Removal of Heavy Metals from Mine Tailings in Central Chile Using Solidago chilensis Meyen, Haplopappus foliosus DC, and Lycium chilense Miers ex Bertero
Abstract
1. Introduction
2. Materials and Methods
2.1. Chilean Bushes
2.2. Quality Control and Assurance
2.3. Tailings Samples
2.4. Evaluation of Phytoremediation
2.5. Phytoremediation Tests
2.6. Statistical Treatment of the Data
3. Results
3.1. Blank Samples and Initial Concentrations
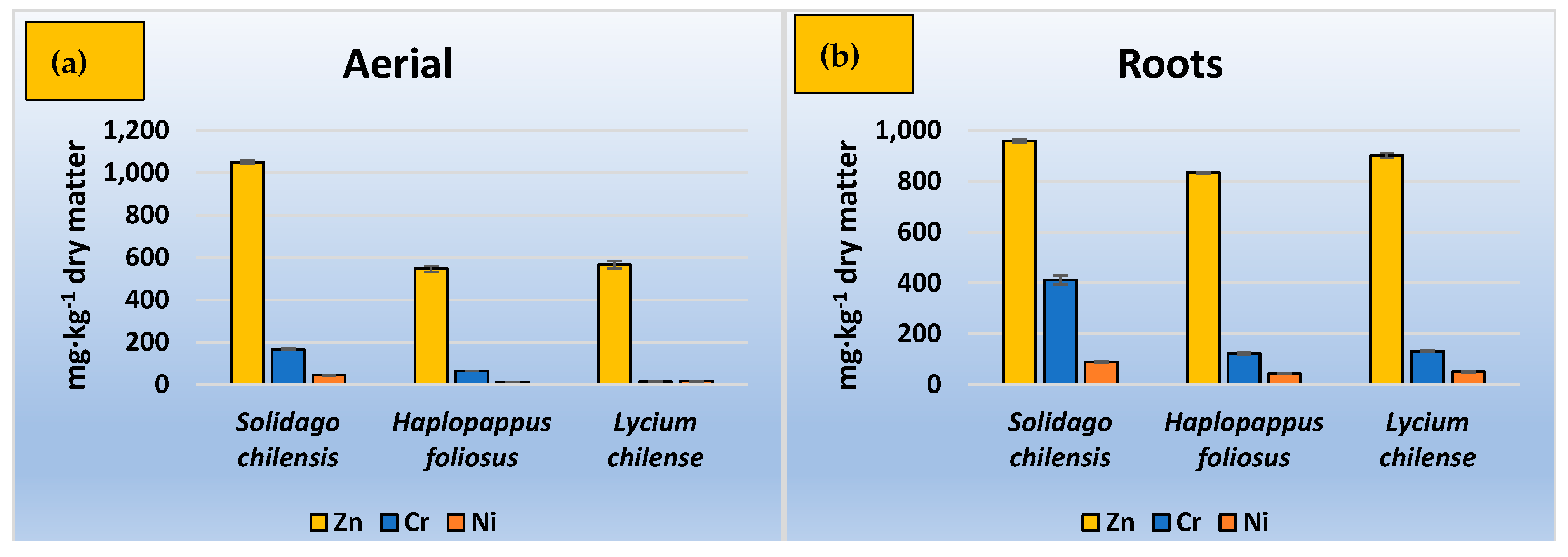
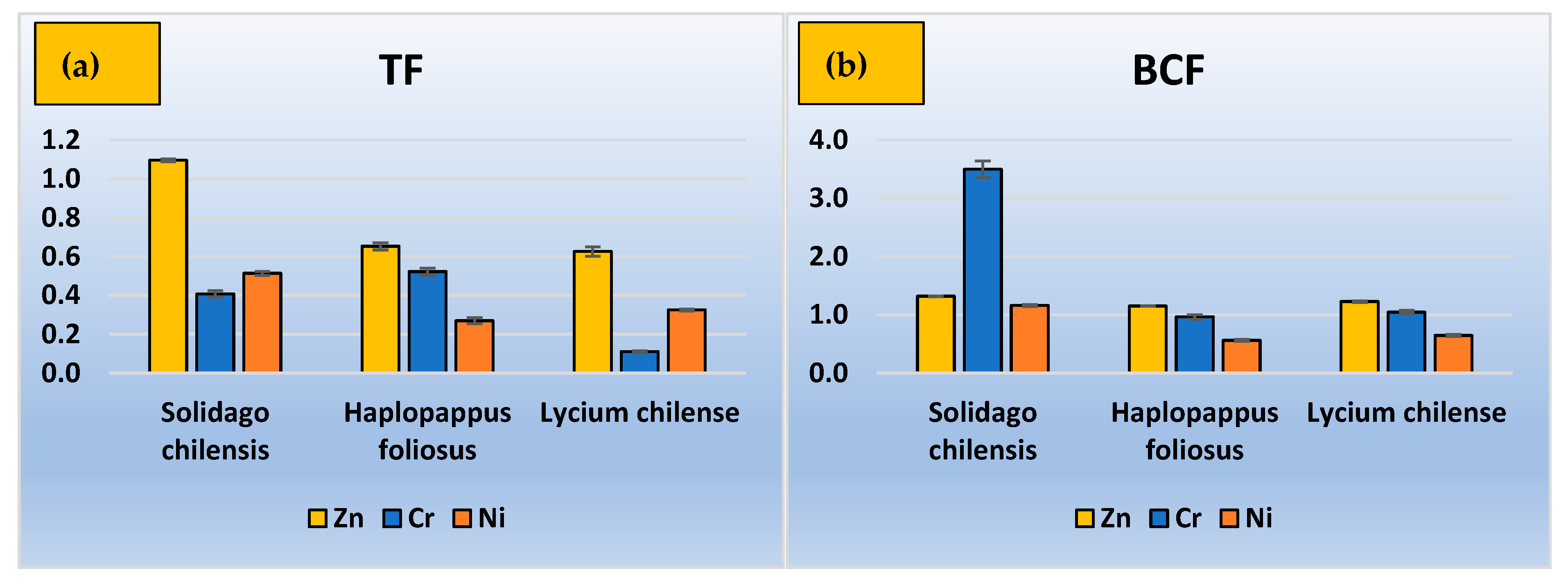
3.2. TF, BCF, and Removal Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministerio de Minería. Reglamento para la aprobación de proyectos de diseño, construcción, operación y cierre de los depósitos de relaves; Decreto Supremo N°248; Ministerio de Minería: Santiago, Chile, 2017.
- Plan Nacional de Depósitos de Relaves para una Minería Sostenible, 1st ed.; Ministerio de Minería, Ministerio de Chile: Santiago, Chile, 2019.
- Neaman, A.; Valenzuela, P.; Tapia-Gatica, J.; Selles, I.; Novoselov, A.; Dovletyarova, E.A.; Yáñez, C.; Krutyakov, Y.A.; Stuckey, J.W. Chilean regulations on metal-polluted soils: The need to advance from adapting foreign laws towards developing sovereign legislation. Environ. Res. 2020, 185, 109429. [Google Scholar] [CrossRef]
- David, A.J.; Leventhal, J.S. Chapter 2: Bioavailability of metals. In Preliminary Compilation of Descriptive Geoenvironmental Mineral Deposit Models; Open-File Report; Department of the Interior, U.S. Geological Survey: Reston, VA, USA, 1995; pp. 95–831. [Google Scholar]
- Karaca, O.; Cameselle, C.; Reddy, K.R. Mine tailing disposal sites: Contamination problems, remedial options and phytocaps for sustainable remediation. Rev. Environ. Sci. Bio. Technol. 2018, 17, 205–228. [Google Scholar] [CrossRef]
- Rodríguez, L.; Ruiz, E.; Alonso-Azcárate, J.; Rincón, J. Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J. Environ. Manag. 2009, 90, 1106–1116. [Google Scholar] [CrossRef]
- Radziemska, M.; Vaverková, M.D.; Baryła, A. Phytostabilization—Management Strategy for Stabilizing Trace Elements in Contaminated Soils. Int. J. Environ. Res. Public Health 2017, 14, 958. [Google Scholar] [CrossRef]
- Huang, L.-M.; Deng, C.-B.; Huang, N.; Huang, X.-J. Multivariate statistical approach to identify heavy metal sources in agricultural soil around an abandoned Pb–Zn mine in Guangxi Zhuang Autonomous Region, China. Environ. Earth Sci. 2013, 68, 1331–1348. [Google Scholar] [CrossRef]
- Sun, Z.; Xie, X.; Wang, P.; Hu, Y.; Cheng, H. Heavy metal pollution caused by small-scale metal ore mining activities: A case study from a polymetallic mine in South China. Sci. Total. Environ. 2018, 639, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Concas, A.; Ardau, C.; Cristini, A.; Zuddas, P.; Cao, G. Mobility of heavy metals from tailings to stream waters in a mining activity contaminated site. Chemosphere 2006, 63, 244–253. [Google Scholar] [CrossRef]
- Parviainen, A. Tailings Mineralogy and Geochemistry at the Abandoned Haveri Au–Cu Mine, SW Finland. Mine Water Environ. 2009, 28, 291–304. [Google Scholar] [CrossRef]
- Naz, A.; Chowdhury, A.; Mishra, B.K.; Gupta, S.K. Metal pollution in water environment and the associated human health risk from drinking water: A case study of Sukinda chromite mine, India. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1433–1455. [Google Scholar] [CrossRef]
- Naz, A.; Mishra, B.K.; Gupta, S.K. Human Health Risk Assessment of Chromium in Drinking Water: A Case Study of Sukinda Chromite Mine, Odisha, India. Expo. Health 2016, 8, 253–264. [Google Scholar] [CrossRef]
- Naz, A.; Chowdhury, A.; Mishra, B.K.; Karthikeyan, K. Distribution of heavy metals and associated human health risk in mine, agricultural and roadside soils at the largest chromite mine of India. Environ. Geochem. Health. 2018, 40, 2155–2175. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.; Chowdhury, A.; Maiti, S.K. Ecological risk assessment of mercury and other heavy metals in soils of coal mining area: A case study from the eastern part of a Jharia coal field, India. Hum. Ecol. Risk Assess. Int. J. 2017, 23, 767–787. [Google Scholar] [CrossRef]
- Du, Y.; Chen, L.; Ding, P.; Liu, L.; He, Q.; Chen, B.; Duan, Y. Different exposure profile of heavy metal and health risk between residents near a Pb-Zn mine and a Mn mine in Huayuan county, South China. Chemosphere 2019, 216, 352–364. [Google Scholar] [CrossRef]
- Servicio Nacional de Geología y Minería. Geoquímica de Superficie de Depósitos de Relaves de Chile; Servicio Nacional de Geología y Minería: Santiago, Chile, 2018.
- Naz, A.; Chowdhury, A.; Chandra, R.; Mishra, B.K. Potential human health hazard due to bioavailable heavy metal exposure via consumption of plants with ethnobotanical usage at the largest chromite mine of India. Environ. Geochem. Health 2020, 42, 4213–4231. [Google Scholar] [CrossRef]
- Eisler, R. Cadmium Hazard to Fish, Wildlife, and Invertebrates: A synoptic Review. Contaminant Hazardous Reviews; Report 2; Fish and Wildlife Service, US Department of the Interior: Atlanta, GA, USA, 1985; Volume 85, pp. 1–30.
- Jarup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Toxicological Profile for Chromium; U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012.
- WHO. Permissible Limits of Heavy Metals in Soil and Plants; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Oliveira, H. Chromium as an Environmental Pollutant: Insights on Induced Plant Toxicity. J. Bot. 2012, 2012, 375843. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants: Tansley review. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Sadeghzadeh, B. A review of zinc nutrition and plant breeding. J. Soil Sci. Plant Nutr. 2013, 13, 905–927. [Google Scholar] [CrossRef]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in soils, water and food crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Aamer, M.; Nawaz, M.; Ali, A.; Khan, M.I.; Khan, T.A. Nickel toxicity in plants: Reasons, toxic effects, tolerance mechanisms, and remediation possibilities—A review. Environ. Sci. Pollut. Res. 2019, 26, 12673–12688. [Google Scholar] [CrossRef]
- Lund, L.J.; Page, A.L.; Sposito, G. Determination and production of chemical forms of trace metals in sewage sludges and sludge-amended soils, Final technical reportUnited States Environmental protection Agency, Cincinnati, Ohio. J. Environ. Qual. 1980, 23, 33–38. [Google Scholar]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation techniques for removal of heavy metals from the soil contaminated through different sources: A review. Environ. Sci. Pollut. Res. 2019, 27, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Shmaefsky, B. (Ed.) Phytoremediation: In-situ Applications, Concepts and Strategies in Plant Sciences; Springer Nature: Berlin, Germany, 2020. [Google Scholar] [CrossRef]
- Chaabani, S.; Abdelmalek-Babbou, C.; Ben Ahmed, H.; Chaabani, A.; Sebei, A. Phytoremediation assessment of native plants growing on Pb–Zn mine site in Northern Tunisia. Environ. Earth Sci. 2017, 76, 585. [Google Scholar] [CrossRef]
- Murtić, S.; Zahirović, Ć; Čivić, H.; Sijahović, E.; Jurković, J.; Avdić, J.; Šahinović, E.; Podrug, A. Phytoaccumulation of heavy metals in native plants growing on soils in the Spreča river valley, Bosnia and Herzegovina. Plant Soil Environ. 2021, 67, 533–540. [Google Scholar] [CrossRef]
- Chandra, R.; Kumar, V.; Tripathi, S.; Sharma, P. Heavy metal phytoextraction potential of native weeds and grasses from endocrine-disrupting chemicals rich complex distillery sludge and their histological observations during in-situ phytoremediation. Ecol. Eng. 2018, 111, 143–156. [Google Scholar] [CrossRef]
- Lam, E.J.; Cánovas, M.; Gálvez, M.E.; Montofré, L.; Keith, B.F.; Faz, Á. Evaluation of the phytoremediation potential of native plants growing on a copper mine tailing in northern Chile. J. Geochem. Explor. 2017, 182, 210–217. [Google Scholar] [CrossRef]
- Milla-Moreno, E.; Guy, R.D. Growth response, uptake and mobilization of metals in native plant species on tailings at a Chilean copper mine. Int. J. Phytoremediation 2021, 23, 539–547. [Google Scholar] [CrossRef]
- Chileflora.com. Available online: http://www.chileflora.com/Shome.htm (accessed on 21 February 2022).
- U.S. Environmental Protection Agency. SW-846 Test Method 9045D: Soil and Waste pH. Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-9045d-soil-and-waste-ph (accessed on 21 February 2022).
- Sernageomin. Servicio Nacional de Geología y Minería (Chile). Available online: https://www.sernageomin.cl/datos-publicos-deposito-de-relaves/ (accessed on 21 February 2022).
- Mishra, T.; Pandey, V.C. Chapter 16—Phytoremediation of Red Mud Deposits through Natural Succession. In Phytomanagement of Polluted Sites, Market Opportunities in Sustainable Phytoremediation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 409–424. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, Y.; Chen, Y.; Zhang, Z. Transfer of heavy metals from the polluted rhizosphere soil to Celosia argentea L. in copper mine tailings. Hortic. Environ. Biotechnol. 2017, 58, 93–100. [Google Scholar] [CrossRef]
- Nirola, R.; Megharaj, M.; Palanisami, T.; Aryal, R.; Venkateswarlu, K.; Naidu, R. Evaluation of metal uptake factors of native trees colonizing an abandoned copper mine—A quest for phytostabilization. J. Sustain. Min. 2015, 14, 115–123. [Google Scholar] [CrossRef]
- Afonso, T.F.; Demarco, C.F.; Pieniz, S.; Quadro, M.S.; Camargo, F.A.; Andreazza, R. Bioprospection of indigenous flora grown in copper mining tailing area for phytoremediation of metals. J. Environ. Manag. 2020, 256, 109953. [Google Scholar] [CrossRef]
- Mellem, J.J.; Baijnath, H.; Odhav, B. Translocation and accumulation of Cr, Hg, As, Pb, Cu and Ni byAmaranthus dubius(Amaranthaceae) from contaminated sites. J. Environ. Sci. Health Part A 2009, 44, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Chamba, I.; Gazquez, M.J.; Selvaraj, T.; Calva, J.; Toledo, J.; Armijos, C. Selection of a suitable plant for phytoremediation in mining artisanal zones. Int. J. Phytoremediation 2016, 18, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Rezania, S.; Taib, S.M.; Din, M.F.M.; Dahalan, F.A.; Kamyab, H. Comprehensive review on phytotechnology: Heavy metals removal by diverse aquatic plants species from wastewater. J. Hazard. Mater. 2016, 318, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Wuana, R.; Okieimen, F. Phytoremediation Potential of Maize (Zea mays L.). A Review. Afr. J. Gen. Agric. 2010, 6, 275–287. [Google Scholar]
- Lazo, P.; Lazo, A. Assessment of Native and Endemic Chilean Plants for Removal of Cu, Mo and Pb from Mine Tailings. Minerals 2020, 10, 1020. [Google Scholar] [CrossRef]
- Rijkswaterstaat Ministry of Infrastructure and Environment. Into Dutch Soils; Rijkswaterstaat: Utrecht, The Netherlands, 2014.
- Ministry of Housing Netherlands. Dutch Intervention Values for Soil Remediation (Report HQ 94-021); Environmental Quality Objectives in the Netherlands; Ministry of Housing: Hague, The Netherlands, 1994.
- Lazo, A.; Lazo, P.; Urtubia, A.; Lobos, M.G.; Hansen, H.K.; Gutiérrez, C. An Assessment of the Metal Removal Capability of Endemic Chilean Species. Int. J. Environ. Res. Public Health 2022, 19, 3583. [Google Scholar] [CrossRef]
- Gupta, K.D. Plant-Based Remediation Processes; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2013; Volume 35. [Google Scholar]
- Susana, R.G.; Enrique, M.N.; Inmaculada, V.B.; Susana, R.F. Accumulation and tolerance characteristics of chromium in a cordgrass chromium-hyperaccumulator, Spartina argentinensis. J. Hazard. Mater. 2011, 185, 862–869. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef]
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Davies, F.T.; Puryear, J.D.; Newton, R.J.; Egilla, J.N.; Grossi, J.A.S. Mycorrhizal fungi increase chromium uptake by sunflower plants: Influence on tissue mineral concentration, growth, and gas exchange. J. Plant Nutr. 2002, 25, 2389–2407. [Google Scholar] [CrossRef]
- Calder, L.M. Chromium contamination of groundwater. In Chromium in the Natural and Human Environments; Nriagu, J.O., Nieboer, E., Eds.; John Wiley & Sons: New York, NY, USA, 1988; pp. 215–229. [Google Scholar]
- Stackhouse, R.; Benson, W.H. The effect of humic acid on the toxicity and bioavailability of trivalent chromium. Ecotoxicol. Environ. Saf. 1989, 17, 105–111. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Value | Units |
|---|---|---|
| Specific gravity | 2.78 ± 0.25 | - |
| pH | 7.30 ± 0.10 | - |
| Solid concentration in weight | 82.00 ± 1.00 | % |
| Granulometry d50 | 0.046 ± 0.001 | µm |
| As | 90 ± 4.53 | mg·kg−1 dry tailing |
| Hg | Under detection limit | mg·kg−1 dry tailing |
| Cu | 1582.22 ± 78.31 | mg·kg−1 dry tailing |
| Pb | 228.15 ± 2.79 | mg·kg−1 dry tailing |
| Zn | 869.80 ± 31.54 | mg·kg−1 dry tailing |
| Ni | 94.64 ± 2.57 | mg·kg−1 dry tailing |
| Mo | 3.86 ± 0.17 | mg·kg−1 dry tailing |
| Cd | Under detection limit | - |
| Cr | 154.63 ± 5.41 | mg·kg−1 dry tailing |
| Element | Concentration mg·kg−1 Dry Weight ± Standard Deviation | ||
|---|---|---|---|
| Solidago chilensis | Haplopappus foliosus | Lycium chilense | |
| Cr aerial part t = 0 | <d.l. | <d.l. | <d.l. |
| Cr aerial part t = 7 months | <d.l. | <d.l. | <d.l. |
| Cr roots t = 0 | 0.01 ± 0.00 | 0.01 ± 0.00 | <d.l. |
| Cr roots t = 7 months | 0.01 ± 0.00 | 0.01 ± 0.00 | <d.l. |
| Ni aerial part t = 0 | 3.00 ± 0.11 | 4.59 ± 0.02 | 5.03 ± 0.02 |
| Ni aerial part t = 7 months | 3.25 ± 0.09 | 5.01 ± 0.04 | 5.17 ± 0.17 |
| Ni roots t = 0 | 3.58 ± 0.13 | 5.53 ± 0.14 | 6.46 ± 0.13 |
| Ni roots t = 7 months | 3.67 ± 0.02 | 6.23 ± 0.18 | 6.59 ± 0.10 |
| Zn aerial part t = 0 | 15.04 ± 0.11 | 14.32 ± 0.22 | 15.11 ± 0.09 |
| Zn aerial part t = seven months | 15.15 ± 0.09 | 15.45 ± 0.00 | 15.33 ± 0.19 |
| Zn roots t = 0 | 16.45 ± 0.28 | 18.01 ± 0.22 | 15.98 ± 0.08 |
| Zn roots t = 7 months | 17.05 ± 0.20 | 18.11 ± 0.28 | 16.07 ± 0.12 |
| Parameter | Target Value | Maximum Value for Residential Land Use | Intervention Value | Units |
|---|---|---|---|---|
| Zn | 140 | 200 | 720 | mg·kg−1 dry sediment |
| Ni | 35 | 39 | 100 | mg·kg−1 dry sediment |
| Cr | 100 | - | 380 | mg·kg−1 dry sediment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazo, P.; Lazo, A.; Hansen, H.K.; Ortiz-Soto, R.; Hansen, M.E.; Arévalo, F.; Gutiérrez, C. Removal of Heavy Metals from Mine Tailings in Central Chile Using Solidago chilensis Meyen, Haplopappus foliosus DC, and Lycium chilense Miers ex Bertero. Int. J. Environ. Res. Public Health 2023, 20, 2749. https://doi.org/10.3390/ijerph20032749
Lazo P, Lazo A, Hansen HK, Ortiz-Soto R, Hansen ME, Arévalo F, Gutiérrez C. Removal of Heavy Metals from Mine Tailings in Central Chile Using Solidago chilensis Meyen, Haplopappus foliosus DC, and Lycium chilense Miers ex Bertero. International Journal of Environmental Research and Public Health. 2023; 20(3):2749. https://doi.org/10.3390/ijerph20032749
Chicago/Turabian StyleLazo, Pamela, Andrea Lazo, Henrik K. Hansen, Rodrigo Ortiz-Soto, Marcela E. Hansen, Felipe Arévalo, and Claudia Gutiérrez. 2023. "Removal of Heavy Metals from Mine Tailings in Central Chile Using Solidago chilensis Meyen, Haplopappus foliosus DC, and Lycium chilense Miers ex Bertero" International Journal of Environmental Research and Public Health 20, no. 3: 2749. https://doi.org/10.3390/ijerph20032749
APA StyleLazo, P., Lazo, A., Hansen, H. K., Ortiz-Soto, R., Hansen, M. E., Arévalo, F., & Gutiérrez, C. (2023). Removal of Heavy Metals from Mine Tailings in Central Chile Using Solidago chilensis Meyen, Haplopappus foliosus DC, and Lycium chilense Miers ex Bertero. International Journal of Environmental Research and Public Health, 20(3), 2749. https://doi.org/10.3390/ijerph20032749







