SARS-CoV-2 Infection in Pregnancy: Clues and Proof of Adverse Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- Age > 18 years,
- Unvaccinated pregnant women positive for SARS-CoV-2 infection prior or at the admission (Case group),
- Unvaccinated pregnant women without any previous or ongoing SARS-CoV-2 infection (Control group).
- Exclusion criteria were:
- Age < 18 years,
- Women positive for other infections during pregnancy, such as HIV, Hepatitis, Chickenpox and those related to the STORCH group (Syphilis, Toxoplasmosis, Rubella, Cytomegalovirus, Herpes Simplex virus, Varicella Zoster, Parvovirus B19).
2.2. Sample Storage and Analysis
2.3. SARS-CoV-2 RNA Detection
2.4. Anti-SARS-CoV-2 Antibody Determination
2.5. Histologic Examination of Placental Tissues
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abu-Raya, B.; Madhi, S.A.; Omer, S.B.; Amirthalingam, G.; Giles, M.L.; Flanagan, K.L.; Zimmermann, P.; O’Ryan, M.; Safadi, M.A.; Papaevangelou, V.; et al. Global Perspectives on Immunization Against SARS-CoV-2 during Pregnancy and Priorities for Future Research: An International Consensus Paper from the World Association of Infectious Diseases and Immunological Disorders. Front. Immunol. 2021, 12, 808064. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19); StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Weinberger, B. Vaccines and Vaccination against SARS-CoV-2: Considerations for the Older Population. Vaccines 2021, 9, 1435. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Madjunkov, M.; Dviri, M.; Librach, C. A comprehensive review of the impact of COVID-19 on human reproductive biology, assisted reproduction care and pregnancy: A Canadian perspective. J. Ovarian Res. 2020, 13, 140. [Google Scholar] [CrossRef]
- Verma, S.; Carter, E.B.; Mysorekar, I.U. SARS-CoV-2 and pregnancy: An invisible enemy? Am. J. Reprod. Immunol. 2020, 84, e13308. [Google Scholar] [CrossRef] [PubMed]
- Metz, T.D.; Clifton, R.G.; Hughes, B.L.; Sandoval, G.J.; Grobman, W.A.; Saade, G.R.; Manuck, T.A.; Longo, M.; Sowles, A.; Clark, K.; et al. Association of SARS-CoV-2 Infection with Serious Maternal Morbidity and Mortality from Obstetric Complications. JAMA 2022, 327, 748–759. [Google Scholar] [CrossRef]
- Chi, J.; Gong, W.; Gao, Q. Clinical characteristics and outcomes of pregnant women with COVID-19 and the risk of vertical transmission: A systematic review. Arch. Gynecol. Obstet. 2021, 303, 337–345. [Google Scholar] [CrossRef]
- Gao, Y.; Ye, L.; Zhang, J.; Yin, Y.; Liu, M.; Yu, H.; Zhou, R. Clinical features and outcomes of pregnant women with COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2020, 20, 564. [Google Scholar] [CrossRef]
- Matar, R.; Alrahmani, L.; Monzer, N.; Debiane, L.G.; Berbari, E.; Fares, J.; Fitzpatrick, F.; Murad, M.H. Clinical Presentation and Outcomes of Pregnant Women with Coronavirus Disease 2019: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2021, 72, 521–533. [Google Scholar] [CrossRef]
- Bellos, I.; Pandita, A.; Panza, R. Maternal and perinatal outcomes in pregnant women infected by SARS-CoV-2: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 194–204. [Google Scholar] [CrossRef]
- Chi, H.; Chiu, N.C.; Tai, Y.L.; Chang, H.Y.; Lin, C.H.; Sung, Y.H.; Tseng, C.Y.; Liu, L.Y.; Lin, C.Y. Clinical features of neonates born to mothers with coronavirus disease-2019: A systematic review of 105 neonates. J. Microbiol. Immunol. Infect. 2021, 54, 69–76. [Google Scholar] [CrossRef]
- Dashraath, P.; Wong, J.L.J.; Lim, M.X.K.; Lim, L.M.; Li, S.; Biswas, A.; Choolani, M.; Mattar, C.; Su, L.L. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020, 222, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Di Toro, F.; Gjoka, M.; Di Lorenzo, G.; De Santo, D.; De Seta, F.; Maso, G.; Risso, F.M.; Romano, F.; Wiesenfeld, U.; Levi-D’Ancona, R.; et al. Impact of COVID-19 on maternal and neonatal outcomes: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, D.J.; Rasmussen, S.A. An update on COVID-19 and pregnancy. Am. J. Obstet. Gynecol. 2022, 226, 177–186. [Google Scholar] [CrossRef]
- Thomas, P.; Alexander, P.E.; Ahmed, U.; Elderhorst, E.; El-Khechen, H.; Mammen, M.J.; Debono, V.B.; Aponte Torres, Z.; Aryal, K.; Brocard, E.; et al. Vertical transmission risk of SARS-CoV-2 infection in the third trimester: A systematic scoping review. J. Matern.-Fetal Neonatal Med. 2022, 35, 2387–2394. [Google Scholar] [CrossRef]
- Timircan, M.; Bratosin, F.; Vidican, I.; Suciu, O.; Tirnea, L.; Avram, V.; Marincu, I. Exploring Pregnancy Outcomes Associated with SARS-CoV-2 Infection. Medicina 2021, 57, 796. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Von Dadelszen, P.; Draycott, T.; Ugwumadu, A.; O’Brien, P.; Magee, L. Change in the Incidence of Stillbirth and Preterm Delivery During the COVID-19 Pandemic. JAMA 2020, 324, 705. [Google Scholar] [CrossRef]
- Mendoza, M.; Garcia-Ruiz, I.; Maiz, N.; Rodo, C.; Garcia-Manau, P.; Serrano, B.; Lopez-Martinez, R.; Balcells, J.; Fernandez-Hidalgo, N.; Carreras, E.; et al. Pre-eclampsia-like syndrome induced by severe COVID-19: A prospective observational study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 1374–1380. [Google Scholar] [CrossRef]
- Smith, V.; Seo, D.; Warty, R.; Payne, O.; Salih, M.; Chin, K.L.; Ofori-Asenso, R.; Krishnan, S.; Da Silva Costa, F.; Vollenhoven, B.; et al. Maternal and neonatal outcomes associated with COVID-19 infection: A systematic review. PLoS ONE 2020, 15, e0234187. [Google Scholar] [CrossRef]
- Wong, S.F.; Chow, K.M.; Leung, T.N.; Ng, W.F.; Ng, T.K.; Shek, C.C.; Ng, P.C.; Lam, P.W.Y.; Ho, L.C.; To, W.W.K.; et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004, 191, 292–297. [Google Scholar] [CrossRef]
- Buonsenso, D.; Raffaelli, F.; Tamburrini, E.; Biasucci, D.G.; Salvi, S.; Smargiassi, A.; Inchingolo, R.; Scambia, G.; Lanzone, A.; Testa, A.C.; et al. Clinical role of lung ultrasound for diagnosis and monitoring of COVID-19 pneumonia in pregnant women. Ultrasound Obstet. Gynecol. 2020, 56, 106–109. [Google Scholar] [CrossRef]
- Porpora, M.G.; Merlino, L.; Masciullo, L.; D’Alisa, R.; Brandolino, G.; Galli, C.; De Luca, C.; Pecorini, F.; Fonsi, G.B.; Mingoli, A.; et al. Does Lung Ultrasound Have a Role in the Clinical Management of Pregnant Women withSARS-CoV-2 Infection? Int. J. Environ. Res. Public Health 2021, 18, 2762. [Google Scholar] [CrossRef]
- Cui, D.; Liu, Y.; Jiang, X.; Ding, C.; Poon, L.C.; Wang, H.; Yang, H. Single-cell RNA expression profiling of SARS-CoV-2-related ACE2 and TMPRSS2 in human trophectoderm and placenta. Ultrasound Obstet. Gynecol. 2021, 57, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.; Anastasi, E.; Brandolino, G.; Brunelli, R.; Di Pietro, M.; Filardo, S.; Masciullo, L.; Terrin, G.; Viscardi, M.F.; Porpora, M.G. What is the Hidden Biological Mechanism Underlying the Possible SARS-CoV-2 Vertical Transmission? A Mini Review. Front. Physiol. 2022, 13, 875806. [Google Scholar] [CrossRef]
- Sessa, R.; Masciullo, L.; Filardo, S.; Di Pietro, M.; Brandolino, G.; Brunelli, R.; Galoppi, P.; Terrin, G.; Viscardi, M.F.; Anastasi, E.; et al. SARS-CoV-2 vertical transmission in a twin-pregnant woman: A case report. Int. J. Infect. Dis. 2022, 125, 192–194. [Google Scholar] [CrossRef]
- Fenizia, C.; Biasin, M.; Cetin, I.; Vergani, P.; Mileto, D.; Spinillo, A.; Gismondo, M.R.; Perotti, F.; Callegari, C.; Mancon, A.; et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat. Commun. 2020, 11, 5128. [Google Scholar] [CrossRef]
- Karimi-Zarchi, M.; Neamatzadeh, H.; Dastgheib, S.A.; Abbasi, H.; Mirjalili, S.R.; Behforouz, A.; Ferdosian, F.; Bahrami, R. Vertical Transmission of Coronavirus Disease 19 (COVID-19) from Infected Pregnant Mothers to Neonates: A Review. Fetal Pediatr. Pathol. 2020, 39, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Baldewijns, M.; Benachi, A.; Bugatti, M.; Bulfamante, G.; Cheng, K.; Collins, R.R.J.; Debelenko, L.; De Luca, D.; Facchetti, F.; et al. Hofbauer Cells and COVID-19 in Pregnancy. Arch. Pathol. Lab. Med. 2021, 145, 1328–1340. [Google Scholar] [CrossRef] [PubMed]
- Shook, L.L.; Sullivan, E.L.; Lo, J.O.; Perlis, R.H.; Edlow, A.G. COVID-19 in pregnancy: Implications for fetal brain development. Trends Mol. Med. 2022, 28, 319–330. [Google Scholar] [CrossRef]
- Vivanti, A.J.; Vauloup-Fellous, C.; Prevot, S.; Zupan, V.; Suffee, C.; Do Cao, J.; Benachi, A.; De Luca, D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020, 11, 3572. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef]
- Eberle, C.; James-Todd, T.; Stichling, S. SARS-CoV-2 in diabetic pregnancies: A systematic scoping review. BMC Pregnancy Childbirth 2021, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Romero, R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2022, 226, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. Can. Med. Assoc. J. 2021, 193, E540–E548. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, A.; Jędruchniewicz, N.; Torelli, A.; Kaczmarzyk-Radka, A.; Coluccio, R.; Kłak, M.; Konieczny, A.; Ferenc, S.; Witkiewicz, W.; Montomoli, E.; et al. Antibodies specific to SARS-CoV-2 proteins N, S and E in COVID-19 patients in the normal population and in historical samples. J. Gen. Virol. 2021, 102, 001692. [Google Scholar] [CrossRef]
- Kotlyar, A.M.; Grechukhina, O.; Chen, A.; Popkhadze, S.; Grimshaw, A.; Tal, O.; Taylor, H.S.; Tal, R. Vertical transmission of coronavirus disease 2019: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021, 224, 35–53.e3. [Google Scholar] [CrossRef]
- Baud, D.; Greub, G.; Favre, G.; Gengler, C.; Jaton, K.; Dubruc, E.; Pomar, L. Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection. J. Am. Med. Assoc. 2020, 323, 2198–2200. [Google Scholar] [CrossRef]
- Di Gioia, C.; Zullo, F.; Bruno Vecchio, R.C.; Pajno, C.; Perrone, G.; Galoppi, P.; Pecorini, F.; Di Mascio, D.; Carletti, R.; Prezioso, C.; et al. Stillbirth and fetal capillary infection by SARS-CoV-2. Am. J. Obstet. Gynecol. MFM 2022, 4, 100523. [Google Scholar] [CrossRef]
- Li, M.; Chen, L.; Zhang, J.; Xiong, C.; Li, X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS ONE 2020, 15, e0230295. [Google Scholar] [CrossRef]
- Bwire, G.M.; Njiro, B.J.; Mwakawanga, D.L.; Sabas, D.; Sunguya, B.F. Possible vertical transmission and antibodies against SARS-CoV-2 among infants born to mothers with COVID-19: A living systematic review. J. Med. Virol. 2021, 93, 1361–1369. [Google Scholar] [CrossRef]
- Taglauer, E.S.; Wachman, E.M.; Juttukonda, L.; Klouda, T.; Kim, J.; Wang, Q.; Ishiyama, A.; Hackam, D.J.; Yuan, K.; Jia, H. Acute Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Pregnancy Is Associated with Placental Angiotensin-Converting Enzyme 2 Shedding. Am. J. Pathol. 2022, 192, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A. An Analysis of 38 Pregnant Women With COVID-19, Their Newborn Infants, and Maternal-Fetal Transmission of SARS-CoV-2: Maternal Coronavirus Infections and Pregnancy Outcomes. Arch. Pathol. Lab. Med. 2020, 144, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guo, J.; Fan, C.; Juan, J.; Yu, X.; Li, J.; Feng, L.; Li, C.; Chen, H.; Qiao, Y.; et al. Coronavirus disease 2019 in pregnant women: A report based on 116 cases. Am. J. Obstet. Gynecol. 2020, 223, 111.e1–111.e14. [Google Scholar] [CrossRef] [PubMed]
- Beesley, M.; Davidson, J.; Panariello, F.; Shibuya, S.; Scaglioni, D.; Jones, B.; Maksym, K.; Ogunbiyi, O.; Sebire, N.; Cacchiarelli, D.; et al. COVID-19 and vertical transmission: Assessing the expression of ACE2/TMPRSS2 in the human fetus and placenta to assess the risk of SARS-CoV-2 infection. BJOG Int. J. Obstet. Gynaecol. 2022, 129, 256–266. [Google Scholar] [CrossRef] [PubMed]
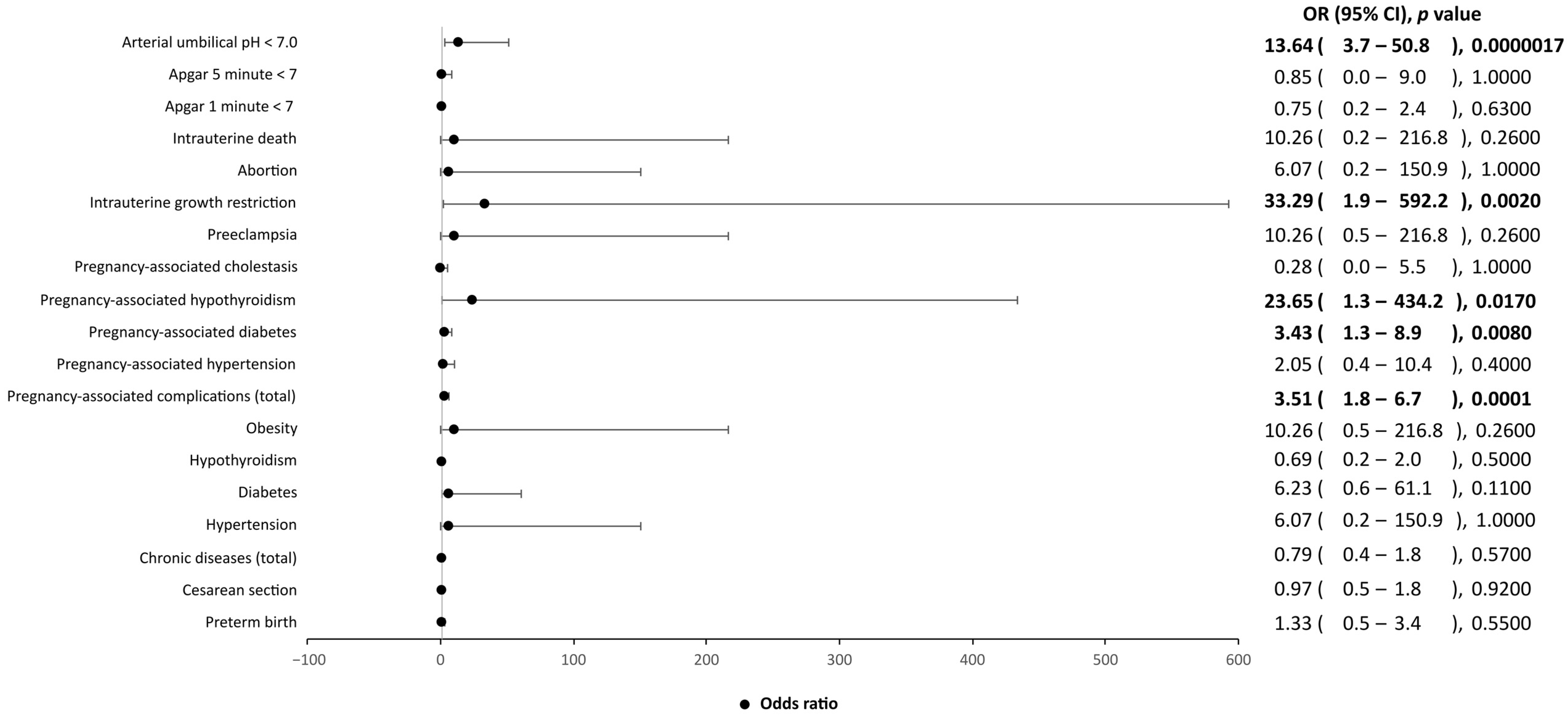

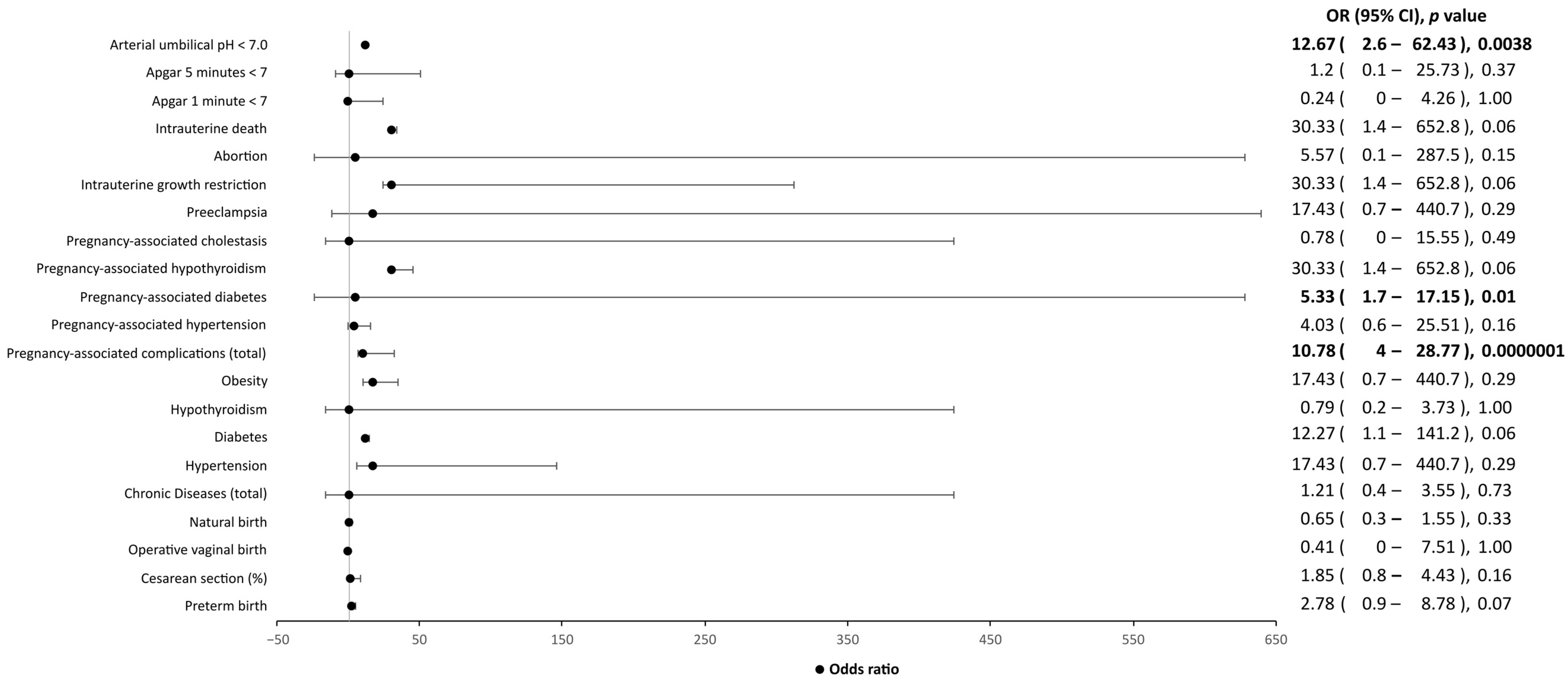

| SARS-CoV-2-positive women (n = 68) | |
| Age (average ± SD) | 29.99 ± 5.30 |
| Gestational age at first positivity (%) | |
| I trimester | 2.99 |
| II trimester | 13.43 |
| III trimester | 83.58 |
| Prevalence of chronic diseases (%) | |
| total | 14.71 |
| Hypertension | 1.47 |
| Diabetes | 4.41 |
| Hypothyroidism | 7.35 |
| Obesity | 2.94 |
| Prevalence of pregnancy-associated complications (%) | |
| total | 44.12 |
| Hypertension | 4.41 |
| Diabetes | 17.65 |
| Hypothyroidism | 7.35 |
| Cholestasis | 0.00 |
| Preeclampsia | 2.94 |
| Intrauterine growth restriction | 10.29 |
| Abortion | 1.47 |
| Intrauterine death | 2.94 |
| Non-specific phlogosis indices (%) | |
| Altered | 43.28 |
| Normal | 55.22 |
| Gestational length (%) | |
| Preterm | 12.31 |
| Full-term | 87.69 |
| Type of delivery (%) | |
| Cesarean section | 38.24 |
| Instrumental delivery | 2.94 |
| Vaginal delivery | 58.82 |
| SARS-CoV-2 manifestations (%) | |
| Symptomatic | 35.29 |
| Asymptomatic | 64.71 |
| SARS-CoV-2 antibody anti-N protein (%) | |
| >1 U/mL | 48.48 |
| <1 U/mL | 51.52 |
| SARS-CoV-2 antibody anti-S protein (%) | |
| >0.8 U/mL | 63.27 |
| <0.8 U/mL | 36.73 |
| Placenta hemorrhagic necrosis (%) | |
| Presence | 24.49 |
| Absence | 75.51 |
| Acute intervillositis (%) | |
| Presence | 8.16 |
| Absence | 91.84 |
| Placental malperfusion (%) | |
| Presence | 8.16 |
| Absence | 91.84 |
| Children from SARS-CoV-2-positive mothers (n = 66) | |
| Weight (g, average ± SD) | 2982.27 ± 590.41 |
| Apgar 1 min (%) | |
| <7 | 6.15 |
| ≥7 | 93.85 |
| Apgar 5 min (%) | |
| <7 | 0.00 |
| ≥7 | 100.00 |
| Arterial umbilical cord pH (%) | |
| Acidic (<7.0) | 23.53 |
| Basic (≥7.0) | 76.47 |
| SARS-CoV-2 antibody anti-N protein (%) | |
| >1 U/mL | 39.58 |
| <1 U/mL | 60.42 |
| SARS-CoV-2 antibody anti-S protein (%) | |
| >0.8 U/mL | 45.83 |
| <0.8 U/mL | 54.17 |
| SARS-CoV-2 uninfected women (n = 136) | |
| Age (average ± SD) | 32.82 ± 5.76 |
| Prevalence of chronic diseases (%) | |
| total | 17.91 |
| Hypertension | 0.00 |
| Diabetes | 0.74 |
| Hypothyroidism | 10.29 |
| Obesity | 0.00 |
| Prevalence of pregnancy-associated complications (%) | |
| total | 18.38 |
| Hypertension | 2.21 |
| Diabetes | 5.88 |
| Hypothyroidism | 0.00 |
| Cholestasis | 2.21 |
| Preeclampsia | 0.00 |
| Intrauterine growth restriction | 0.00 |
| Abortion | 0.00 |
| Intrauterine death | 0.00 |
| Gestational length (%) | |
| Preterm | 9.56 |
| Full-term | 90.44 |
| Type of delivery (%) | |
| Cesarean section | 38.97 |
| Operative vaginal birth | 4.41 |
| Natural birth | 56.62 |
| Children from SARS-CoV-2 uninfected mothers (n = 136) | |
| Weight (g, average ± SD) | 3229.46 ± 457.84 |
| Apgar 1 min (%) | |
| <7 | 8.09 |
| ≥7 | 91.91 |
| Apgar 5 min (%) | |
| <7 | 1.47 |
| ≥7 | 98.53 |
| Arterial umbilical cord pH (%) | |
| Acidic (<7.2) | 2.21 |
| Basic (≥7.2) | 97.79 |
| SARS-CoV-2-Positive Pregnant Women (n = 49) | |||||||
|---|---|---|---|---|---|---|---|
| Symptomatic (n = 16) | Asymptomatic (n = 33) | p-Value | Odds Ratio | 95% C.I. | |||
| Antibody anti-N protein (%) | 62.50 | 48.48 | 0.36 | 1.77 | 0.52 | - | 6.00 |
| Antibody anti-S protein (%) | 68.75 | 60.61 | 0.58 | 1.43 | 0.40 | - | 5.08 |
| Children from SARS-CoV-2-Positive Mothers (n = 49) | |||||||
| Symptomatic (n = 15) | Asymptomatic (n = 33) | p-Value | Odds Ratio | 95% C.I. | |||
| Antibody anti-N protein (%) | 33.33 | 42.42 | 0.75 | 0.68 | 0.19 | - | 2.43 |
| Antibody anti-S protein (%) | 33.33 | 51.52 | 0.24 | 0.47 | 0.13 | - | 1.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sessa, R.; Filardo, S.; Masciullo, L.; Di Pietro, M.; Angeloni, A.; Brandolino, G.; Brunelli, R.; D’Alisa, R.; Viscardi, M.F.; Anastasi, E.; et al. SARS-CoV-2 Infection in Pregnancy: Clues and Proof of Adverse Outcomes. Int. J. Environ. Res. Public Health 2023, 20, 2616. https://doi.org/10.3390/ijerph20032616
Sessa R, Filardo S, Masciullo L, Di Pietro M, Angeloni A, Brandolino G, Brunelli R, D’Alisa R, Viscardi MF, Anastasi E, et al. SARS-CoV-2 Infection in Pregnancy: Clues and Proof of Adverse Outcomes. International Journal of Environmental Research and Public Health. 2023; 20(3):2616. https://doi.org/10.3390/ijerph20032616
Chicago/Turabian StyleSessa, Rosa, Simone Filardo, Luisa Masciullo, Marisa Di Pietro, Antonio Angeloni, Gabriella Brandolino, Roberto Brunelli, Rossella D’Alisa, Maria Federica Viscardi, Emanuela Anastasi, and et al. 2023. "SARS-CoV-2 Infection in Pregnancy: Clues and Proof of Adverse Outcomes" International Journal of Environmental Research and Public Health 20, no. 3: 2616. https://doi.org/10.3390/ijerph20032616
APA StyleSessa, R., Filardo, S., Masciullo, L., Di Pietro, M., Angeloni, A., Brandolino, G., Brunelli, R., D’Alisa, R., Viscardi, M. F., Anastasi, E., & Porpora, M. G. (2023). SARS-CoV-2 Infection in Pregnancy: Clues and Proof of Adverse Outcomes. International Journal of Environmental Research and Public Health, 20(3), 2616. https://doi.org/10.3390/ijerph20032616










