The Mechanism of Aerobic Exercise Regulating the PI3K/Akt-mTOR Signaling Pathway Intervenes in Hippocampal Neuronal Apoptosis in Vascular Dementia Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Handling
2.2. Establishment of the Vascular Dementia Model
2.3. Aerobic Exercise Program
2.4. Neurobehavioral Assessment
2.5. Hippocampal Tissue Sampling
2.6. Immunohistochemical Detection of the LC3II Protein Expression
2.7. Apoptosis Detection of Hippocampal Neurons with TUNEL
2.8. RT-PCR Detection of the Related Gene Expression
2.9. Western Blot
2.10. Statistical Analysis
3. Results
3.1. Neurobehavioral Assessment
3.2. The Results of Apoptosis Detection of Hippocampal Neurons
3.3. Expression of the LC3Ⅱ Protein in the Hippocampus
3.4. Expression Results of the PI3K/Akt/mTOR Pathway and Its Related Factors
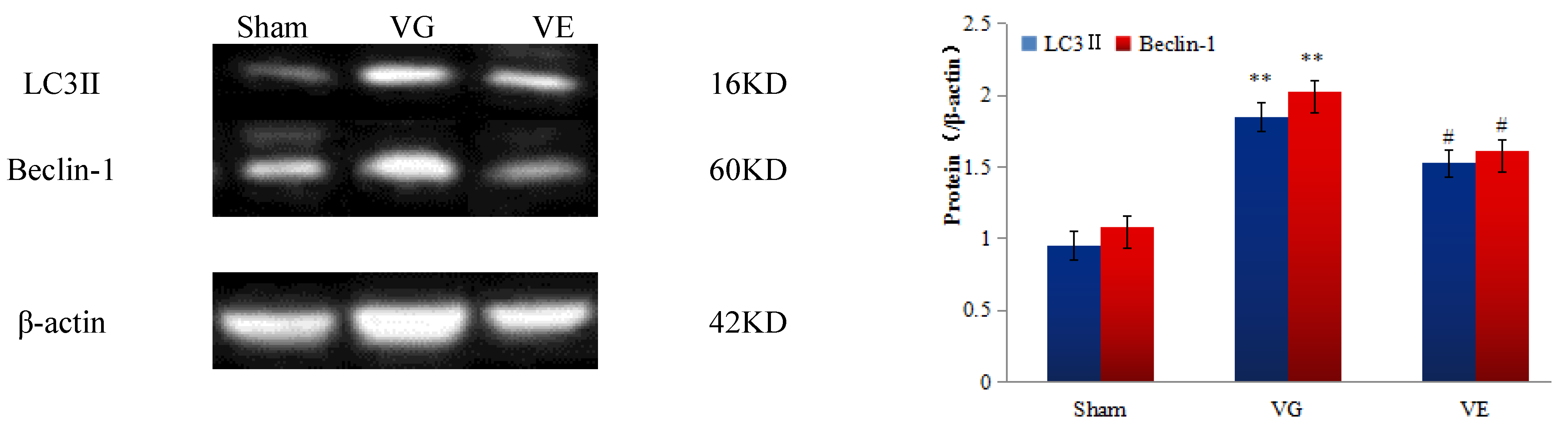
3.5. The Results of the Western Blot
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, X.; Yi, L.L.; Wang, Y. Thioredoxin-1 improving the cognitive function and nerve injury of vascular dementia rats by regulating the IRE1-JNK signaling pathway. Chin. J. Gerontol. 2020, 40, 181–185. [Google Scholar]
- Vinters, H.V.; Zarow, C.; Borys, E.; Whitman, J.D.; Tung, S.; Ellis, W.G.; Zheng, L.; Chui, H.C. Review: Vascular dementia: Clinicopathologic and genetic considerations. Neuropathol. Appl. Neurobiol. 2018, 44, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.R.; Zhang, H.; Deng, C.; Chen, D.F.; Xu, Y.Y.; Xiong, D.; Zou, Z.J.; Tan, J. Effects of electroacupuncture on ROS-NLRP3 inflammatory pathway and autophagy related proteins in hippocampus of vascular dementia rats. Acupunct. Res. 2022, 47, 298–304. [Google Scholar]
- Jiang, X.; Niu, X.L.; Guo, Q.J.; Dong, Y.; Xu, J.; Yin, N.; Qi, Q.; Jia, Y.; Gao, L.; He, Q.; et al. FoxO1-mediated autophagy plays an important role in the neuroprotective effects of hydrogen in a rat model of vascular dementia. Behav. Brain Res. 2019, 356, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Yoshimori, T.; Tooze, S.A. The autophagosome: Origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 2013, 14, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.X.; Wang, Y.L.; Shan, M.Y.; Chen, J.; Wang, H.N.; Sun, B.Q.; Jin, C.; Li, X.; Yin, Y.; Song, C.; et al. Apelin receptor homodimer inhibits apoptosis in vascular dementia. Exp. Cell Res. 2021, 407, 112739. [Google Scholar] [CrossRef] [PubMed]
- Spilman, P.; Podlutskaya, N.; Hart, M.J.; Debnath, J.; Gorostiza, O.; Bredesen, D.; Richardson, A.; Strong, R.; Galvan, V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS ONE 2015, 5, e9979. [Google Scholar] [CrossRef]
- Maiese, K. mTOR: Driving apoptosis and autophagy ofr neurocardiac complications of diabetes mellitus. World J. Diabetes 2015, 6, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, J.W.; Lin, L.T.; Huang, J.; Wang, X.R.; Su, X.T.; Cao, Y.; Fisher, M.; Liu, C.Z. Acupuncture Attenuates Inflammation in Microglia of Vascular Dementia Rats by Inhibiting miR-93-Mediated TLR4/MyD88/NF-κB Signaling Pathway. Oxidative Med. Cell. Longev. 2020, 9, 1–15. [Google Scholar] [CrossRef]
- Ghanbarabadi, M.; Falanji, F.; Rad, A.; Chazani Sharahi, N.; Amoueian, S.; Amin, M.; Molavi, M.; Amin, B. Neuroprotective effects of clavulanic acid following permanent bilateral common carotid artery occlusion in rats. Drug Dev. Res. 2019, 80, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Bedford, T.G.; Tipton, C.M.; Willson, N.C.; Oppliger, R.A.; Gisolfi, C.V. Maximum oxygen consumption of rats and its changes with various ex-perimental procedures. J. Appl. Physiol. 1979, 47, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Topuz, R.D.; Gunduz, O.; Tastekin, E.; Karadag, C.H. Effects of hippocampal histone acetylation and HDAC inhibition on spatial learning and memory in the Morris water maze in rats. Fundam. Clin. Pharmacol. 2019, 34, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Alcalá, J.A.; Callejas-Aguilera, J.E.; Nelson, J.B.; Rosas, J.M. Reversal training facilitates acquisition of new learning in a Morris water maze. Learn. Behav. 2020, 48, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, Y.Z. Effect of Fuzhi Capsule on Apoptotic Timing of Hippocampal Neurons in Rats with Vascular Dementia. Acta Chin. Med. 2019, 34, 222–228. [Google Scholar]
- Wang, B. Effects of endocannabinoid receptor CB1 and chronic intermittent hypoxia on the expression of autophagy related proteins LC3 II and BECLIN-1 in rat nerve cells. Sleep Med. 2019, 64, S409. [Google Scholar] [CrossRef]
- Takemura, G.Z.; Kanamori, H.; Okada, H.; Miyazaki, N.; Watanabe, T.; Tsujimoto, A.; Goto, K.; Maruyama, R.; Fujiwara, T.; Fujiwara, H. Anti-apoptosis in nonmyocytes and pro-autophagy in cardiomyocytes: Two strategies against postinfarction heart failure through regulation of cell death/degeneration. Heart Fail. Rev. 2018, 23, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.L.; Yan, Y.; Liu, X.M.; Shen, W.; Liu, X.H.; Ma, Q.K.; Ying, W.; Jin, X. Effect of medicated thread moxibustion on apoptosis of hippocampal neurons in rat models of chronic cerebral ischemic vascular dementia. Cell. Mol. Biol. 2018, 64, 107–112. [Google Scholar]
- Li, L.; Han, X.F.; Gao, Y.N.; Diao, Q.C.; Xiao, Y.J. Ethanol extract of Gynura bicolor (GB) protects against UVB-induced photodamage of skin by inhibiting P53-mediated Bcl-2/BAX/Caspase-3 apoptosis pathway. Arch. Dermatol. Res. 2020, 312, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.L.; Zhang, Q.X.; Wang, N.B.; Deng, M.Z.; Fang, Y.Q. β-Asarone Regulates ER Stress and Autophagy Via Inhibition of the PERK/CHOP/Bcl-2/Beclin-1 Pathway in 6-OHDA-Induced Parkinsonian Rats. Neurochem. Res. 2019, 44, 1159–1166. [Google Scholar] [CrossRef]
- Heydarabadi, F.H.; Abdoli, A.; Gharibzadeh, S.; Sayyah, M.; Bashar, R.; Sheikholeslami, F. Role of autophagy in nerve cell apoptosis in mice infected with street rabies virus. Arch. Virol. 2020, 165, 2857–2867. [Google Scholar] [CrossRef]
- Luo, C.; Ouyang, M.W.; Fang, Y.Y.; Li, S.J.; Zhou, Q.; Fan, J.; Qin, Z.S.; Tao, T. Dexmedetomidine Protects Mouse Brain from Ischemia-Reperfusion Injury via Inhibiting Neuronal Autophagy through Up-Regulating HIF-1α. Front. Cell. Neurosci. 2017, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Aboutaleb, N.; Shamsaei, N.; Rajabi, H.; Khaksari, M.; Erfani, S.; Nikbakht, F.; Motamedi, P.; Shahbazi, A. Protection of Hippocampal CA1 Neurons against Ischemia/Reperfusion Injury by Exercise Preconditioning via Modulation of Bax/Bcl-2 Ratio and Prevention of Caspase-3 Activation. Basic Clin. Neurosci. 2016, 7, 21–29. [Google Scholar] [PubMed]
- Li, Q.R.; Han, Y.; Du, J.B.; Jin, H.F.; Zhang, J.; Niu, M.M.; Qin, J. Alterations of apoptosis and autophagy in developing brain of rats with epilepsy: Changes in LC3, P62, Beclin-1 and Bcl-2 levels. Neurosci. Res. 2018, 130, 47–55. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhang, B.; Xia, R.; Jia, Q. Inflammation, apoptosis and autophagy as critical players in vascular dementia. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9601–9614. [Google Scholar] [PubMed]
- Gui, X.; Yang, H.; Li, T.; Tan, X.J.; Shi, P.Q.; Li, M.H.; Du, F.; Chen, Z.J. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature 2019, 567, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Wang, X.X.; Chen, L.; Tang, J.; Xia, Y.F.; Qian, K.; Qin, Z.H.; Waeber, C.; Sheng, R. A sphingosine kinase 2-mimicking TAT-peptide protects neurons against ischemia-reperfusion injury by activating BNIP3-mediated mitophagy. Neuropharmacology 2020, 181, 108326. [Google Scholar] [CrossRef] [PubMed]
- Shaerzadeh, F.; Motamedi, F.; Dariush, M.T.; Khodagholi, F. Monitoring of Neuronal Loss in the Hippocampus of Aβ-Injected Rat: Autophagy, Mitophagy, and Mitochondrial Biogenesis Stand Against Apoptosis. Neuromol. Med. 2014, 16, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhang, X.L.; Xia, J.; Yan, Q.W.; Xu, B. Effects of 12-week Aerobic Treadmill Exercise on Autophagy Activity in the Hippocampus of APP/PS1 Mice. China Sport Sci. 2019, 39, 43–53. [Google Scholar]
- Li, L.C.; Li, X.Y.; Du, X.H. Acupuncture improves cognitive function of vascular dementia rats by regulating PI3K/Akt/mTOR pathway. Acupunct. Res. 2021, 46, 851–856. [Google Scholar]
- Zhao, Y.J.; Guo, W.Y.; Gu, X.L.; Chang, C.; Wu, J.E. Repression of deoxynivalenol-triggered cytotoxicity and apoptosis by mannan/β-glucans from yeast cell wall: Involvement of autophagy and PI3K-AKT-mTOR signaling pathway. Int. J. Biol. Macromol. 2020, 164, 1413–1421. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequence | Length (bp) |
|---|---|---|
| Caspase-3 | Sense primer 5’-GTACAGAGCTGGACTGCGGTATTG-3’ Anti-sense primer 5’-AGTCGGCCTCCACTGGTATCTTC-3’ | 84 |
| Bcl-2 | Sense primer 5’-CGGGAGAACAGGGTATGA-3’ Anti-sense primer 5’-CAGAGACAGCCAGGAGAA-3’ | 631 |
| Bax | Sense primer 5’-AGGGTTTCATTCCAGGATCGAGC-3’ Anti-sense primer 5’-AGGCGGTGAGGACTCCAGCC-3’ | 468 |
| Beclin-1 | Sense primer 5’-GCTCAGTACCAGCGAGA-3’ Anti-sense primer 5’-ACAGTACAACGGCAACTC | 381 |
| PI3K | Sense primer 5’-GAGGGGCTACGAGTGGGATA-3’ Anti-sense primer 5’-CAGGCTGGAAGGAGAAGATG-3’ | 81 |
| Akt | Sense primer 5’-AGGACCCTACACAGAGGCT-3’ Anti-sense primer 5’-ACACGATGTTGGCAAAGAA-3’ | 81 |
| mTOR | Sense primer 5’-AAGAAGGTCACTGAGGATT-3’ Anti-sense primer 5’-GGAGATAGAACGGAAGAAG-3’ | 81 |
| LC3Ⅱ | Sense primer 5’-CGGGTTGAGGAGACACACAA-3’ Anti-sense primer 5’-ATGAGCCGGACATCTTCCAC-3’ | 220 |
| GAPDH | Sense primer 5’-GTTACCAGGGTTTCCCGT-3’ Anti-sense primer 5’-GATGGTGATGGGTTTCCCGT-3’ | 177 |
| Group | Latency (s) | Crossing Frequency | Swimming Time a (%) |
|---|---|---|---|
| Sham | 49.500 ± 5.161 | 5.917 ± 1.379 | 44.833 ± 8.632 |
| VG | 93.583 ± 12.206 ** | 2.500 ± 1.446 ** | 26.750 ± 6.905 * |
| VE | 57.417 ± 8.017 *,## | 4.500 ± 1.446 *,## | 41.833 ± 10.983 # |
| Group | TUNEL-Positive Cell Rates (%) | Caspase-3 | Bcl-2 | Bax |
|---|---|---|---|---|
| Sham | 17.667 ± 2.658 | 0.582 ± 0.092 | 0.827 ± 0.118 | 1.283 ± 0.157 |
| VG | 46.500 ± 8.313 ** | 0.951 ± 0.053 ** | 0.359 ± 0.061 ** | 2.213 ± 0.192 ** |
| VE | 23.500 ± 4.416 *,## | 0.825 ± 0.057 ## | 0.645 ± 0.072 *,## | 1.575 ± 0.131 *,## |
| Group | PI3K | Akt | mTOR | LC3Ⅱ | Beclin-1 |
|---|---|---|---|---|---|
| Sham | 1.601 ± 0.136 | 1.710 ± 0.110 | 2.013 ± 0.042 | 0.166 ± 0.039 | 0.301 ± 0.067 |
| VG | 0.962 ± 0.116 ** | 1.413 ± 0.127 * | 1.621 ± 0.059 | 0.749 ± 0.078 ** | 0.752 ± 0.133 ** |
| VE | 1.546 ± 0.121 ## | 1.830 ± 0.182 ## | 3.086 ± 0.230 ## | 0.219 ± 0.024 ## | 0.468 ± 0.159 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Liu, F.; Liu, R. The Mechanism of Aerobic Exercise Regulating the PI3K/Akt-mTOR Signaling Pathway Intervenes in Hippocampal Neuronal Apoptosis in Vascular Dementia Rats. Int. J. Environ. Res. Public Health 2023, 20, 1893. https://doi.org/10.3390/ijerph20031893
Gao L, Liu F, Liu R. The Mechanism of Aerobic Exercise Regulating the PI3K/Akt-mTOR Signaling Pathway Intervenes in Hippocampal Neuronal Apoptosis in Vascular Dementia Rats. International Journal of Environmental Research and Public Health. 2023; 20(3):1893. https://doi.org/10.3390/ijerph20031893
Chicago/Turabian StyleGao, Lei, Fushun Liu, and Ruilian Liu. 2023. "The Mechanism of Aerobic Exercise Regulating the PI3K/Akt-mTOR Signaling Pathway Intervenes in Hippocampal Neuronal Apoptosis in Vascular Dementia Rats" International Journal of Environmental Research and Public Health 20, no. 3: 1893. https://doi.org/10.3390/ijerph20031893
APA StyleGao, L., Liu, F., & Liu, R. (2023). The Mechanism of Aerobic Exercise Regulating the PI3K/Akt-mTOR Signaling Pathway Intervenes in Hippocampal Neuronal Apoptosis in Vascular Dementia Rats. International Journal of Environmental Research and Public Health, 20(3), 1893. https://doi.org/10.3390/ijerph20031893






