Abstract
The optimal structure of the acute ischaemic stroke treatment network is unknown and eagerly sought. To make it most effective, different treatment and transportation strategies have been developed and investigated worldwide. Since only a fraction of acute stroke patients with large vessel occlusion are treated, a new entity—thrombectomy-capable stroke centre (TCSC)—was introduced to respond to the growing demand for timely endovascular treatment. The purpose of this study was to present the early experience of the first 70 patients treated by mechanical means in a newly developed cardiac Cathlab-based TCSC. The essential safety and efficacy measures were recorded and compared with those reported in the invasive arm of the HERMES meta-analysis—the largest published dataset on the subject. We found no significant differences in terms of clinical and safety outcomes, such as early neurological recovery, level of functional independence at 90 days, symptomatic intracranial haemorrhage, parenchymal haematoma type 2, and mortality. These encouraging results obtained in the small endovascular centre may be an argument for the introduction of the TCSC into operating stroke networks to increase patient access to timely treatment and to improve clinical outcomes.
1. Introduction
Stroke is one of the leading causes of disability and death throughout the civilised world. Mechanical thrombectomy (MT) has revolutionised the treatment of acute ischaemic stroke patients with large vessel occlusion (LVO) [1,2,3,4,5]. It is estimated that 10%–12% of ischaemic stroke patients may be candidates for interventional therapy, which starkly contrasts with the numbers of MT provided with median access of 2.76% worldwide [6,7,8,9].
Changes to stroke network structure and operator training are introduced to meet the growing demand for timely thrombectomy [10,11]. As a result, a new level of stroke care certification in the US was introduced in 2018—a thrombectomy-capable stroke centre (TCSC). Since then, 44 such units have been registered in the US to support 262 comprehensive stroke centres (CSC). Since the introduction of the TCSC, a drop in case fatality rate was observed, while the outcomes achieved were better than that of primary stroke centres (PSC) and comparable to CSC [12,13]. The acute ischaemic stroke system in Poland suffers from small MT penetration (roughly 4% of all ischaemic strokes treated with MT in 2021) and considerable delays due to long distances to CSC [14]. Therefore, we believe there is an urgent need for change in the Polish MT network structure and propose the introduction of thrombectomy-capable stroke centres (TCSC) based on existing upgraded primary stroke centres (PSC). To support the idea, we present the experience of our institution that underwent such a transformation recently [15].
The short- and medium-term results of our first 70 acute LVO ischaemic stroke patients treated by mechanical reperfusion techniques were compared with the data available from the HERMES meta-analysis of five randomised trials (MR CLEAN, ESCAPE, REVASCAT, SWIFT PRIME, and EXTEND IA) [16]. Safety and efficacy results support the idea that experienced and integrated multidisciplinary teams may be a suitable platform to transform PSC into TCSC.
2. Materials and Methods
2.1. Patients
All patients were scanned with CT/CTA or MRI before the intervention. Our analysis included patients with acute ischaemic strokes due to LVO (plus M2). LVO was defined as the occlusion of the internal carotid artery (ICA), the middle cerebral artery (MCA) at the M1 level, the intracranial vertebral artery (VA), and the basilar artery (BA) in computed tomography angiography (CTA). Other inclusion criteria were as follows: age > 18 years, National Institutes of Health Stroke Scale (NIHSS) ≥ 6 or isolated aphasia, prior functional independence with the modified ranking scale − mRS ≤ 2, time from onset to presentation <6 h. In addition, we also included four patients classified as ‘wake-up’ strokes with time of presentation 6–24 h and a confirmed mismatch between ischaemic core and penumbra on magnetic resonance imaging (MRI). All eligible patients received intravenous thrombolysis (IVT) with recombinant tissue plasminogen activator (rt-PA) prior to intervention. In the case of contraindication to IVT, we proceeded with MT after computed tomography (CT) and CTA. The intervention decision was made by a team consisting of a neurologist, a radiologist, and an interventionalist. We excluded patients with demarcated massive strokes on scanning (more than 1/3 of the territory of the MCA). All patients were Caucasian. Informed consent was taken from all patients before treatment initiation.
2.2. MT Technique
A senior interventional cardiologist and a neurologist performed all interventions. The team comprised two interventional cardiologists, an interventional neurologist in training and a vascular surgeon in training. All procedures were performed in an angio suite on a Siemens Artis Zee monoplane system. MT was performed either under conscious sedation (CS) or general anaesthesia (GA). In cooperative patients (except tandem lesions, dissections, and M2 occlusions), conscious sedation was preferred. Balloon guide catheter (BGC) placement in the internal carotid artery (ICA) was our initial strategy; in the cases of difficult access or intervention in the posterior circulation, we used long interventional 6F sheaths. In all the procedures, a distal access catheter (DAC) was introduced intracranially, and in the case of proximal thrombus location, one pass with a direct aspiration first pass technique (ADAPT) technique was attempted. Our default strategy was the stent-retriever assisted vacuum-locked extraction (SAVE) technique, where thrombus extraction was performed using 2-point active suction on both BGC and DAC, combined with a stent retriever [17]. After the procedure, the groin puncture site was rechecked by angiography and, if possible, secured with a vascular plug (or otherwise by manual compression).
2.3. Procedure Safety and Efficacy
Angiograms were assessed and classified by 2 interventionalists. We used the modified treatment in cerebral infarction (mTICI) scale to score pre- and post-procedural cerebral flow, with success defined as the TICI 2b/3 score [18]. All patients were scanned by CT or MRI within 24 h post-procedure to assess the extent of the infarcted area and the presence of complications. In all patients who received stents, CT was performed immediately after the procedure. CT was also urgently performed in cases of clinical deterioration. We classified intracranial haemorrhage (ICH) according to the Heidelberg bleeding classification [19]. Symptomatic intracranial haemorrhage (sICH) was defined as ICH with a concomitant drop in NIHSS by at least 4 points. Safety outcomes were classified as the proportion of patients with sICH, parenchymal haematoma type 2 (PH2) within 48 h post-procedure, and 90-day mortality. Efficacy outcomes were determined by estimation of the mean NIHSS score at 24 h, a change in the mean NIHSS score from the baseline to 24 h, and early neurological recovery (ENR) at 24 h (NIHSS drop of ≥8), together with a degree of disability measured by the modified Rankin score at 90 days.
2.4. Patient Evaluation
A neurologist assessed patients according to the acute stroke protocol used at our institution. At the baseline—apart from age, sex, and standard clinical data (arterial hypertension, diabetes mellitus, atrial fibrillation, and smoking)—we measured initial NIHSS and the mRS scale. We noted the location of LVO and measured NIHSS at 24 h, as well as mRS at 90 days post-procedure. Crucial patient care times were noted: onset-to-LVO detection on the CTA scan, onset-to-IVT administration, and onset-to-reperfusion on final angiography. Control head CT was routinely performed 24 h post-procedure and urgently in the above-mentioned cases.
2.5. Data Analysis
Descriptive data were presented as mean±standard deviation (SD)/number of participants in the group (N) or % (n/N). HERMES data were partially presented as the median (interquartile range, IQR), which was transformed into mean ± SD [20]. The Shapiro–Wilk test was performed to assess data normality, and data were deemed to be normally distributed if p > 0.05. To compare two cohorts treated with MT, we performed independent sample t tests or U-Mann Whitney tests, as applicable for continuous data, and chi-square tests for categorical data [21].
3. Results
We compared the safety and efficacy results of the first 70 acute stroke patients treated with endovascular methods in our institution between August 2020 and September 2022, with 634 patients pooled in the invasive arm of the HERMES meta-analysis. During that period, 1224 patients were admitted to our unit with a stroke diagnosis (see Figure 1), 1059 of which were ischaemic. IVT was given to 271 patients (25%), while 77 patients (7%) were qualified for endovascular treatment (EVT). Note that 7 out of 77 patients were not treated on-site but transferred to the nearest CSC for various technical reasons (patients qualified for MT at the same time, angiosuite unavailable, staffing problems) and thus excluded from the analysis. Six patients had EVT for acute stroke due to a carotid stenosis/occlusion (without intracranial intervention); all of these patients received carotid stents.
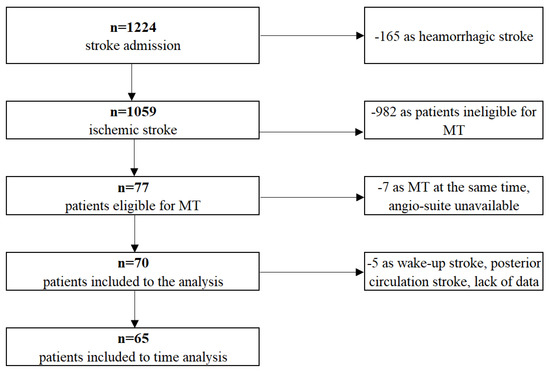
Figure 1.
Our group of patients flow chart.
We noted that mean age, number of men and women, and baseline mean NIHSS were comparable in the two cohorts. Arterial hypertension and atrial fibrillation were more prevalent in our group compared to HERMES. GA was applied in 51% of patients, and conversion from CS to GA was necessary for 3 of the 70 patients (4%). Groin puncture (our default access) was performed under ultrasound guidance in 76% of patients, and in two instances, brachial access was necessary. Regarding the occlusion site, patients with cervical lesions were much more prevalent in our group than in HERMES (62% vs. 21%, p < 0.0001), most likely due to tandem lesions being excluded in the latter cohort. At the same time, M1 occlusion was less frequent in our study compared to HERMES (38.6% vs. 69%, respectively). Distal MCA occlusions (M2) were encountered in similar ratios in both groups (7% vs. 8%, respectively). We treated only one patient with an occlusion site other than anterior circulation, namely, the basilar artery (1%), compared to 2% of such cases observed in HERMES (see Table 1). IVT (rt-PA) was administered in the identical percentage of patients in both groups (83%). We calculated crucial interventional times: onset-to-LVO, onset-to-IVT, and onset-to-reperfusion (five patients, three with ‘wake-up’ strokes and two with missing precise timing data, were excluded from the analysis). The onset-to-LVO detection time (onset to randomisation in the HERMES analysis) was significantly shorter in our group (104 vs. 199 min, p < 0.001), while the onset-to-IVT time was longer (120 vs. 103 min, p = 0.007), most likely as a result of the fact that the vast majority of patients in our institution were primarily treated in the mothership paradigm (see Table 2 below). The onset-to-reperfusion times were comparable in both cohorts (272 vs. 286 min, p = 0.3585, respectively).

Table 1.
Baseline clinical patient characteristics of both groups (mean age, men, women, hypertension, diabetes mellitus, atrial fibrillation, known history of smoking, baseline NIHSS, occlusion location, IV thrombolysis). Data are presented as mean ± SD/N or % (n/N).

Table 2.
Process times comparison (three wake-up stroke patients’ data excluded, two patients with missing data excluded). Data are presented as mean ± SD/N.
By measuring the percentage of patients across mRS groups (0–6) after 90 days, we assessed the efficacy of treatment. We also measured the ratio of patients with excellent clinical outcome (mRS 0–1) at 90 days, good clinical outcome (mRS 0–2) at 90 days, mean NIHSS drop within 24 h, and early neurological recovery at 24 h.
The mean baseline NIHSS, mean NIHSS at 24 h, change in mean NIHSS score from baseline to 24 h were all comparable (see Table 3). Early neurological recovery was nonsignificantly lower in our group (46.2% vs. 50.2%, p = 0.6336).

Table 3.
Comparison of early efficacy outcomes (mean NIHSS score at 24 h, change in mean NIHSS score from baseline to 24 h, early neurological recovery at 24 h).
In the presented cohort, we observed similar ratios of patients with excellent clinical outcomes (mRS 0–1) at 90 days (30% vs. 26.9%, p = 0.6748, respectively) and nonsignificantly more patients with good clinical outcomes (mRS 0–2) at 90 days (55.7% vs. 46%, p = 0.1545, respectively) compared to HERMES (see Table 4).

Table 4.
Comparison of long-term clinical outcomes (mRS, mRS 0–1, and mRS 0–2 at 90 days) and safety outcomes at 90 days (symptomatic intracranial haemorrhage, parenchymal haematoma type 2, and mortality).
Regarding the safety outcomes, we compared the rate of symptomatic ICH, parenchymal haematoma type 2, and mortality at 90 days in both groups. We observed a nonsignificantly higher prevalence of sICH in our group compared to HERMES (10% vs. 4.4%, p = 0.0801). We noted a similar incidence of PH2 (7.1% vs. 5.1%, p = 0.6547) and no difference in mortality at 90 days (17% vs. 15%, 0.8177).
4. Discussion
In Poland, a heated interdisciplinary discussion has been taking place concerning the future shape of the acute ischaemic stroke network. As great proponents of the idea of wide MT-network expansion, we aimed to show that the safety and efficacy results achieved in our recently developed, small cardiac Cathlab-based thrombectomy-capable stroke centre were comparable to those reported in the HERMES meta-analysis [15,16]. In this study, we compared the early and late safety and efficacy results of the cohort of the first 70 LVO stroke patients treated by MT in our centre with the pooled data of 634 patients from the invasive arm of the HERMES study. The main findings of this retrospective analysis may be summarised as follows: (1) the safety outcomes measured at 90 days (sICH, PH type 2, and mortality) were comparable in both groups, and (2) the efficacy outcomes, both early (NIHSS at 24 h, NIHSS change at 24 h, and ENR) and late (mRS 0–1 and mRS 0–2 at 90 days), were comparable in both cohorts. In detail, we observed similar ratios of patients with excellent (mRS 0–1) and good (mRS 0–2) clinical outcomes at 90 days.
Compared to HERMES, a nonsignificantly higher prevalence of sICH was noted, and this may be associated with much higher incidence, present in our cohort, of ICA occlusion [22]. A similar incidence of PH2 and no difference in mortality at 90 days were observed in compared groups. A limitation of this study is the lack of access to the HERMES analysis raw data, which prevented us from checking data distribution and using nonparametric tests if required.
Since ‘time is brain’, timely reperfusion has become the Holy Grail of modern acute ischaemic stroke care. In patients with severe strokes due to LVO occlusion, MT has revolutionised the treatment and, with time, has become faster, safer, and more widely accessible. Today, 5%–10% of acute ischaemic stroke patients are treated invasively, but treatment availability differs globally [6,7,8,9]. The main issue to be solved is creating effective acute stroke networks, enabling fast selection of LVO patients, and admitting them to thrombectomy centres in time. Considerable delays are the Achilles heel of many healthcare systems, despite the introduction of various transportation models [23,24,25]. These are applied and further researched according to the local environment in terms of stroke network characteristics and its logistics associated with specific geography [26,27]. There are various transportation strategies: drip-and-ship (DS), mothership (MS), and drive-a-doctor (DD). The DS provides fairly fast access to diagnostics and IVT but at the cost of considerable delays in thrombectomy. The MS improves timely access to mechanical intervention, but it is unavailable for many patients who live in rural areas. The DD, while feasible, is probably the least practical solution due to the questionable availability of ‘on-call’ specialists and the suboptimal procedure environment of an unfamiliar team and angio suite [28,29,30]. Acute ischaemic stroke treatment is highly time-sensitive, and there is evidence that cutting down the time to reperfusion improves the outcomes in patients with LVO [31,32]. Moreover, in many cases, direct transport to locally available thrombectomy facilities makes it possible to invasively treat patients with LVO; otherwise, they would be denied treatment due to lengthy transportation times in the DS model [7]. In stroke communities throughout the world, there is an ongoing discussion on how to improve results in the treatment of ischaemic stroke patients. The key question is how to set up a network that would provide timely treatment for both LVO and non-LVO cohorts. Proper balance between the fast IVT treatment for the majority of stroke patients and prompt access to mechanical intervention for MT candidates is crucial. Ideally, all patients should be treated in a closely located institution that provides both treatments in the MS model [29]. However, this solution is practically limited to big agglomerations, while for the patients living in the countryside, transfer times to CSC are often long. Which of the two models (DS vs. MS) provides better outcomes in this scenario is still a matter of debate [33,34]. Most likely, the good results observed in the MS paradigm have a chance to be replicated in the DS model only in the exceptionally well-developed stroke network logistics, which in most cases is unattainable [35,36].
Fast access to a local PSC with prompt IVT is essential for the majority of stroke patients [37]. Yet, for 10%–20% of the most disabling LVO stroke patients, such a model (DS) provides inferior results since delays associated with inter-hospital transportation are considerable [24,32,38].
We believe that stroke networks should undergo a crucial transformation, which might be achieved by creating a middle institution, a thrombectomy-capable stroke centre. It would be based on existing PSC and allow considerable improvement while preserving the best features of both transportation models. TCSC would enjoy inherited PSC’s short time to IVT and bring MT closer to the patients, thus eliminating transfer delays. It is by no means a new concept since the creation of TCSC has been postulated and introduced throughout the world [6,10,11,25,39]. According to recent research, TCSC teams performing above 35 MT per year (15 MT per operator) achieve excellent results, and in the US, they are called ‘high volume’ [6].
Apart from creating a network of TCSC, there is an urgent need for further improvements in stroke services. It is crucial to shorten time intervals in both pre-hospital and in-hospital phases [25]. Neither the Polish Stroke Society in its recommendations on ischaemic stroke treatment nor the European Stroke Organisation (ESO) in the guidelines on mechanical thrombectomy in acute ischaemic stroke specify time limits for acute stroke patient travel time to the hospital [40,41]. The American Heart Association and American Stroke Association in their quality improvement initiative Mission: Lifeline Stroke set the time limit for hospital arrival at 30 min. The National Health Service (NHS) in the UK recommends the optimal transport times to be 30 min and no more than 60 min, and the stroke network should be organised accordingly. In the US, only one-quarter of patients have direct access (within a 15-min drive) to the nearest EVT centre, while approximately 30% of the population live beyond a 1-h travel time [7,25]. Various improvement models have been postulated, and two solutions were investigated: transforming PSC into TCSC or bypassing PSC. The latter option could be contemplated only if the additional time to reach a MT centre is less than 15 min. The result of the modelling process was as follows: when 10% of PSC were transformed into TCSC, fast MT access was achieved for a further 7% of the population. Yet, it was almost doubled for an alternative strategy: bypass transfer to an MT centre [25].
Since it is rather difficult to envisage widespread utilisation of costly mobile CT scanners, the immediate identification of the potential LVO stroke patients should be performed by trained ambulance staff, using validated clinical scales [42]. Even though not supported by the present ESO recommendations, introducing one of them on the national level would certainly simplify training and improve communication between teams. Establishing good lines of communication between stroke centres and ambulances is crucial; the role of neuro tele-consultations is indisputable since it shortens times to treatment [43]. Such a strategy enables the funnelling of patients with large deficits to MT-capable centres, while patients with smaller deficits may be directed to the closest stroke units. Proper patient selection in the field is essential since stroke mimics may constitute up to 25% of patients admitted as acute stroke patients [44].
Another essential step in service improvement would be creating a dense network of MT centres (TCSC and CSC), geared to shorten times to diagnostics and mechanical intervention. Presently, in systems with few MT centres and a dominant DS transportation model, reduction in crucial times proves difficult due to long door-in–door-out (DIDO) times and transport logistics [45,46]. The mothership model provides faster access to invasive treatment but requires the investment in new MT-capable centres and the training of new teams. Since the number of neurointerventional MT operators is highly unsatisfactory, structured training schemes should be introduced, including specialists from different backgrounds with endovascular experience [11,39,47,48,49,50,51].
Since the intervention in acute ischaemic stroke is highly time dependent, it is necessary for healthcare organisers to establish effective MT networks and ensure crucial balance between fast and efficacious treatment and safety. By introducing thrombectomy-capable stroke centres, it is possible to further extend the MT network with the new entity, which has already proven to be safe and effective [12,13]. This will only be possible within a safe environment of teams consisting of neurologists, radiologists, anaesthetists, and interventionists.
We have previously described in detail our experience in creating such a multidisciplinary team, as well as our very early results [15].
Since 2018, when the Mechanical Thrombectomy Pilot Study in Poland was launched, the number of MT centres has been steadily increasing to reach 27 centres per 38 million people in 2022. In 2021, roughly 4% of all Polish acute ischaemic stroke patients were treated by mechanical means [14]. Recognising the mothership transportation paradigm as the closest to ideal, we believe that, in the long term, Poland should strive for a ‘mothership for all’ policy and create a dense network of TCSCs so that patients can receive effective treatment within 1 h of first medical contact. To reduce the time to intervention and provide MT to possibly as many as 12% of all ischaemic stroke patients, Poland needs to triple its network strength and significantly increase the number of MT centres [6,52].
Limitations of the Study
The main limitation of this study is the relatively small number of patients included and the fact that it was not an all-comer population. We recognize the time difference from symptom onset to randomization (in the HERMES population, up to 12 h; in our group, within 6 h) as a significant limitation. The other shortcoming is the unavailability of the ASPECTS score on the initial CT scan to make a better comparison with the HERMES cohort. Another limitation to consider is the lack of access to the HERMES analysis raw data.
5. Conclusions
In this study, we present the results of the first 70 LVO ischaemic stroke patients treated invasively in our small cardiac Cathlab-based TCSC and compare them with the outcomes reported in the intervention arm of the HERMES meta-analysis. Our results, in terms of safety and efficacy, are comparable with those observed in huge neurointerventional centres assessed in HERMES. The encouraging results of this study should inspire further discussions regarding the place of TCSC in future models of acute ischaemic stroke treatment.
Author Contributions
Conceptualization, K.P. and M.S.; formal analysis, A.P.; methodology, A.D. and A.P.; resources, K.P. and A.D.; software, A.D. and A.P.; supervision, A.M. and M.S.; validation, K.P., A.D. and M.S.; writing—original draft, K.P.; writing—review and editing, J.K. All authors will be informed about each step of manuscript processing, including submission, revision, revision reminder, etc., via emails from our system or assigned Assistant Editor. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the report are available from Marek Szołkiewicz (e.mars@wp.pl) on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fransen, P.S.; Beumer, D.; Berkhemer, O.A.; van den Berg, L.A.; Lingsma, H.; van der Lugt, A.; van Zwam, W.H.; van Oostenbrugge, R.J.; Roos, Y.B.; Majoie, C.B.; et al. MR CLEAN, a multicenter randomised clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands: Study protocol for a randomised controlled trial. Trials 2014, 15, 343. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. ESCAPE Trial Investigators. Randomised assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.A.; Chamorro, A.; Rovira, À.; De Miquel, M.A.; Serena, J.; Román, L.S.; Jovin, T.G.; Davalos, A.; Cobo, E. REVASCAT: A randomised trial of revascularisation with SOLITAIRE FR device vs. best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eight-hours of symptom onset. Int. J. Stroke 2015, 10, 619–626. [Google Scholar] [CrossRef]
- Goyal, M.; Jadhav, A.; Bonafe, A.; Diener, H.; Pereira, V.M.; Levy, E.; Baxter, B.; Jovin, T.; Jahan, R.; Menon, B.; et al. SWIFT PRIME investigators. Analysis of Workflow and Time to Treatment and the Effects on Outcome in Endovascular Treatment of Acute Ischemic Stroke: Results from the SWIFT PRIME Randomized Controlled Trial. Radiology 2016, 279, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.; Mitchell, P.; Kleinig, T.; Dewey, H.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.; Parsons, M.; Oxley, T.; et al. EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Bulwa, Z.; Chen, M. Stroke Center Designations, Neurointerventionalist Demand, and the Finances of Stroke Thrombectomy in the United States. Neurology 2021, 97 (Suppl. S2), S17–S24. [Google Scholar] [CrossRef]
- Aroor, S.R.; Asif, K.S.; Potter-Vig, J.; Sharma, A.; Menon, B.K.; Inoa, V.; Zevallos, C.B.; Romano, J.G.; Ortega-Gutierrez, S.; Goldstein, L.B.; et al. Mechanical Thrombectomy Access for All? Challenges in Increasing Endovascular Treatment for Acute Ischemic Stroke in the United States. J. Stroke 2022, 24, 41–48. [Google Scholar] [CrossRef]
- de Sousa, D.A.; von Martial, R.; Abilleira, S.; Gattringer, T.; Kobayashi, A.; Gallofré, M.; Fazekas, F.; Szikora, I.; Feigin, V.; Caso, V.; et al. Access to and delivery of acute ischaemic stroke treatments: A survey of national scientific societies and stroke experts in 44 European countries. Eur. Stroke J. 2019, 4, 13–28. [Google Scholar] [CrossRef]
- Focus on Mission Thrombectomy 2020+ with, Dr. Dileep R. Yavagal. Available online: https://www.linnc.com/News/2022/WorldStrokeDay2022/Focus-on-Mission-Thrombectomy-2020 (accessed on 10 December 2022).
- Baker, D.W.; Tschurtz, B.A.; Aliaga, A.E.; Williams, S.C.; Jauch, E.C.; Schwamm, L.H. Determining the Need for Thrombectomy-Capable Stroke Centers Based on Travel Time to the Nearest Comprehensive Stroke Center. Jt. Commun. J. Qual. Patient Saf. 2020, 46, 501–505. [Google Scholar] [CrossRef]
- Pruvo, J.-P.; Berge, J.; Kuchcinski, G.; Bretzner, M.; Leclerc, X.; Hacein-Bey, L. Health Care Organization for the Management of Stroke: The French Perspective. Neuroimaging Clin. N Am. 2018, 28, 691–698. [Google Scholar] [CrossRef]
- Park, E.H.; Gil, Y.J.; Kim, C.; Kim, B.J.; Hwang, S.-S. Presence of Thrombectomy-capable Stroke Centers Within Hospital Service Areas Explains Regional Variation in the Case Fatality Rate of Acute Ischemic Stroke in Korea. J. Prev. Med. Public Health 2021, 54, 385–394. [Google Scholar] [CrossRef] [PubMed]
- ‘New Study Finds Patients Treated at Advanced Stroke Centers Had Better Outcomes’—American Stroke Association International Stroke Conference (5–7 November 2022), Oral Presentation I: LB9. Available online: https://newsroom.heart.org/news/new-study-finds-patients-treated-at-advanced-stroke-centers-had-better-outcomes (accessed on 10 December 2022).
- 7th Online Symposium on Ischemic Stroke in Poland 2–4 December 2021. Available online: https://live.medorado.com/archive/337/C8A23jvfHLrVupaEwSxSxvpv1rdc7G (accessed on 4 December 2022).
- Pawłowski, K.; Klaudel, J.; Dziadkiewicz, A.; Mączkowiak, A.; Szołkiewicz, M. Cardiac CathLab-based stroke thrombectomy routine service by the BRAIN team in a recently established Thrombectomy-Capable Stroke Center in Poland. Kardiol. Pol. 2021, 79, 684–686. [Google Scholar] [CrossRef]
- Goyal, M.; Menon, B.; van Zwam, W.; Dippel, D.; Mitchell, P.; Demchuk, A.; Dávalos, A.; Majoie, C.; van der Lugt, A.; de Miquel, M.; et al. HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Maus, V.; Henkel, S.; Riabikin, A.; Riedel, C.; Behme, D.; Tsogkas, I.; Hesse, A.C.; Abdullayev, N.; Jansen, O.; Wiesmann, M.; et al. The SAVE Technique: Large-Scale Experience for Treatment of Intracranial Large Vessel Occlusions. Clin. Neuroradiol. 2019, 29, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Zaidat, O.; Yoo, A.; Khatri, P.; Tomsick, T.; von Kummer, R.; Saver, J.; Marks, M.; Prabhakaran, S.; Kallmes, D.; Fitzsimmons, B.-F.; et al. Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularisation working group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force. Recommendations on angiographic revascularisation grading standards for acute ischemic stroke: A consensus statement. Stroke 2013, 44, 2650–2663. [Google Scholar]
- von Kummer, R.; Broderick, J.; Campbell, B.; Demchuk, A.; Goyal, M.; Hill, M.; Treurniet, K.; Majoie, C.; Marquering, H.; Mazya, M.; et al. The Heidelberg Bleeding Classification: Classification of Bleeding Events After Ischemic Stroke and Reperfusion Therapy. Stroke 2015, 46, 2981–2986. [Google Scholar] [CrossRef]
- Available online: https://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html (accessed on 10 December 2022).
- Available online: www.statstodo.com (accessed on 10 December 2022).
- Dong, S.; Yu, C.; Wu, Q.; Xia, H.; Xu, J.; Gong, K.; Wang, T. Predictors of Symptomatic Intracranial Hemorrhage after Endovascular Thrombectomy in Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Cerebrovasc Dis. 2022, 1–13. [Google Scholar] [CrossRef]
- Snyder, T.; Agarwal, S.; Huang, J.; Ishida, K.; Flusty, B.; Frontera, J.; Lord, A.; Torres, J.; Zhang, C.; Rostanski, S.; et al. Stroke Treatment Delay Limits Outcome After Mechanical Thrombectomy: Stratification by Arrival Time and ASPECTS. J. Neuroimaging 2020, 30, 625–630. [Google Scholar] [CrossRef]
- Asif, K.S.; Lazzaro, M.A.; Zaidat, O. Identifying delays to mechanical thrombectomy for acute stroke: Onset to door and door to clot times. J. Neurointerv. Surg. 2014, 6, 505–510. [Google Scholar] [CrossRef]
- Sarraj, A.; Savitz, S.; Pujara, D.; Kamal, H.; Carroll, K.; Shaker, F.; Reddy, S.; Parsha, K.; Fournier, L.; Jones, E.; et al. Endovascular Thrombectomy for Acute Ischemic Strokes: Current US Access Paradigms and Optimisation Methodology. Stroke 2020, 51, 1207–1217. [Google Scholar] [CrossRef]
- Holodinsky, J.; Williamson, T.; Demchuk, A.; Zhao, H.; Zhu, L.; Francis, M.; Goyal, M.; Hill, M.; Kamal, N. Modeling Stroke Patient Transport for All Patients With Suspected Large-Vessel Occlusion. JAMA Neurol. 2018, 75, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Milne, M.; Holodinsky, J.; Hill, M.; Nygren, A.; Qiu, C.; Goyal, M.; Kamal, N. Drip’n Ship Versus Mothership for Endovascular Treatment: Modeling the Best Transportation Options for Optimal Outcomes. Stroke 2017, 48, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, M.; Biguzzi, S.; Cordici, F.; Romoli, M.; Altini, M.; Agnoletti, V.; Fabbri, A.; Francesconi, R.; Menarini, M.; Perin, T.; et al. Drip-and-ship toward mothership model for mechanical thrombectomy during COVID-19 pandemic: A retrospective analysis. Neurol Sci. 2023, 44, 1–7. [Google Scholar] [CrossRef]
- Mohamed, A.; Fatima, N.; Shuaib, A.; Saqqur, M. Comparison of mothership versus drip-and-ship models in treating patients with acute ischemic stroke: A systematic review and meta-analysis. Int. J. Stroke 2022, 17, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Seker, F.; Möhlenbruch, M.; Nagel, S.; Ulfert, C.; Schönenberger, S.; Pfaff, J.; Ringleb, P.; Steiner, T.; Bendszus, M.; Herweh, C. Clinical results of a new concept of neurothrombectomy coverage at a remote hospital-”drive the doctor”. Int. J. Stroke 2018, 13, 696–699. [Google Scholar] [CrossRef]
- Saver, J.; Goyal, M.; van der Lugt, A.; Menon, B.; Majoie, C.; Dippel, D.; Campbell, B.; Nogueira, R.; Demchuk, A.; Tomasello, A.; et al. HERMES Collaborators. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. JAMA 2016, 316, 1279–1288. [Google Scholar] [CrossRef]
- Jahan, R.; Saver, J.; Schwamm, L.; Fonarow, G.; Liang, L.; Matsouaka, R.; Xian, Y.; Holmes, D.; Peterson, E.; Yavagal, D.; et al. Association Between Time to Treatment With Endovascular Reperfusion Therapy and Outcomes in Patients With Acute Ischemic Stroke Treated in Clinical Practice. JAMA 2019, 322, 252–263. [Google Scholar] [CrossRef]
- Wu, X.; Wira, C.R.; Matouk, C.C.; Forman, H.P.; Gandhi, D.; Sanelli, P.C.; Schindler, J.; Malhotra, A. Drip-and-ship versus mothership for endovascular treatment of acute stroke: A comparative effectiveness analysis. Int. J. Stroke 2022, 17, 315–322. [Google Scholar] [CrossRef]
- Taschner, C.; Trinks, A.; Bardutzky, J.; Brich, J.; Hartmann, R.; Urbach, H.; Niesen, W.-D. Drip-and-Ship for Thrombectomy Treatment in Patients With Acute Ischemic Stroke Leads to Inferior Clinical Outcomes in a Stroke Network Covering Vast Rural Areas Compared to Direct Admission to a Comprehensive Stroke Center. Front. Neurol. 2021, 12, 743151. [Google Scholar] [CrossRef]
- Brochado, A.P.; Muras, A.C.; Oyarzun-Irazu, I.; Rodriguez-Sainz, A.; Caballero-Romero, I.; Aguilera-Irazabal, B.; García-Sánchez, J.M.; Sustatxa-Zárraga, I.; Martínez-Condor, D.; Gutierrez-Albizuri, C.; et al. Drip and ship and mothership models of mechanical thrombectomy result in similar outcomes in acute ischemic stroke of the anterior circulation. J. Stroke Cerebrovasc. Dis. 2022, 31, 106733. [Google Scholar] [CrossRef]
- de la Ossa, N.P.; Abilleira, S.; Jovin, T.; García-Tornel, Á.; Jimenez, X.; Urra, X.; Cardona, P.; Cocho, D.; Purroy, F.; Serena, J.; et al. RACECAT Trial Investigators. Effect of Direct Transportation to Thrombectomy-Capable Center vs Local Stroke Center on Neurological Outcomes in Patients With Suspected Large-Vessel Occlusion Stroke in Nonurban Areas: The RACECAT Randomized Clinical Trial. JAMA 2022, 327, 1782–1794. [Google Scholar] [CrossRef]
- Saver, J.; Fonarow, G.; Smith, E.; Reeves, M.; Grau-Sepulveda, M.; Pan, W.; Olson, D.; Hernandez, A.; Peterson, E.; Schwamm, L. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013, 309, 2480–2488. [Google Scholar] [CrossRef] [PubMed]
- Seker, F.; Bonekamp, S.; Rode, S.; Hyrenbach, S.; Bendszus, M.; Möhlenbruch, M. Direct Admission vs. Secondary Transfer to a Comprehensive Stroke Center for Thrombectomy: Retrospective Analysis of a Regional Stroke Registry with 2797 Patients. Clin. Neuroradiol. 2020, 30, 795–800. [Google Scholar] [CrossRef]
- Behzadi, G.N.; Fjetland, L.; Advani, R.; Kurz, M.W.; Kurz, K.D. Endovascular stroke treatment in a small-volume stroke center. Brain Behav. 2017, 7, e00642. [Google Scholar] [CrossRef] [PubMed]
- Błażejewska-Hyzorek, B.; Czernuszenko, A.; Członkowska, A.; Ferens, A.; Gąsecki, D.; Kaczorowski, R.; Karaszewski, B.; Karliński, M.; Kaźmierski, R.; Kłysz, B.; et al. Wytyczne postępowania w udarze mózgu. Pol. Prz. Neurol. 2019, 15, 1–156. (In Polish) [Google Scholar] [CrossRef]
- Turc, G.; Bhogal, P.; Fischer, U.; Khatri, P.; Lobotesis, K.; Mazighi, M.; Schellinger, P.; Toni, D.; de Vries, J.; White, P.; et al. European Stroke Organisation (ESO)—European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischaemic StrokeEndorsed by Stroke Alliance for Europe (SAFE). Eur. Stroke J. 2019, 4, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, M.V.; Hemendinger, M.L.; Baird, G.L.; Yaghi, S.; Cutting, S.; Saad, A.; Siket, M.; E Madsen, T.; Williams, K.; Rhodes, J.; et al. Field triage for endovascular stroke therapy: A population-based comparison. J. Neurointerv. Surg. 2020, 12, 233–239. [Google Scholar] [CrossRef]
- Al Kasab, S.; Almallouhi, E.; Grant, C.; Hewitt, D.; Hewitt, J.; Baki, M.; Sabatino, P.; Jones, D.; Holmstedt, C. Telestroke Consultation in the Emergency Medical Services Unit: A Novel Approach to Improve Thrombolysis Times. J. Stroke Cerebrovasc. Dis. 2021, 30, 105710. [Google Scholar] [CrossRef]
- Mechanical Thrombectomy for Acute Ischaemic Stroke: An Implamantation Guide for the UK. Available online: https://arc-swp.nihr.ac.uk/publications/mechanical-thrombectomy-for-acute-ischaemic-stroke-an-implementation-guide-for-the-uk/ (accessed on 10 December 2022).
- McTaggart, R.; Moldovan, K.; Oliver, L.; Dibiasio, E.; Baird, G.; Hemendinger, M.; Haas, R.; Goyal, M.; Wang, T.; Jayaraman, M. Door-in-Door-Out Time at Primary Stroke Centers May Predict Outcome for Emergent Large Vessel Occlusion Patients. Stroke 2018, 49, 2969–2974. [Google Scholar] [CrossRef]
- Froehler, M.; Saver, J.; Zaidat, O.; Jahan, R.; Aziz-Sultan, M.A.; Klucznik, R.; Haussen, D.; Hellinger, F.; Yavagal, D.; Yao, T.; et al. STRATIS Investigators. Interhospital Transfer Before Thrombectomy Is Associated With Delayed Treatment and Worse Outcome in the STRATIS Registry (Systematic Evaluation of Patients Treated With Neurothrombectomy Devices for Acute Ischemic Stroke). Circulation 2017, 136, 2311–2321. [Google Scholar] [CrossRef]
- Fjetland, L.; Roy, S.; Kurz, K.; Larsen, J.P.; Kurz, M. Endovascular acute stroke treatment performed by vascular interventional radiologists: Is it safe and efficacious? Cardiovasc. Intervent. Radiol. 2012, 35, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Landwehr, P.; Reimer, P.; Bücker, A.; Berlis, A.; Weber, W. DeGIR-/DGNR training programme in interventional radiology and neuroradiology. Vasa 2017, 46, 494–495. [Google Scholar] [CrossRef] [PubMed]
- Lanzer, P.; Cremonesi, A.; Widimský, P. Perspectives on training requirements for interventional cardiologists to perform endovascular interventions for acute ischaemic stroke. EuroIntervention 2019, 14, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Wita, K.; Kułach, A.; Wilkosz, K.; Wybraniec, M.; Wojakowski, W.; Kuczmik, W.; Lelek, M.; Tomalski, W.; Ochała, A.; Uchwat, U.; et al. Mechanical Thrombectomy in Acute Ischemic Stroke-The Role of Interventional Cardiologists: A Prospective Single-Center Study. JACC Cardiovasc. Interv. 2022, 15, 550–558. [Google Scholar] [CrossRef]
- Widimsky, P.; Koznar, B.; Peisker, T.; Vasko, P.; Rohac, F.; Vavrova, J.; Kroupa, J.; Stetkarova, I. Feasibility and safety of direct catheter-based thrombectomy in the treatment of acute ischaemic stroke. Cooperation among cardiologists, neurologists and radiologists. Prospective registry PRAGUE-16. EuroIntervention 2017, 13, 131–136. [Google Scholar] [CrossRef]
- Pawlowski, K.; Dziadkiewicz, A.; Klaudel, J.; Mączkowiak, A.; Szołkiewicz, M. Acute ischemic stroke treatment model for Poland in the mechanical thrombectomy era—Which way to go? Postep. Kardiol Interwencyjnej 2022, 18, 4–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).