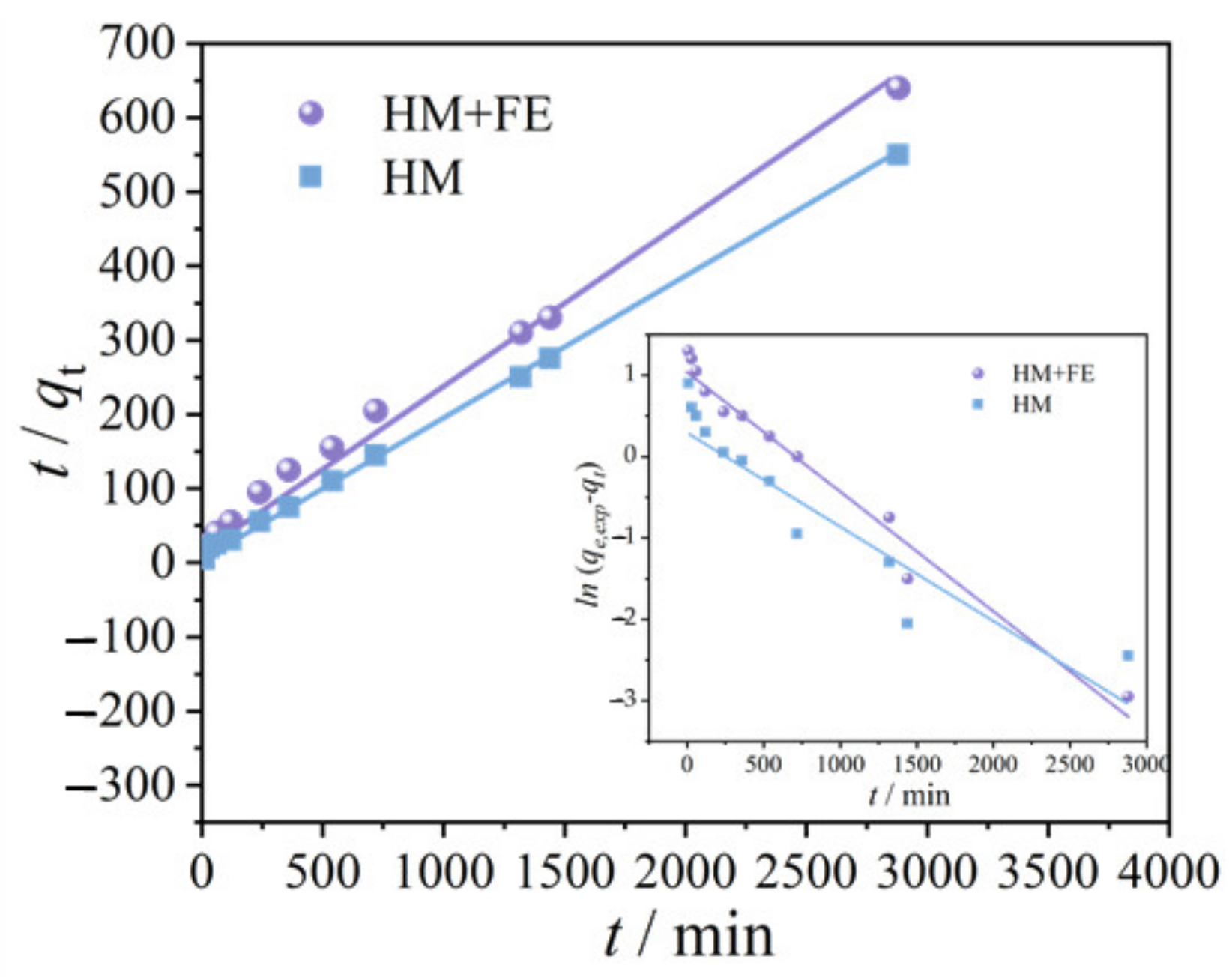
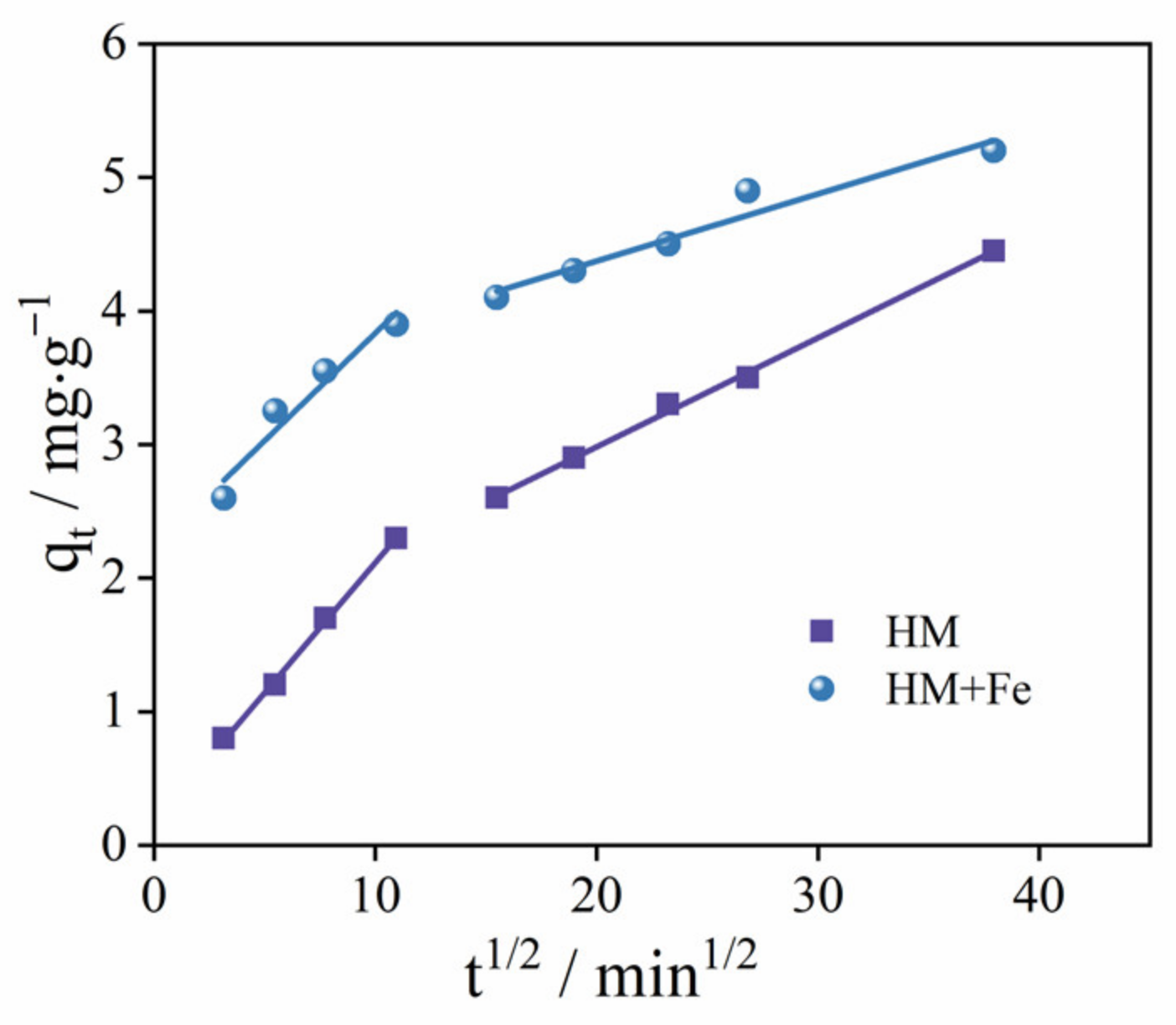
3.2. Adsorption Kinetic Model
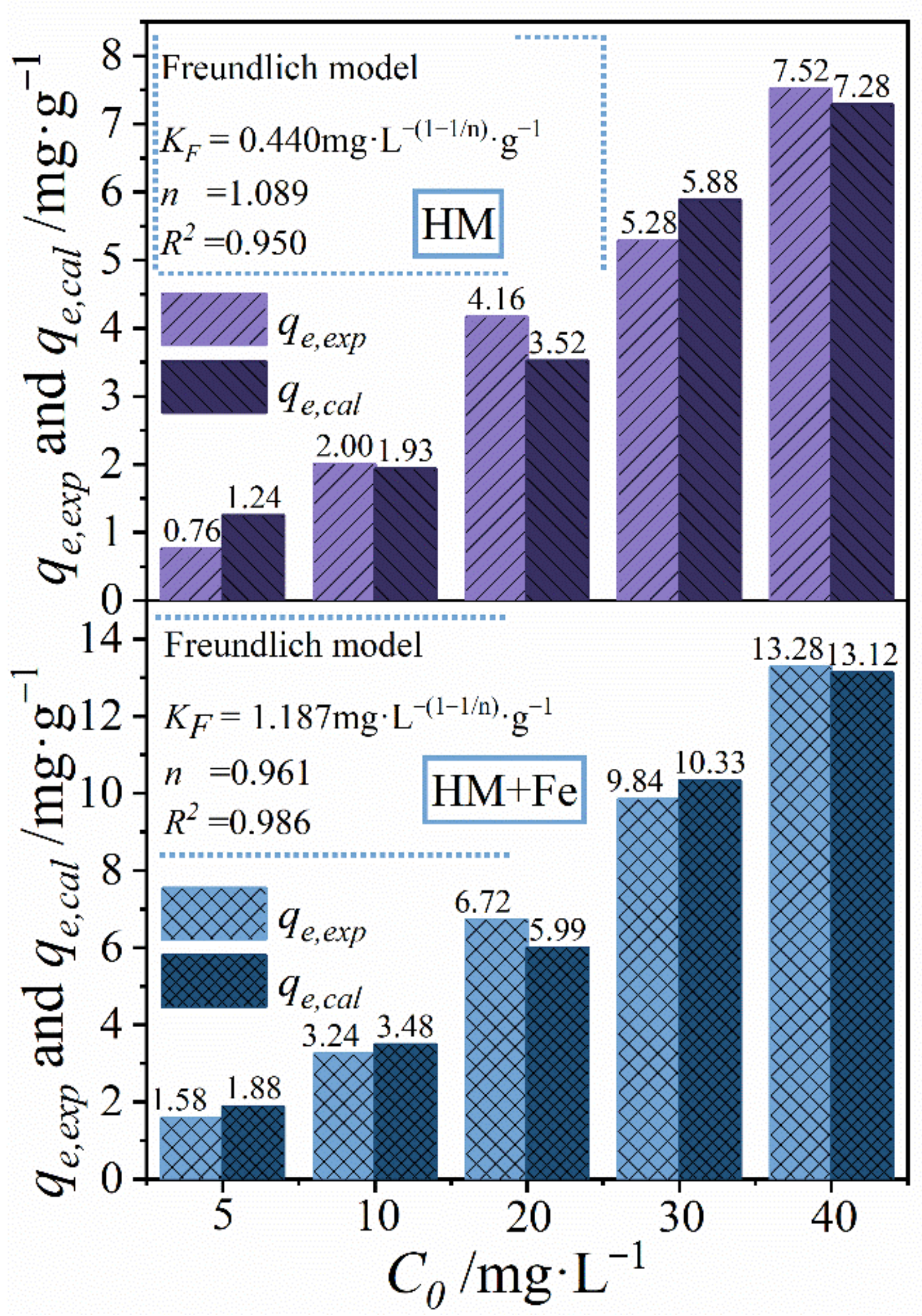
3.3. Adsorption Isotherm Model
3.4. Influence Mechanism of Aging on Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, S.W.; Ma, Y.B.; Xu, M.G. Ageing of added copper in bentonite without and with humic acid. Chem. Speciat. Bioavailab. 2009, 21, 175–184. [Google Scholar] [CrossRef]
- Dar, A.A.; Pan, B.; Qin, J.; Zhu, Q.; Lichtfouse, E.; Usman, M.; Wang, C. Sustainable ferrate oxidation: Reaction chemistry, mechanisms and removal of pollutants in wastewater. Environ. Pollut. 2021, 290, 117957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Wang, P.; Keller, A.A. Sorption and desorption of atrazine and diuron onto water dispersible soil primary size fractions. Water Res. 2009, 43, 1448–1456. [Google Scholar] [CrossRef]
- Delle Site, A. Factors affecting sorption of organic compounds in natural sorbent/water systems and sorption coefficients for selected pollutants. A review. Ournal Phys. Chem. Ref. Data 2001, 30, 187–439. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Zhang, X.; Meng, Q.; Gao, F.; Zhang, Y. Characterization of spectral responses of dissolved organic matter (DOM) for atrazine binding during the sorption process onto black soil. Chemosphere 2017, 180, 531–539. [Google Scholar] [CrossRef]
- Fan, Y.; Zheng, C.; Huo, A.; Wang, Q.; Shen, Z.; Xue, Z.; He, C. Investigating the binding properties between antimony(V) and dissolved organic matter (DOM) under different pH conditions during the soil sorption process using fluorescence and FTIR spectroscopy. Ecotoxicol. Environ. Saf. 2019, 181, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Zhang, X.; Li, R.; Chen, Y.; Meng, Q. Investigating the behavior of binding properties between dissolved organic matter (DOM) and Pb (II) during the soil sorption process using parallel factor analysis (PARAFAC) and two-dimensional correlation spectroscopy (2D-COS). Environ. Sci. Pollut. Res. 2017, 24, 25156–25165. [Google Scholar] [CrossRef]
- See, H.; Bronk, D.A. Changes in C:N ratios and chemical structures of estuarine humic substances during aging. Mar. Chem. 2005, 97, 334–346. [Google Scholar] [CrossRef]
- Yu, Y.; Wan, Y.; Camara, A.Y.; Li, H. Effects of the addition and aging of humic acid-based amendments on the solubility of Cd in soil solution and its accumulation in rice. Chemosphere 2018, 196, 303–310. [Google Scholar] [CrossRef]
- Regitano, J.B.; Koskinen, W.C.; Sadowsky, M.J. Influence of soil aging on sorption and bioavailability of simazine. J. Agric. Food Chem. 2006, 54, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, P.; Stagnitti, F.; Ye, J.; Dong, D.; Zhang, Y.; Li, P. Effects of aging and freeze-thawing on extractability of pyrene in soil. Chemosphere 2009, 76, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Xing, B.; Tai, P.; Yang, K.; Li, H.; Zhang, L.; Li, P. Effect of freeze-thawing cycles on aging behavior of phenanthrene, pyrene and their mixture in soil. Sci. Total Environ. 2013, 452, 246–252. [Google Scholar] [CrossRef]
- An, X.J.; Xiao, B.H.; Di, X.Y.; Dong, H.; Tang, H.M.; Wu, S.S. Effect of Inorganic Ion Precipitation on Hydrophobic Organic Pollutant Adsorption by Non-extracted Soil Organic Matters. Earth Environ. 2016, 5, 572–580. [Google Scholar]
- ESPAUR (English Surveillance Programme for Antimicrobial Utilization and Resistance). 2018. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/759975/ESPAUR_2018_report.pdf (accessed on 15 February 2018).
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.M.; Lv, L.; Qiao, X.L. Progress in Sorption of Antibiotics to Soils. Soils 2009, 41, 703–708. [Google Scholar]
- Dar, A.A.; Usman, M.; Zhang, W.; Zhu, Q.; Pan, B.; Sial, A.; Wang, C. Synergistic Degradation of 2,4,4′-Trihydroxybenzophenone UsingCarbon Quantum Dots, Ferrate, and Visible Light Irradiation: Insights into Electron Generation/Consumption Mechanism. ACS EST Eng. 2022, 2, 1942–1952. [Google Scholar] [CrossRef]
- Dar, A.A.; Chen, J.; Shad, A.; Pan, X.; Yao, J.; Bin-Jumah, M.; Wang, Z. A combined experimental and computational study on the oxidative degradation of bromophenols by Fe(VI) and the formation of self-coupling products. Environ. Pollut. 2020, 258, 11678. [Google Scholar] [CrossRef]
- Cheng, X.; Hou, H.; Li, R.; Zheng, C.; Liu, H. Adsorption behavior of tetracycline on the soil and molecular insight into the effect of dissolved organic matter on the adsorption. J. Soils Sediments 2020, 20, 1846–1857. [Google Scholar] [CrossRef]
- Zhang, C.; Katayama, A. Humin as an electron mediator for microbial reductive dehalogenation. Environ. Sci. Technol. 2012, 46, 6575–6583. [Google Scholar] [CrossRef]
- Wang, H. Effects and Mechanisms of Fe(II/III) on the Degradation of Tetracycline Antibiotics in Water; Beijing Jiaotong University: Beijing, China, 2016; pp. 45–71. [Google Scholar]
- Wierup, M. The Swedish experience of the 1986-year ban of antimicrobial growth promoters, with special reference to animal health, disease prevention, productivity, and usage of antimicrobials. Microb. Drug Resist. 2001, 7, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zheng, C.; Shen, Z.; Lu, Q.; He, C.; Zhang, T.C.; Liu, J. Polyethyleneimine and carbon disulfide co-modified alkaline lignin for removal of Pb2+ ions from water. Chem. Eng. 2019, 359, 265–274. [Google Scholar] [CrossRef]
- Cheng, X.; Zheng, C.; Lu, Q.; Liu, J.; Wang, Q.; Fan, Y.; Zhang, J. Adsorption of Furazolidone, D-Cycloserine, and Chloramphenicol on Granular Activated Carbon Made from Corn Stover. J. Environ. Eng. 2019, 145, 04019038. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Q.; Zheng, C.; He, C. Sorption of Sulfadiazine, Norfloxacin, Metronidazole, and Tetracycline by Granular Activated Carbon: Kinetics, Mechanisms, and Isotherms. Water Air Soil Pollut. 2017, 228, 129. [Google Scholar] [CrossRef]
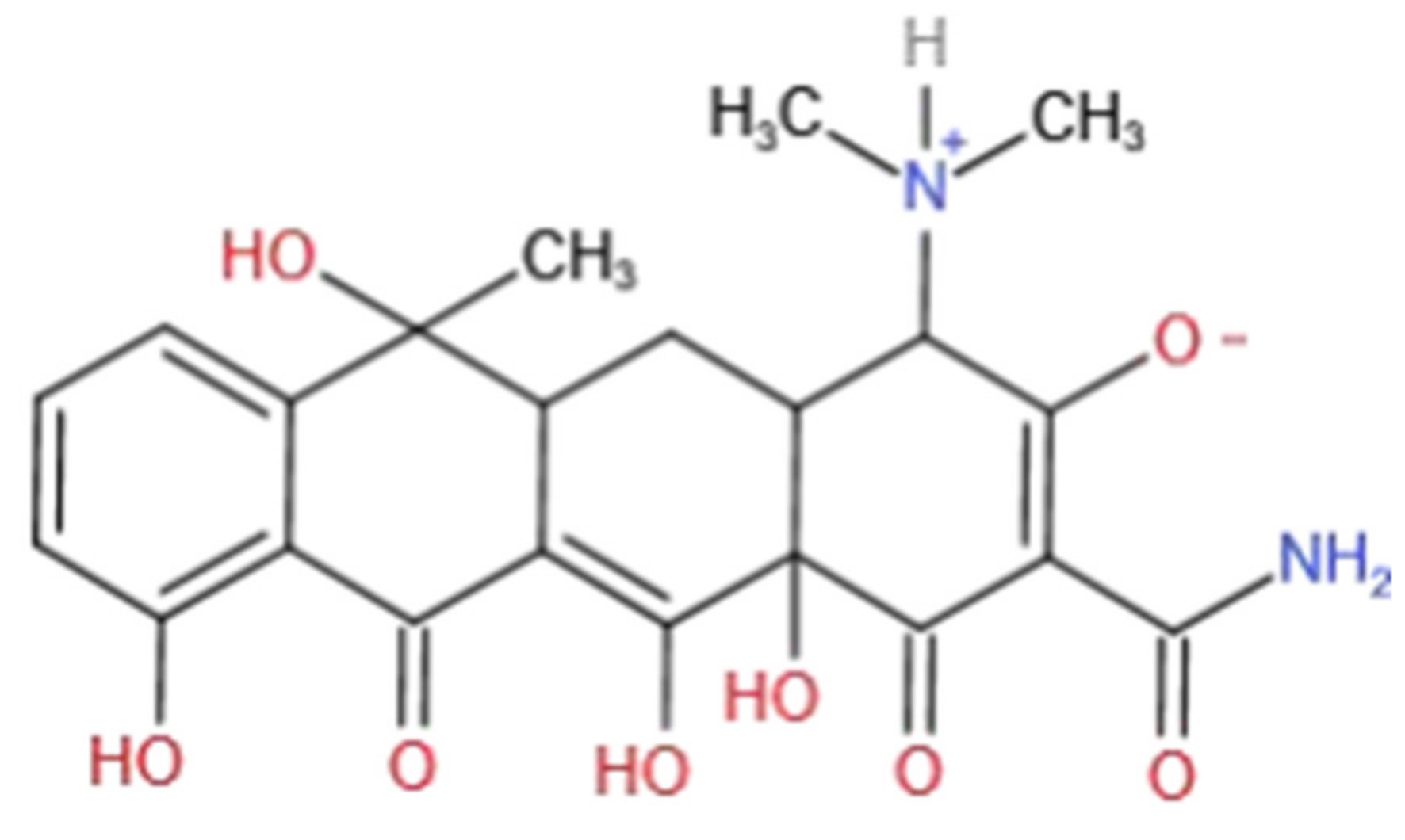
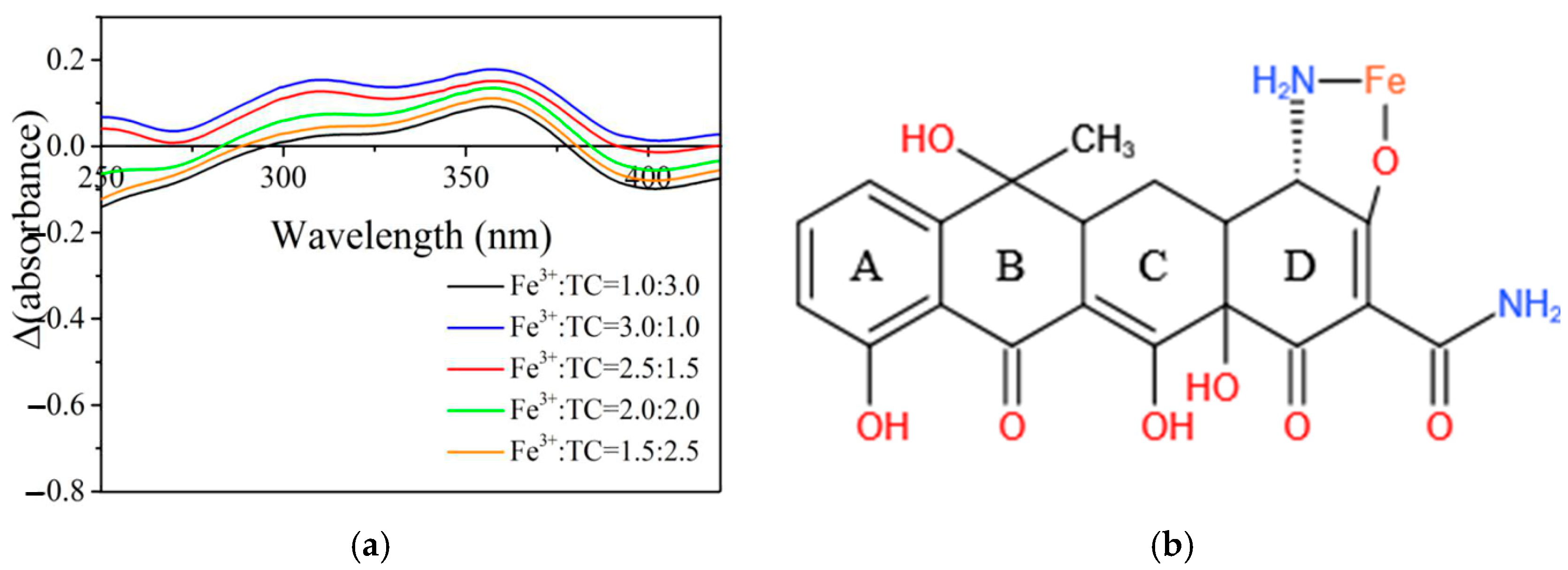
| Model | Parameter | HM | HM+Fe |
|---|---|---|---|
| Pseudo-first-order kinetic model | qe,cal/mg·g−1 | 3.0 | 1.57 |
| K1/h−1 | 0.0015 | 0.0012 | |
| R2 | 0.974 | 0.861 | |
| K2/g mg−1·min−1 | 0.002 | 0.005 | |
| Pseudo-second-order kinetic model | qe,cal/mg·g−1 | 5.263 | 4.650 |
| K2q2e,cal/mg·g−1·min−1 | 0.036 | 0.132 | |
| R2 | 0.997 | 0.996 |
| Model | Parameter | HM | HM+Fe |
|---|---|---|---|
| Weber–Morris intraparticle diffusion model | Kid 1/mg·g−1·min−1/2 | 0.1949 | 0.1611 |
| I1/mg·g−1 | 0.1682 | 2.2238 | |
| R2 | 0.9984 | 0.9426 | |
| Kid 2/mg·g−1·min−1/2 | 0.0816 | 0.0503 | |
| I2/mg·g−1 | 1.3507 | 3.3687 | |
| R2 | 0.9977 | 0.9460 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, H.; Xu, G.; He, F.; Pan, H. Effects of Aging on Adsorption of Tetracycline Hydrochloride by Humin. Int. J. Environ. Res. Public Health 2023, 20, 2901. https://doi.org/10.3390/ijerph20042901
Hou H, Xu G, He F, Pan H. Effects of Aging on Adsorption of Tetracycline Hydrochloride by Humin. International Journal of Environmental Research and Public Health. 2023; 20(4):2901. https://doi.org/10.3390/ijerph20042901
Chicago/Turabian StyleHou, Hongbo, Guoliang Xu, Fei He, and Hua Pan. 2023. "Effects of Aging on Adsorption of Tetracycline Hydrochloride by Humin" International Journal of Environmental Research and Public Health 20, no. 4: 2901. https://doi.org/10.3390/ijerph20042901
APA StyleHou, H., Xu, G., He, F., & Pan, H. (2023). Effects of Aging on Adsorption of Tetracycline Hydrochloride by Humin. International Journal of Environmental Research and Public Health, 20(4), 2901. https://doi.org/10.3390/ijerph20042901






